Published online Sep 7, 2008. doi: 10.3748/wjg.14.5210
Revised: August 14, 2008
Accepted: August 21, 2008
Published online: September 7, 2008
AIM: To evaluate the efficacy and safety of gemcitabine-oxaliplatin (GEMOX) combined with huachansu (cinobufagin) injection treatment in patients with locally advanced or metastatic gallbladder carcinoma (GBC), and to assess the quality of life (QOL) of such patients.
METHODS: Twenty-five patients with locally advanced or metastatic GBC were treated with intravenous gemcitabine (1000 mg/m2) over 30 min on days 1 and 8, 2 h infusion of oxaliplatin (120 mg/m2) on day 1, and 2-3 h infusion of huachansu (20 mL/m2) on days -3-11, every 3-4 wk. Treatment was continued until occurrence of unacceptable toxicity or disease progression. QOL of patients was assessed by the EORTC QLQ-C30 at baseline, at the end of the first, third and sixth chemotherapy cycles, and 1 mo after the treatment.
RESULTS: Among the 25 patients with a median age of 64 years (range 42-78 years), 23 were evaluable in the study. A total of 137 cycles of therapy were performed and the median cycle was 5 (range 1-8) per patient. Out of the 23 patients whose response could be evaluated, 8 partial responses (PR) were observed (34.8%), while 7 patients (30.4%) demonstrated a stable disease (SD). The disease control rate was 65.2%. Progression of cancer was observed in 8 (34.8%) patients. The median progression-free and overall survival time was 5.8 mo (95% CI: 4.5-7.1 mo) and 10.5 mo, respectively. The therapy was well tolerated, with moderate myelosuppression as the main toxicity. Anemia grade 2 was seen in 16.0%, neutropenia grade 3 in 8.0% and thrombocytopenia grade 3 in 24.0% of patients, respectively. Non-hematologic toxicity ranged from mild to moderate. No death occurred due to toxicity. The QOL of patients was improved after chemotherapy, and the scores of QOL were increased by 10 to 20 points.
CONCLUSION: GEMOX combined with huachansu (cinobufagin) injection is well tolerated, effective, thus improving the QOL of patients with advanced GBC.
- Citation: Qin TJ, Zhao XH, Yun J, Zhang LX, Ruan ZP, Pan BR. Efficacy and safety of gemcitabine-oxaliplatin combined with huachansu in patients with advanced gallbladder carcinoma. World J Gastroenterol 2008; 14(33): 5210-5216
- URL: https://www.wjgnet.com/1007-9327/full/v14/i33/5210.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.5210
| Characteristic | Data |
| Total No. of patients (evaluable for response) | 25 (23) |
| Sex (female/male) | 15/10 |
| Median age (range) | 64 (42-78) |
| Nevin tumor stage | n (%) |
| III | 4 (16.0) |
| IV | 12 (48.0) |
| V | 9 (36.0) |
| ECOG performance status at baseline | |
| 0 | 3 (12.0) |
| 1 | 17 (68.0) |
| 2 | 5 (20.0) |
| Site of metastasis | |
| Liver | 7 (28.0) |
| Lung | 2 (8.0) |
| Lymph nodes | 12 (48.0) |
| Peritoneum | 3 (12.0) |
| Bone | 2 (8.0) |
| Recurrence after surgical resection | 5 (20.0) |
| Pre-treatment requiring stent or percutaneous transhepatic drainage because of obstructive jaundice, | 4 (16.0) |
| Pre-treatment chemotherapy | 4 (16.0) 5-FU-based chemotherapy |
| Liver and renal functions | |
| Total bilirubin (6-20.5 μmol/L1) | |
| Median | 18 |
| Range | 7-58 |
| Gamma-glutamyltransferase (183.4-833.5 nkat/L1) | |
| Median | 1 033.5 |
| Range | 283.4-2 583.9 |
| Aspartate aminotransferase/Alanine aminotransferase (0-666.8 nkat/L1) | |
| Median | 950.2 |
| Range | 383.4-1 600.3 |
| Blood urea nitrogen (3.2-7.0 mmol/L1) | |
| Median | 6.2 |
| Range | 3.6-7.8 |
| Serum creatinine (44-140 μmol/L1) | |
| Median | 78 |
| Range | 48-121 |
| CA19-9 (0-39 kU/L1) | |
| Median | 496 |
| Range | 7-10 463 |
| Toxicity | NCI-CTC grade | |||
| 1 | 2 | 3 | 4 | |
| Leukopenia | 9 (36.0) | 5 (20.0) | 2 (8.0) | 0 |
| Neutropenia | 6 (24.0) | 7 (28.0) | 2 (8.0) | 0 |
| Anemia | 2 (8.0) | 4 (16.0) | 0 | 0 |
| Thrombocytopenia | 7 (28.0) | 5 (20.0) | 6 (24.0) | 2 (8.0) |
| Diarrhea | 1 (4.0) | 0 | 0 | 0 |
| Nausea/emesis | 5 (20.0) | 0 | 0 | 0 |
| Peripheral neuropathy | 16 (64.0) | 0 | 0 | 0 |
| Pain | 2 (8.0) | 0 | 0 | 0 |
| Domain/Item | Baseline | First cycle | Third cycle | Sixth cycle | 1 mo |
| Functioning | |||||
| Physical | 65.4 ± 17.0 | 57.0 ± 20.3 | 64.4 ± 21.0 | 70.6 ± 18.3 | 77.5 ± 16.4a |
| Role | 57.1 ± 26.3 | 55.0 ± 24.3 | 58.5 ± 26.2 | 60.4 ± 25.4 | 63.4 ± 27.2 |
| Emotional | 54.3 ± 33.0 | 47.2 ± 36.8 | 59.4 ± 39.1 | 70.5 ± 24.2 | 69.4 ± 30.1a |
| Cognitive | 66.1 ± 24.6 | 68.1 ± 30.2 | 77.4 ± 26.3 | 79.1 ± 24.0 | 79.5 ± 21.7a |
| Social | 47.2 ± 30.4 | 39.4 ± 33.6 | 44.1 ± 29.0 | 41.1 ± 30.7 | 48.2 ± 32.6 |
| Global QOL | 52.3 ± 20.0 | 41.4 ± 24.3 | 50.6 ± 29.7 | 55.4 ± 30.0 | 65.3 ± 22.4a |
| symptoms | |||||
| Fatigue | 43.3 ± 31.2 | 51.2 ± 33.4 | 40.1 ± 27.4 | 42.1 ± 22.0 | 38.2 ± 28.6 |
| Nausea and vomiting | 7.6 ± 6.2 | 9.0 ± 5.1 | 7.4 ± 4.3 | 5.4 ± 2.5 | 3.2 ± 2.1a |
| Pain | 44.5 ± 21.6 | 28.0 ± 18.3 | 25.5 ± 12.4 | 24.3 ± 9.1 | 26.0 ± 10.4a |
There is a marked worldwide geographic variation in gallbladder carcinoma (GBC) incidence, which correlates with the prevalence of cholelithiasis, and the highest prevalence of GBC is in Israel, Mexico, Chile, Japan, and America[1,2]. The incidence and mortality of GBC in China has had a tendency to increase in recent years[3].
The early symptoms of GBC are similar to those of other gallbladder diseases, such as gallstones or infection. But, no characteristic symptoms could be observed at its early stage. In fact, early gallbladder cancer is discovered often when the gallbladder is removed as a treatment for gallstones. Otherwise, gallbladder cancer is often at its advanced stage at the time when it is diagnosed, and has a postoperative 5-year survival rate of less than 5%[4] with a high relapse rate.
Conventional surgery is considered the most effective treatment for GBC. But, many cases are inoperable at the time of its diagnosis. Chemotherapy has recently shown its effect on gallbladder cancer. The most commonly used chemotherapeutic agent is 5-fluorouracil (5-FU), which is often used alone or in combination with leucovorin. Several small trials of combined regimens for GBC, using 5-FU, cisplatin, mitomycin and/or leucovorin, have been reported to have mixed results. Other chemotherapeutic agents that are now in clinical trials include capecitabine, oxaliplatin[5], gemcitabine[6-8], erlotinib[9], etc. In addition, more and more people have paid their close attention to Chinese medicines for the prevention and treatment of cancer. Huachansu (cinobufagin) is just a widely used antitumor agent of traditional Chinese medicine in China[10,11].
Because the clinical data have demonstrated the effectiveness of gemcitabine on pancreatic cancer, and gallbladder shares a common embryological origin with the exocrine pancreas, we used gemcitabine-oxaliplatin (GEMOX) combined with huachansu injection (cinobufagin) in treatment of advanced GBC.
Since the prognosis of GBC patients is usually poor, it is important to maintain their health-related quality of life (HRQOL). The QOL of patients has been an important endpoint in assessment of GBC treatment. However, to our knowledge, only a few studies discussing QOL of patients with GBC, and there is no report on GEMOX and huachansu regimen for GBC patients. The aim of this study was to evaluate the efficacy and safety of GEMOX plus huachansu treatment of GBC, and to assess the QOL of GBC patients.
From January 2003 to July 2005, of the 25 enrolled patients (10 males and 15 females) with locally advanced or metastatic GBC, 23 were assessable. The patients were required to have histologically confirmed diagnosis, measurable computed tomography (CT) scan or magnetic resonance imaging (MRI), Eastern Cooperative Oncology Group (ECOG) performance status (PS) 0-2, age < 78 years, a life expectancy > 3 mo. No patient underwent anticancer procedures within 1 mo before the present study. The laboratory criteria were leukocyte count ≥ 4.0 × 109/L, neutrophils ≥ 1.5 × 109/L, platelet count ≥ 100 × 109/L, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) ≤ 2.5 × upper limit of normal (ULN), serum creatinine < 1.5 × ULN, serum bilirubin value < 2.5 × ULN. Basic CA19-9 level was recorded. Patients with active infections, unstable cardiovascular conditions, brain metastases, other malignancy or serious medical illnesses were excluded from this study. The pre-treatment characteristics are listed in Table 1.
Tumor size was assessed by CT scan or MRI within 3 wk prior to the first cycle, and baseline biological analysis was performed within 1 wk. A physical examination and complete blood cell count were performed 2-3 d before each cycle. Blood count was obtained each week to determine the level of myelosuppression. After every three cycles, a full clinical evaluation including performance status and physical examination was performed.
All patients received intravenous gemcitabine (1000 mg/m2) over 30 min on days 1 and 8, 2 h oxaliplatin infusion (120 mg/m2) on day 1 (oxaliplatin was discontinued if specific cumulative peripheral sensory neuropathy of NCI CTC grade 3 occurred), and 2-3 h huachansu infusion (20 mL/m2) (0.5 g/mL) in 500 mL of 50 g/L glucose solution on days -3-11. Treatment was repeated every 3-4 wk until limiting toxicity or disease progression occurred, or further treatments were refused by patients. Biliary bypass or stenting was required in four patients before the treatment.
After two cycles of chemotherapy, patients were followed up every 4-6 wk till February 2007.
Tumor response was evaluated every three cycles by CT scan or MRI, using standard RECIST criteria[12]. Complete response (CR) was defined as a disappearance of all signs and symptoms of disease. Partial response (PR) was defined as a decrease > 30% of the sum of the largest diameters of target (measurable) lesions without appearance of new lesions or progression of non-target (evaluable) lesions. To be assigned a response status, changes in tumor measurement were confirmed by a repeated assessment performed no less than 4 wk after the criteria for response were first met. Stable disease (SD) was defined as no sufficient shrinkage to qualify partial response or less than a 20% increase in the sum of the largest diameters of target lesions without appearance of new lesions or progression of non-target lesions. Progressive disease (PD) was defined as a 20% increase in the sum of the largest diameters of target lesions or as appearance of new lesions or as progression of non-target lesions. Disease control was defined as the absence of tumor progression (i.e. complete and partial response and SD) for at least 2 mo. Progression-free survival (PFS) was determined from the first day of treatment until clinical progression or tumor progression assessed by CT scan. Overall survival (OS) was determined from the first day of treatment until the date of death. OS and PFS were analyzed using the Kaplan-Meier method. Toxicity was evaluated at each cycle according to the NCI CTC version 2.0.
The validated traditional Chinese version of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30 (EORTC QLQ-C30) was used to measure the patients’ QOL. Patients completed the EORTC QLQ-C30 at baseline, at the end of the first, third and sixth chemotherapeutic cycles, and 1 mo after completion of chemotherapy.
The QLQ-C30 is a 30-item standardized measure that includes a global QOL/ overall health status scale, five functioning scales (physical, role, emotional, cognitive, and social), three multi-item symptom scales (fatigue, pain, nausea and vomiting) and six single items (dyspnea, sleep disturbance, appetite loss, diarrhea, constipation, and financial impact). The raw scores obtained from the EORTC questionnaire were converted to scores ranging from 0 to 100 using linear transformation according to the scoring procedure. Higher scores on the functioning scales, and the overall health status indicate a higher functioning level and a better QOL. Higher scores on the symptom scales or single item scales represent a higher level of symptoms or problems.
QOL scores were determined in our study in the following domains: physical, role, emotional, cognitive and social function, global QOL/overall health status, fatigue, nausea and vomiting, and pain.
The objective response rate, progression-free survival, overall survival, toxicity and QOL were observed, and the 95% confidence interval (CI) was calculated using the method of Clopper and Pearson. Overall survival, progression-free survival, death or last follow-up was made using the Kaplan-Meier method using SPSS version 12.0 software. The life table method was used to evaluate the 1-year survival rate. Data were expressed as mean ± SD. QOL score analysis was done using the paired t-test for comparison between baseline and 1 mo after completing chemotherapy. P < 0.05 was considered statistically significant, whereas a mean difference of 10 or more points in QOL scales represents a clinically significant/relevant difference[13].
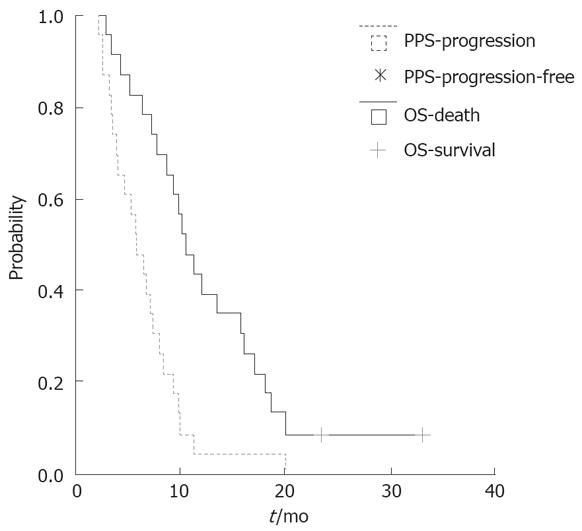
A total of 25 patients (10 males and 15 females) were included this trial and 23 were assessable. Twenty-one patients (84.0%) received GEMOX plus huachansu injection as the first-line and 4 (16.0%) as the second-line chemotherapy. A total of 137 cycles of therapy were performed, the median cycle was 5 (range 1-8) per patient. Up to February 2007, two patients were still alive. The median follow-up time was 11.5 (range, 2-33) mo. The one-year survival rate was 39.1%. There was no complete response. However, out of the 23 patients whose response could be evaluated, 8 (34.8%) went into PR, 7 (30.4%) demonstrated SD, 8 (34.8%) had progression of the disease. The disease control rate was 65.2%. The median progression-free and overall survival was 5.8 mo (95% CI: 4.5-7.1 mo) and 10.5 mo, respectively (Figure 1).
Four patients (16.0%) required biliary tract decompression by endoscopic or percutaneous stenting because of obstructive jaundice before chemotherapy.
Basic CA19-9 levels were recorded in all patients (median 496; range 7-10 463 kU/L), and 15 patients (60%) had elevated baseline levels. Serum CA19-9 levels were measured every 3-4 wk. The relative changes of CA19-9 during chemotherapy was negatively correlated with the baseline and progression-free survival (P = 0.03).
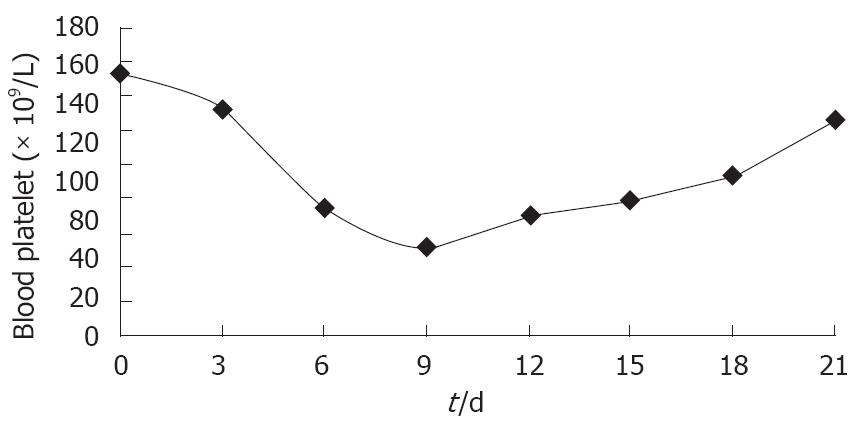
All patients were evaluable for toxicity. Gemcitabine in combination with oxaliplatin induced neutropenia, thrombocytopenia, nausea/vomiting, and peripheral neuropathy due their toxicity. It was observed by us that gemcitabine-based therapy was associated with a higher rate of thrombocytopenia among Chinese patients with cancer. Myelosuppression was the most frequent side-effect in our study. Anemia grade 2, neutropenia grade 3 and thrombocytopenia grade 3 were observed in 16%, 8% and 24% of patients, respectively. The nadir of thrombocytes appeared on days 6-12 [(51.0 ± 19.4) × 109/L, mean ± SD]. Recombinant human interleukin-11 (IL-11) was used to treat chemotherapy-induced thrombocytopenia. The platelet count returned to normal [(126.0 ± 18.2) × 109/L] after 3-8 d of IL-11 treatment at a dose of 25-50 μg/kg per day (Figure 2).
The symptoms induced by grade 1 non-hematologic toxicity included nausea/emesis (20.0%), diarrhea (4.0%), pain (8.0%), and peripheral neuropathy (64.0%). Sixteen patients (64.0%) felt anaesthesia of hands and feet, which was mild and did not interfere with their functions.
GEMOX combined with huachansu injection regimen was well tolerated (Table 2) with moderate myelosuppression as the main toxicity. No treatment-related death occurred.
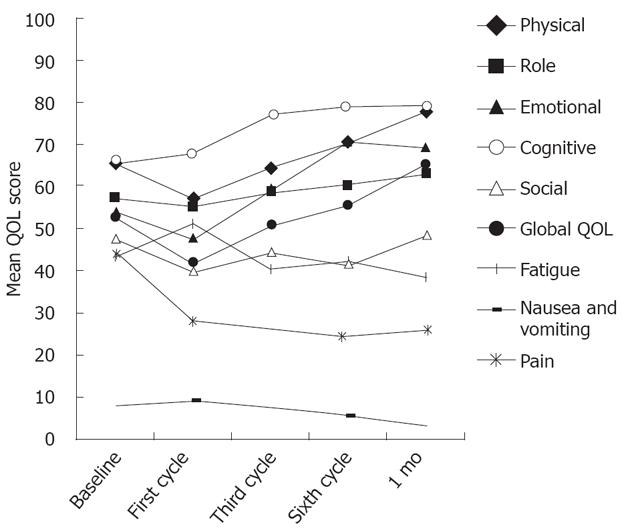
The completion rate of QOL questionnaire declined from 96.0% at baseline to 73.9%, 1 mo after completion of chemotherapy. The mean score and standard deviation for the EORTC QLQ-C30 are presented in Table 3. An improvement of more than 10 points was found in the global QOL, physical, cognitive and emotional functioning, whereas symptoms such as pain decreased more than 18 points after chemotherapy. One month after the completion of treatment, physical functioning (PF) increased over baseline levels by approximately 12 points, and the global QOL score increased from 52.3 ± 20.0 at baseline to 65.3 ± 22.4, 1 mo after completion of chemotherapy. As compared with baseline, the patients reported fatigue that was worse immediately after the first cycle. However, fatigue then improved from the end of the first cycle to 1 mo after treatment. The five functional scales, global QOL, and multi-item symptom scales are shown in Figure 3. One month after treatment, the trend to improve the functioning, global QOL and symptoms was significant.
Due to the high mortality rate of GBC in China, we made this study to test the efficacy and safety of GEMOX combined with huachansu injection (cinobufagin) in patients with locally advanced or metastatic GBC and to assess the patients’ QOL.
GBC, though rare, has a very poor prognosis. There is no generally accepted standard chemotherapy for advanced, non-resectable cancer of the gallbladder or biliary tree. The median survival time of advanced GBC patients who receive best supportive care is approximately 6 mo[14].
Some new chemotherapeutic agents have been used in treatment of patients with advanced biliary tract cancer recently, such as gemcitabine, 5-fluorouracil[15-17], capecitabine[18], cisplatin[19,20], oxaliplatin or carboplatin. It was reported that gemcitabine is active against pancreatic and advanced biliary tract adenocarcinoma, and able to induce a response rate of 8.0%-60.0%[21,22].
Gallardo[23] conducted a phase II trial with gemcitabine (1 000 mg/m2 over 30 min weekly for 3 wk followed by a week of rest) in patients with locally advanced or metastatic GBC, and found that the overall response rate was 36.0%. The cancer remained stable in 6 (25.0%) patients, and progressed in 10 (40.0%) patients. The median survival time was 30 wk. In another phase II trial with 24-h gemcitabine infusion weekly in patients with advanced gallbladder and biliary tract carcinoma, 18 patients were evaluated for response. One partial response was observed (6.0%), the disease control rate was 61.0%, the median time of tumor progression was 3.6 mo and the median overall survival time was 7.5 mo[24].
Andre et al[25] used GEMOX regimen to treat patients with advanced biliary tract adenocarcinoma, and found that the objective response was 36%, the median PFS time was 5.7 mo, the overall survival time was 15.4 mo in group A (PS 0-2), while the objective response was 22.0%, the median PFS time was 3.9 mo and the median OS time was 7.6 mo in group B (PS > 2).
Huachansu, one of the most widely studied traditional Chinese medicine, is a water soluble extract from Bufo toad skin, and can be sued in treatment of cancer, especially liver and pancreatic cancer. Some studies demonstrated that huachansu injection can improve the QOL of patients and has been used as a treatment of cancer in China[26-28]. It was reported that digitalis-like cinobufagin can protect prostate cancer cells from proliferation. The protein expression of active caspase 3 in LNCaP, DU145, and PC3 cells was increased after treated with combined cinobufagin and EGTA, the expression of Fas was increased, the expression of Bax was down-regulated in nuclei, and the protein expression of cytosolic cytochrom C was also increased after treatment with cinobufagin in these cell lines[29]. It has been shown that bufalin or cinobufagin increases Ca2+ and apoptosis in cancer cells, caspase 3 activities in DU145 and PC3 cells, and caspase 9 activities in LNCaP cells after a 24 h culture[30].
QOL is a multidimensional concept including physical, emotional, social, and daily-life functioning as well as disease symptoms and treatment from the patient's perspective. Improving QOL and disease-associated symptoms is increasingly important for patients with advanced GBC. QOL is now regarded as a biologically and clinically meaningful outcome that is as important as disease-free, and overall survival with regard to anticancer treatment. Huachansu injection was given 3 d before GEMOX in our study, and its effectiveness on pain relief appeared 1-2 d later (9/14 patients). The overall life quality of patients entered this trial improved 10 to 20 points. The patients undergoing GEMOX and huachansu regimen achieved better QOL outcomes. The partial response rate was 34.8%, and the disease control rate was 65.2%. The median progression-free and median overall survival was 5.8 mo and 10.5 mo, respectively. The patients tolerated the treatment well with moderate myelosuppression as the main toxicity. No treatment-related deaths occurred.
In conclusion, combined GEMOX and huachansu injection regimen can be used in treatment of GBC patients, especially those with advanced or metastatic GBC.
The difficulty in early diagnosis of gallbladder carcinoma (GBC) is its poor specificity in clinical and ambiguous early symptoms, thus affecting its prognosis. Some patients are found having GBC only when other diseases are diagnosed and treated. Surgery is the only curative treatment for gallbladder cancer. However, because of frequent local and distant recurrence, radical surgery at its advanced stage is often unsuccessful. Thus, chemotherapy for patients with advanced GBC seems to be a better choice of treatment. However, no standard chemotherapy for GBC has yet been established.
The increasing number of papers on chemotherapy for GBC emphasizes the need of a new standard beyond 5-FU. At present, clinical studies on the treatment of GBC with new chemotherapeutic agents are underway. Further clinical trials, especially large multi-institutional RCTs (phase III studies) using novel agents such as gemcitabine, should be performed in order to establish a standard treatment for GBC.
In China, huachansu used in treatment of patients with lung cancer and hepatocellular carcinoma has achieved rather good results in suppressing the growth of cancer, alleviating of pain and fatigue, and improving the function of patients’ immune system. Our findings provide certain possible evidence that combined gemcitabine-oxaliplatin (GEMOX) and huachansu chemotherapy may improve the survival and QOL of GBC patients.
The results of this study indicate that combined GEMOX and huachansu chemotherapy is an effective regimen for metastatic GBC. The patients can tolerate it well. Toxicities are mostly hematological and easily manageable. If this combined regimen could be applied in clinic practice, the patients would have a longer survival time.
The World Health Organization (WHO) defines QOL as" an individual's perception of his or her position in life in the context of culture and value system in which he or she lives in relation to his or her goal, expectation, standard and concern. It is a broad-ranging concept affected in a complex way by the person's physical health, psychological state, independence, social relationships, and his or her relationship to salient feature of his or her environment." Studies showed that QOL is associated to the survival of cancer patients.
This is an interesting study. The authors evaluated the efficacy and safety of combined GEMOX and huachansu injection (cinobufagin) treatment in patients with locally advanced or metastatic gallbladder carcinoma, and assessed the patients’ QOL. The study was well designed.
Peer reviewer: Nahum Méndez-Sánchez, PhD, Department of Gastroenterology and Liver Unit, Medica Sur Clinic & Foundation, Puente de Piedra 150, Col. Toriello Guerra, Mexico City 14050, Mexico
S- Editor Li DL L- Editor Wang XL E- Editor Lin YP
| 1. | Yang DZ, He J, Zhang JC, Wang ZR. Expression of angiostatin cDNA in human gallbladder carcinoma cell line GBC-SD and its effect on endothelial proliferation and growth. World J Gastroenterol. 2006;12:2762-2766. |
| 2. | Eckel F, Schmid RM. Chemotherapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Br J Cancer. 2007;96:896-902. |
| 3. | Xiao WD, Peng CH, Zhou GW, Wu WD, Shen BY, Yan JQ, Yang WP, Li HW. Surgical treatment for Nevin stage IV and V gallbladder carcinoma: report of 70 cases. Hepatobiliary Pancreat Dis Int. 2005;4:589-592. |
| 4. | Levy AD, Murakata LA, Rohrmann CA Jr. Gallbladder carcinoma: radiologic-pathologic correlation. Radiographics. 2001;21:295-314; questionnaire, 549-555. |
| 5. | Androulakis N, Aravantinos G, Syrigos K, Polyzos A, Ziras N, Tselepatiotis E, Samonis G, Kentepozidis N, Giassas S, Vamvakas L. Oxaliplatin as first-line treatment in inoperable biliary tract carcinoma: a multicenter phase II study. Oncology. 2006;70:280-284. |
| 6. | Eng C, Ramanathan RK, Wong MK, Remick SC, Dai L, Wade-Oliver KT, Mani S, Kindler HL. A Phase II trial of fixed dose rate gemcitabine in patients with advanced biliary tree carcinoma. Am J Clin Oncol. 2004;27:565-569. |
| 7. | Park JS, Oh SY, Kim SH, Kwon HC, Kim JS, Jin-Kim H, Kim YH. Single-agent gemcitabine in the treatment of advanced biliary tract cancers: a phase II study. Jpn J Clin Oncol. 2005;35:68-73. |
| 8. | Tsavaris N, Kosmas C, Gouveris P, Gennatas K, Polyzos A, Mouratidou D, Tsipras H, Margaris H, Papastratis G, Tzima E. Weekly gemcitabine for the treatment of biliary tract and gallbladder cancer. Invest New Drugs. 2004;22:193-198. |
| 9. | Philip PA, Mahoney MR, Allmer C, Thomas J, Pitot HC, Kim G, Donehower RC, Fitch T, Picus J, Erlichman C. Phase II study of erlotinib in patients with advanced biliary cancer. J Clin Oncol. 2006;24:3069-3074. |
| 10. | Zhang P, Cui Z, Liu Y, Wang D, Liu N, Yoshikawa M. Quality evaluation of traditional Chinese drug toad venom from different origins through a simultaneous determination of bufogenins and indole alkaloids by HPLC. Chem Pharm Bull (Tokyo). 2005;53:1582-1586. |
| 11. | Dai LP, Wang ZM, Gao HM, Jiang X, Ding GZ. [Determination of bufothionine in skin of Bufo bufo gargarizans and Huachansu injection]. Zhongguo Zhongyao Zazhi. 2007;32:224-226. |
| 12. | Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205-216. |
| 13. | Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16:139-144. |
| 14. | Nehls O, Oettle H, Hartmann JT, Hofheinz RD, Hass HG, Horger MS, Koppenhofer U, Hochhaus A, Stieler J, Trojan J. Capecitabine plus oxaliplatin as first-line treatment in patients with advanced biliary system adenocarcinoma: a prospective multicentre phase II trial. Br J Cancer. 2008;98:309-315. |
| 15. | Murad AM, Guimaraes RC, Aragao BC, Rodrigues VH, Scalabrini-Neto AO, Padua CA, Moore FC. Phase II trial of the use of gemcitabine and 5-fluorouracil in the treatment of advanced pancreatic and biliary tract cancer. Am J Clin Oncol. 2003;26:151-154. |
| 16. | Knox JJ, Hedley D, Oza A, Siu LL, Pond GR, Moore MJ. Gemcitabine concurrent with continuous infusional 5-fluorouracil in advanced biliary cancers: a review of the Princess Margaret Hospital experience. Ann Oncol. 2004;15:770-774. |
| 17. | Hsu C, Shen YC, Yang CH, Yeh KH, Lu YS, Hsu CH, Liu HT, Li CC, Chen JS, Wu CY. Weekly gemcitabine plus 24-h infusion of high-dose 5-fluorouracil/leucovorin for locally advanced or metastatic carcinoma of the biliary tract. Br J Cancer. 2004;90:1715-1719. |
| 18. | Knox JJ, Hedley D, Oza A, Feld R, Siu LL, Chen E, Nematollahi M, Pond GR, Zhang J, Moore MJ. Combining gemcitabine and capecitabine in patients with advanced biliary cancer: a phase II trial. J Clin Oncol. 2005;23:2332-2338. |
| 19. | Thongprasert S, Napapan S, Charoentum C, Moonprakan S. Phase II study of gemcitabine and cisplatin as first-line chemotherapy in inoperable biliary tract carcinoma. Ann Oncol. 2005;16:279-281. |
| 20. | Doval DC, Sekhon JS, Gupta SK, Fuloria J, Shukla VK, Gupta S, Awasthy BS. A phase II study of gemcitabine and cisplatin in chemotherapy-naive, unresectable gall bladder cancer. Br J Cancer. 2004;90:1516-1520. |
| 21. | Scheithauer W. Review of gemcitabine in biliary tract carcinoma. Semin Oncol. 2002;29:40-45. |
| 22. | Eckel F, Schmelz R, Erdmann J, Mayr M, Lersch C. Phase II trial of a 24-hour infusion of gemcitabine in previously untreated patients with advanced pancreatic adenocarcinoma. Cancer Invest. 2003;21:690-694. |
| 23. | Gallardo JO, Rubio B, Fodor M, Orlandi L, Yanez M, Gamargo C, Ahumada M. A phase II study of gemcitabine in gallbladder carcinoma. Ann Oncol. 2001;12:1403-1406. |
| 24. | von Delius S, Lersch C, Schulte-Frohlinde E, Mayr M, Schmid RM, Eckel F. Phase II trial of weekly 24-hour infusion of gemcitabine in patients with advanced gallbladder and biliary tract carcinoma. BMC Cancer. 2005;5:61. |
| 25. | Andre T, Tournigand C, Rosmorduc O, Provent S, Maindrault-Goebel F, Avenin D, Selle F, Paye F, Hannoun L, Houry S. Gemcitabine combined with oxaliplatin (GEMOX) in advanced biliary tract adenocarcinoma: a GERCOR study. Ann Oncol. 2004;15:1339-1343. |
| 26. | Ye M, Qu G, Guo H, Guo D. Specific 12 beta-hydroxylation of cinobufagin by filamentous fungi. Appl Environ Microbiol. 2004;70:3521-3527. |
| 27. | Ma XC, Xin XL, Liu KX, Han J, Guo DA. Microbial transformation of cinobufagin by Syncephalastrum racemosum. J Nat Prod. 2008;71:1268-1270. |
| 28. | Ye M, Guo H, Guo H, Han J, Guo D. Simultaneous determination of cytotoxic bufadienolides in the Chinese medicine ChanSu by high-performance liquid chromatography coupled with photodiode array and mass spectrometry detections. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;838:86-95. |