Published online Sep 14, 2008. doi: 10.3748/wjg.14.5265
Revised: August 18, 2008
Accepted: August 25, 2008
Published online: September 14, 2008
Wireless capsule endoscopy has become the first imaging tool for small bowel examination. Recently, new capsule endoscopy applications have been developed, such as esophageal capsule endoscopy and colon capsule endoscopy. Clinical trials results have shown that colon capsule endoscopy is feasible, accurate and safe in patients suffering from colonic diseases. It could be a good alternative in patients refusing conventional colonoscopy or when it is contraindicated. Upcoming studies are needed to demonstrate its utility for colon cancer screening and other indications such us ulcerative colitis. Comparative studies including both conventional and virtual colonoscopy are also required.
- Citation: Fernandez-Urien I, Carretero C, Borda A, Muñoz-Navas M. Colon capsule endoscopy. World J Gastroenterol 2008; 14(34): 5265-5268
- URL: https://www.wjgnet.com/1007-9327/full/v14/i34/5265.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.5265
| Eliakim[10] | Schoofs[9] | Lewis[11] | |
| Day-2 | Low fiber diet | (-) | (-) |
| Day-1 | 19:00-20:00 PEG 2 L | 18:00-21:00 PEG 3 L | 18:00-21:00 PEG 3 L |
| Day 01 | 07:00-08:00 | 06:00-07:00 | 07:00-08:00 |
| PEG 1 L | PEG 1 L | PEG 1 L | |
| + | + | + | |
| 08:15 | 07:45 | 08:15 | |
| Tegaserod 6 mg | Motilium 20 mg | Tegaserod 6 mg | |
| + | + | + | |
| 08:30 | 08:00 | 08:30 | |
| Capsule ingestion | Capsule ingestion | Capsule ingestion | |
| + | + | + | |
| 10:30 | 10:00 | 10:30 | |
| NaP 30 mL2 | NaP 45 mL2 | NaP 30 mL2 | |
| + | + | + | |
| 13:00 | 14:00 | 13:00 | |
| Tegaserod 6 mg | NaP 30 mL | Tegaserod 6 mg | |
| + | + | + | |
| 14:00 | 16:30 | 14:00 | |
| NaP 15 mL | Bisacodyl | NaP 15 mL | |
| + | Suppository 10 mg | + | |
| 16:30 | 16:30 | ||
| Bisacodyl | Bisacodyl | ||
| suppository 10 mg | suppository 10 mg |
Colorectal cancer (CRC) is the second most frequent cause of cancer-related death in western countries -skin tumors excluded-, after lung cancer in men and breast cancer in women. One out of three patients suffering from CRC will not survive[1]. Nevertheless, it can be considered as a preventable and curable condition. Firstly a preventable condition because in most cases, it develops from colonic adenomas. In fact, colonic adenomas are found in 11% to 40% of average risk population[2-4]. And secondly, a curable condition, because the 5-year survival rate in early stages can reach 90%[1]. For these reasons, conventional colonoscopy is suggested to be the optimal technique to be used for CRC screening programs in high-risk population, allowing a 90% decrease in CRC incidence[5]. However, it has to be considered that no more than 25% of compliance has been achieved in screening programs[5]. This low compliance can be explained by the drawbacks of conventional colonoscopy, such as being painful, patient’s embarrassment or the need of sedation. Non-invasive techniques for colonoscopy, such as CT colonography[6-8] and Colon Capsule Endoscopy[9-11] are currently being evaluated as alternatives to conventional colonoscopy in order to improve the compliance to screening programs.
A large number of clinical trials have been performed testing different capsule designs in healthy volunteers. Finally, Given Imaging Ltd. has developed the final prototype for colon examination, which is called PillCam™ Colon. The PillCam™ Colon capsule has some differences from those used to study the small bowel and the esophagus. It measures 31 mm in length (4 mm longer than the PillCam™ ESO and SB) and 11 mm in diameter (the same as PillCam™ ESO and SB).
Figure 1 shows some morphologic differences between the three capsules commercially available. The PillCam™ Colon capsule has also some technical improvements, such as being equipped with cameras on both ends taking 4 images per second (2 images per camera). Each camera contains an automatic lighting control and has improved optics, which capture more than twice the coverage area and depth of field of PillCam™ SB resulting in a superior observation field. Other specific features are the presence of a longer battery (lasts 9-10 h on average), which can also “hibernate” minutes to hours after ingestion in order to conserve power before the capsule enters into the colon.
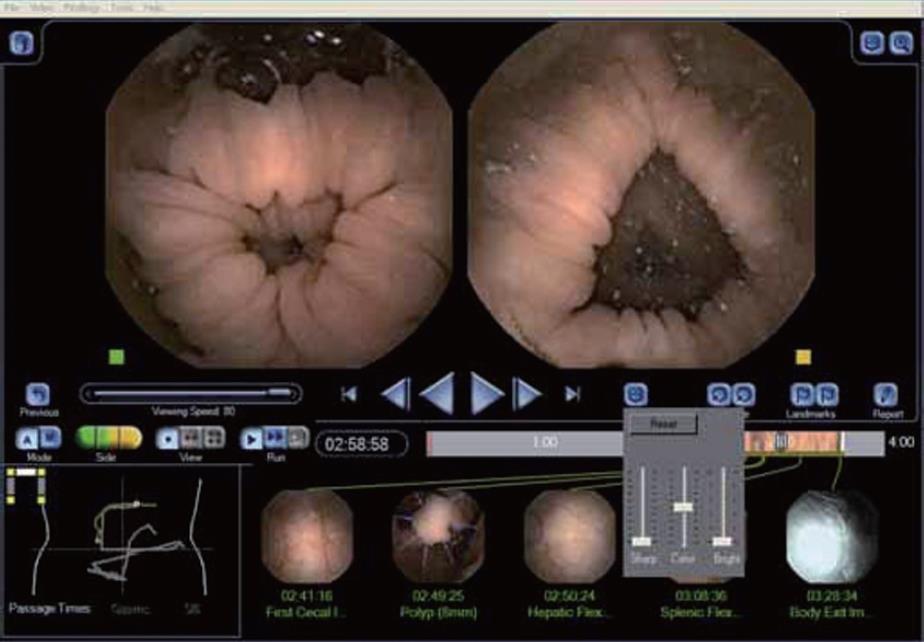
The accessory devices (sensor arrays and Data Recorder) are similar to those ones used by the PillCam™ ESO and SB. The RAPID® software used for images visualization during first clinical trials is a scientific version very similar to RAPID® 4, which includes (I) a larger image display (round-rectangular shape), (II) a complementary capsule localization system and an (III) image enhancement (IE) features (Figure 2). The localization display is similar to the one already in use for the small bowel, but it also includes a schematic diagram of the colon that helps the physician to identify the location of findings, i.e., right, transverse and left colon segments once the main anatomic landmarks (first cecal image, the hepatic flexure, the splenic flexure and the body exit) have been selected. Moreover, this software allows the physician to enhance the appearance of the image by changing their color, brightness and sharpness.
RAPID® Access RT by Given Imaging allows real time visualization of capsule images. This is extremely useful in certain circumstances as the physician can intervene to optimize the procedure by changing patient position or administering medications such as laxatives depending on the images obtained in real time. In the PillCam™ Colon procedure, the importance of the real time viewer is that -as we will see later in more detail- 2 h post PillCam™ Colon Capsule ingestion, the patient has to drink a small amount of Sodium Phosphate. It is well known that Sodium Phosphate can delay gastric emptying time; therefore before giving it to the patient, it is recommended to check if the capsule has left the stomach, which can be easily done with the real time viewer.
The procedure of bowel cleansing until capsule ingestion is similar to that used for traditional colonoscopy. It usually begins one day before capsule ingestion, with the administration of laxatives to the patient. Patients are usually asked to maintain a low fiber diet 2 d before capsule ingestion. After the capsule has been ingested additional laxative and prokinetic agents are provided to the patient in order to (I) maintain the cleanliness of the colon throughout the transit of the capsule and (II) enhance capsule propulsion and excretion within 9-10 h post ingestion. The laxative and prokinetic agents are commercially available, and are provided within their permitted dose. Detailed information of the prep and procedure regimen used in recent trials[9-11] is shown in Table 1.
First results using the same prep as conventional colonoscopy showed low capsule excretion rates (about 20%) which meant low rates of complete colonoscopies. Changes in prep regimens were then introduced (see Table 1) and higher excretion rates were reported by Eliakim et al[9], Schoofs et al[10] and Lewis et al[11] (78%, 84% and 90%, respectively). Moreover, the colon cleansing level reported by Eliakim et al[9] and Schoofs et al[10] was good to excellent in 84.4% and 88% of the patients, respectively. Recently, an undergoing European multicenter study published in abstract form[12] has reported a capsule excretion rate of 93% and good to excellent colon cleansing level in 71% of the patients. All these results are consistent with those obtained by conventional colonoscopy. As the goal of colon capsule endoscopy is to improve patient compliance to CRC screening, other simplified ingestion regimens including Moviprep® as the main laxative product or capsule procedures during the night are currently under evaluation.
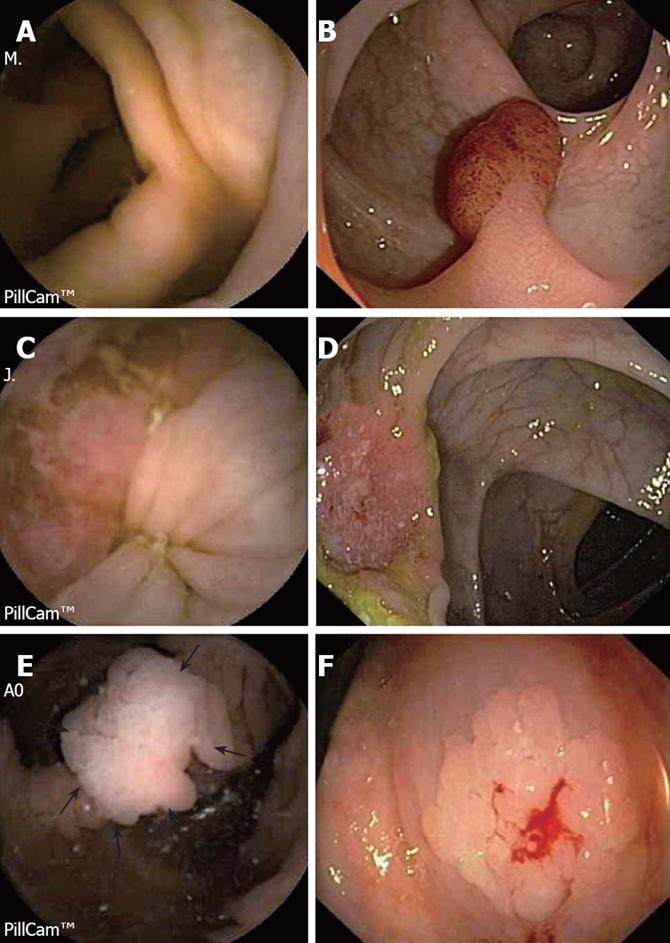
The long term primary objective of the PillCam™ Colon capsule is the average risk population undergoing CRC screening. In order to evaluate the accuracy of the new capsule device, it is being tested in those patients with known or suspected lesions (i.e. polyps or tumors). At the moment, encouraging results has been reported. Two European feasibility studies[9,10] including a total of 132 patients and one American study[11] published in abstract form including 25 patients, have recently evaluated the role of the PillCam™ Colon capsule in detecting colonic lesions. In all of these studies, conventional colonoscopy was considered the gold standard and the American[11] study included also the virtual colonoscopy as an additional comparative procedure. Preliminary results from these studies are resumed in Table 2. The European studies showed a capsule sensitivity (S) for polyps of any size of 69% and 76%, specificity (E) of 81% and 64%, positive predictive value (PPV) of 74% and 83% and negative predictive value (NPV) of 78% and 54%, respectively. Those polyps greater than 6 mm or 3 polyps of 3 mm were considered significant lesions. The accuracy of the colon capsule for significant lesions was very similar as well as for inflammatory lesions (i.e. diverticula, ulcerative colitis, etc). These results are consistent with those obtained in the American study which also showed that conventional colonoscopy was more accurate than colon capsule endoscopy and virtual colonoscopy (81%, 63% and 54%, respectively). In the European multicenter study[12], S, E, PPV and NPV for significant lesions were 66%, 82%, 72% and 77%, respectively; S, E, PPV and NPV for polyps > 6 mm were 64%, 84%, 60% and 86%, respectively and S, E, PPV and NPV for polyps > 10 mm were 60%, 98%, 83% and 93%, respectively. These results are very similar to those obtained by previous studies. On the other hand, the Z line is clearly visualized in 60% of cases by the capsule, even if the capsule is ingested in the standing position[10]. It means that patients undergoing CRC screening by PillCam™ Colon capsule endoscopy could be also screened for Barrett´s esophagus. Figure 3 shows some images from PillCam™ Colon capsule endoscopy.
The capsule colonoscopy seems to be a safe procedure. Capsule or laxatives-related complications during procedures has nor been reported by first feasibility studies[9-11]. On the other hand, 2 of 126 patients (1.6%) were unable to swallow the capsule in the study by Eliakim et al[9]. However, in these patients, the capsule can be easily introduced into the stomach or duodenum by means of the capsule deliver system (US Endoscopy).
As demonstrated by several studies, patients’ compliance for CRC screening is still much lower that for other common neoplastic diseases such as breast and prostate cancer. Therefore, alternative procedures such as colon capsule endoscopy or CT colonography, which may increase patients´ compliance, are welcome. In fact, colon capsule endoscopy is an attractive non-invasive method for CRC screening, especially for those patients who are non-compliant to current screening procedures. Whether colon capsule endoscopy will be cost-effective has not been widely evaluated. However, a recent paper by Hassan et al[13] based on a mathematical Markov model concludes that colon capsule endoscopy may be cost-effective compared with colonoscopy if a 30% patients´ compliance increase is achieved. Moreover, as polyp detection by capsule endoscopy is expected to be more accurate in the future, it may be cost-effective even if compliance rates achieved remains lower than 30%.
Based on current available studies, PillCam™ Colon capsule colonoscopy is a feasible, effective and safe procedure that allows the visualization of the entire colon in most of the cases. It may be complementary to conventional colonoscopy and could be an appropriate exam for those patients who have received incomplete colonoscopy, contraindicated or are unwilling to undergo conventional colonoscopy. Further studies are needed to confirm these results and the possibilities of this new modality for endoscopic examination of the colon and for CRC screening. As colon capsule endoscopy has still some limitations (cannot insufflate air, clean or take biopsies), future capsule prototypes seem to be necessary. Moreover, it is anticipated that future procedures with modified regimens that may be performed at home, possibly over the weekend, can offer a unique method and further enhance patient compliance.
Peer reviewers: Burton I Korelitz, MD, Department of Gastroenterology, Lenox Hill Hospital, 100 East 77th Street, 3 Achelis, New York, N.Y 10021, United States; Francesco Costa, Dr, Dipartimento di Medicina Interna-U.O. di Gastroenterologia Università di Pisa-Via Roma, 67-56122-Pisa, Italy
S- Editor Zhong XY E- Editor Lin YP
| 1. | Ibanez MB, Ribon CC, de la Torre FT, Munoz-Navas M. Evidencia científica en cribado del cáncer colorrectal: manual de actuacion. Madrid: International Marketing & Communication 2006; . |
| 2. | Betes M, Munoz-Navas MA, Duque JM, Angos R, Macias E, Subtil JC, Herraiz M, De La Riva S, Delgado-Rodriguez M, Martinez-Gonzalez MA. Use of colonoscopy as a primary screening test for colorectal cancer in average risk people. Am J Gastroenterol. 2003;98:2648-2654. |
| 3. | Betes Ibanez M, Munoz-Navas MA, Duque JM, Angos R, Macias E, Subtil JC, Herraiz M, de la Riva S, Delgado-Rodriguez M, Martinez-Gonzalez MA. Diagnostic value of distal colonic polyps for prediction of advanced proximal neoplasia in an average-risk population undergoing screening colonoscopy. Gastrointest Endosc. 2004;59:634-641. |
| 4. | Levine JS, Ahnen DJ. Clinical practice. Adenomatous polyps of the colon. N Engl J Med. 2006;355:2551-2557. |
| 5. | Winawer SJ, Zauber AG, Ho MN, O'Brien MJ, Gottlieb LS, Sternberg SS, Waye JD, Schapiro M, Bond JH, Panish JF. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977-1981. |
| 6. | Silva AC, Wellnitz CV, Hara AK. Three-dimensional virtual dissection at CT colonography: unraveling the colon to search for lesions. Radiographics. 2006;26:1669-1686. |
| 7. | Johnson KT, Carston MJ, Wentz RJ, Manduca A, Anderson SM, Johnson CD. Development of a cathartic-free colorectal cancer screening test using virtual colonoscopy: a feasibility study. AJR Am J Roentgenol. 2007;188:W29-W36. |
| 8. | Chaoui AS, Barish MA. Virtual colonoscopy: a new tool for colorectal cancer screening. Curr Opin Gastroenterol. 2001;17:78-85. |
| 9. | Eliakim R, Fireman Z, Gralnek IM, Yassin K, Waterman M, Kopelman Y, Lachter J, Koslowsky B, Adler SN. Evaluation of the PillCam Colon capsule in the detection of colonic pathology: results of the first multicenter, prospective, comparative study. Endoscopy. 2006;38:963-970. |
| 10. | Schoofs N, Deviere J, Van Gossum A. PillCam colon capsule endoscopy compared with colonoscopy for colorectal tumor diagnosis: a prospective pilot study. Endoscopy. 2006;38:971-977. |
| 11. | Lewis B, Rex D, Lieberman D. Capsule Colonoscopy: An Interim Report of a Pilot 3 Arm, Blinded Trial of Capsule Colonoscopy, Virtual Colonoscopy and Colonoscopy. Am J Gastroenterol. 2006;101:S545-S561 (A1470). |
| 12. | Deviere J, Munoz-Navas M, Fernandez-Urien I, Carretero C, Gay G, Delvaux M, Lapalus MG, Ponchon T, Costamagna G, Riccioni ME. PillCam® Colon Capsule Endoscopy Compared to Colonoscopy in Detection of Colon Polyps and Cancers. Gastroenterology. 2008;134:282 (A38). |