Published online Sep 7, 2008. doi: 10.3748/wjg.14.5197
Revised: June 30, 2008
Accepted: July 7, 2008
Published online: September 7, 2008
AIM: To examine whether the sedative effects assessed by psychomotor tests would depend on the cytochrome P450 (CYP) 2C19 genotypes after an infusion regimen of diazepam commonly used for gastrointestinal endoscopy in Japan.
METHODS: Fifteen healthy Japanese volunteers consisting of three different CYP2C19 genotype groups underwent a critical flicker fusion test, an eye movement analysis and a postural sway test as a test for physical sedative effects, and a visual analog scale (VAS) symptom assessment method as a test for mental sedative effects during the 336 h period after the intravenous infusion of diazepam (5 mg).
RESULTS: The physical sedative effects assessed by the critical flicker test continued for 1 h (t values of 5 min, 30 min and 60 min later: 4.35, 5.00 and 3.19, respectively) and those by the moving radial area of a postural sway test continued for 3 h (t values of 5 h, 30 h, 60 min and 3 h later: -4.05, -3.42, -2.17 and -2.58, respectively), which changed significantly compared with the baseline level before infusion (P < 0.05). On the other hand, the mental sedative effects by the VAS method improved within 1 h. The CYP2C19 genotype-dependent differences in the postinfusion sedative effects were not observed in any of the four psychomotor function tests.
CONCLUSION: With the psychomotor tests, the objective sedative effects of diazepam continued for 1 h to 3 h irrespective of CYP2C19 genotype status and the subjective sedative symptoms improved within 1 h. Up to 3 h of clinical care appears to be required after the infusion of diazepam, although patients feel subjectively improved.
- Citation: Sugimoto M, Furuta T, Nakamura A, Shirai N, Ikuma M, Misaka S, Uchida S, Watanabe H, Ohashi K, Ishizaki T, Hishida A. Maintenance time of sedative effects after an intravenous infusion of diazepam: A guide for endoscopy using diazepam. World J Gastroenterol 2008; 14(33): 5197-5203
- URL: https://www.wjgnet.com/1007-9327/full/v14/i33/5197.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.5197
| Homozygous EM | Heterozygous EM | PM | P | |
| Genotype status | Wild-type/Wild-type (n = 5) | Wild-type/CYP2C19 mutation in exon 5 (n = 3) | CYP2C19 mutation in exon 5/CYP2C19 mutation in exon 5 (n = 2) | |
| Wild-type/CYP2C19 mutation in exon 4 (n = 2) | CYP2C19 mutation in exon 4/CYP2C19 mutation in exon 4 (n = 1) | |||
| CYP2C19 mutation in exon 5/CYP2C19 mutation in exon 4 (n = 2) | ||||
| Male/Female | 3/2 | 3/2 | 5/0 | 0.472 |
| Age (yr) | 24 (22-31) | 29 (25-44) | 23 (22-35) | 0.093 |
| Height (cm) | 170 (154-174) | 177 (155-181) | 171 (169-180) | 0.400 |
| Body weight (kg) | 62 (51-67) | 67 (51-78) | 65 (57-78) | 0.424 |
Gastrointestinal endoscopy is commonly performed for the screening and/or diagnosis of upper gastrointestinal disorders. Recent advances in gastrointestinal endoscopy are remarkable, and gastrointestinal endoscopy now plays an important role in the treatment of several upper gastrointestinal disorders (e.g. endoscopic mucosal resection of gastric cancer in the early stage and treatment of haemorrhage from peptic ulcer). However, gastrointestinal endoscopy is invasive and accompanied with distress of patients. Therefore, benzodiazepines, such as diazepam and midazolam, are commonly used as a premedication for gastrointestinal endoscopy[1,2]. The administration of benzodiazepines as premedications for endoscopy reduces distress of patients and results in a better patient acceptance and compliance for endoscopy[1,2]. However, use of benzodiazepines sometimes causes undesirable events; e.g. residual benzodiazepine after endoscopy may cause sustained distraction, which leads to some accidents. For the prevention of such undesirable accidents, the dose of benzodiazepines should, therefore, be minimized or optimized. However, a reduced dose of benzodiazepines sometimes results in an insufficient sedation for endoscopy, which is a dilemma for performing the safe and comfortable endoscopy.
Diazepam is metabolized by cytochrome P450 (CYP) 3A4 to temazepam, and by CYP3A4 and CYP2C19 to N-desmethyldiazepam[3,4]. There are genetic differences in the activity of CYP2C19[3,4]. The pharmacokinetics of diazepam significantly depend on CYP2C19 genotype status[5-9], as observed in proton pump inhibitors, such as omeprazole and rabeprazole[10,11]. The CYP2C19 genotypes are classified into the three groups: homozygous extensive metabolizers (EMs), heterozygous EMs, and poor metabolizers (PMs)[12-14]. In PMs, the plasma diazepam concentrations are markedly increased due to an impaired metabolism of diazepam in comparison with those in EMs[3-7]. However, whether the pharmacodynamic effects of an intravenous infusion of diazepam would differ between the CYP2C19 EMs and PMs remain, to our knowledge, unknown.
Psychomotor tests, such as a critical flicker fusion test, an eye movement analysis, a postural sway test and a visual analog scale (VAS) symptom assessment method are well known as psychometric markers and are commonly used to quantify the pharmacodynamic responses associated with an administration of benzodiazepine sedatives[15-17]. Indeed, plasma or serum diazepam concentrations were significantly correlated with psychomotor test scores[18,19].
In this study, we aimed to examine the objective and subjective sedative effects of an intravenous 5-mg dose of diazepam commonly used for gastrointestinal endoscopy in relation to CYP2C19 genotypes.
Fifteen healthy Japanese volunteers whose CYP2C19 genotypes had been determined by a PCR-RFLP method[20,21] were enrolled in this study (5 homozygous EMs, 5 heterozygous EMs and 5 PMs of CYP2C19) (Table 1). A written informed consent was obtained from each subject.
All subjects were given a single intravenous infusion of Diazepam (Cercine®, Takeda Pharmaceutical Co. Ltd., Osaka, Japan) 5 mg at 8:00 am, which was infused over 1 min. Four psychomotor tests, such as a critical flicker fusion test, an eye movement analysis, a postural sway test and a VAS symptom assessment method[15-17], were performed at the pre- and postinfusion time points of diazepam as follows: before the infusion and 5 min and 30 min, and 1 h, 3 h, 6 h, 10 h, 24 h, 72 h and 336 h postinfusion.
The critical flicker fusion test was measured by the discrimination of fused flickering red light (DF-1, Shibata Chemical, Tokyo, Japan). The value used for the fusion time was flickers per second. The results of an eye movement test used the saccadic latency (per second), which is time from the displacement of red light signal to the response of eye movement. The stimulated horizontal displacement of red lights at random intervals was recorded (DP1200A, Nihon Denki Sanei, Tokyo, Japan). The subjects responded to and followed a signal for 30 s as quickly as possible. The moving radial area (cm2) for 60 s by a postural sway test was measured by a sway meter (G5500, Anima, Tokyo, Japan) for the 60 s period with eyes closed. The radial area was determined by calculating the radial distance to the center of pressure at each sampling interval from the geometric center of the stance. A VAS symptom assessment method consisted of questions of both the two parameters of mental sedation and physical sedation. Subjects had to mark on the 100 mm line to show the degree of their feeling.
All subjects were provided with three meals a day (breakfast at 7:30 am, lunch at 12:30 pm, and supper at 6:00 pm). Mineral water was allowed ad libitum. But, no other beverages including caffeine-containing and grapefruit juice-related products were permitted. None of them drank alcohol or had a smoking habit. None had taken any drugs for 1 mo before the study, nor did they take any during it. The subjects stayed in the translational research (TR) unit of Hamamatsu University School of Medicine during the first 24 h postinfusion period. Four psychomotor tests at 72 h and 336 h postinfusion were performed in the TR unit.
The protocol was approved in advance by the Human Institutional Review Board of the Hamamatsu University School of Medicine. Written informed consent was obtained from each subject before participation in the study.
The median values of four psychomotor tests and median percent changes from the baseline value (before Diazepam infusion) were determined. Statistically significant differences in the median pharmacodynamic parameters among the 3 different CYP2C19 genotype groups at the pre- and postinfusion time points were determined by the Mann-Whitney U-test, when a significant difference was obtained by the Kruskal-Wallis test. Statistical differences with the median parameters between the different pre- and postinfusion time points in all of the subjects enrolled in this study were determined by using the Wilcoxon’s signed rank test, when significant differences were obtained by the Friedmann’s test. All P values were two-sided, and P < 0.05 was taken to indicate statistical significance.
There were no statistically significant differences in the demographic characteristics, such as age, body weight, height and sex ratio among the 3 different CYP2C19 genotype groups (Table 1).
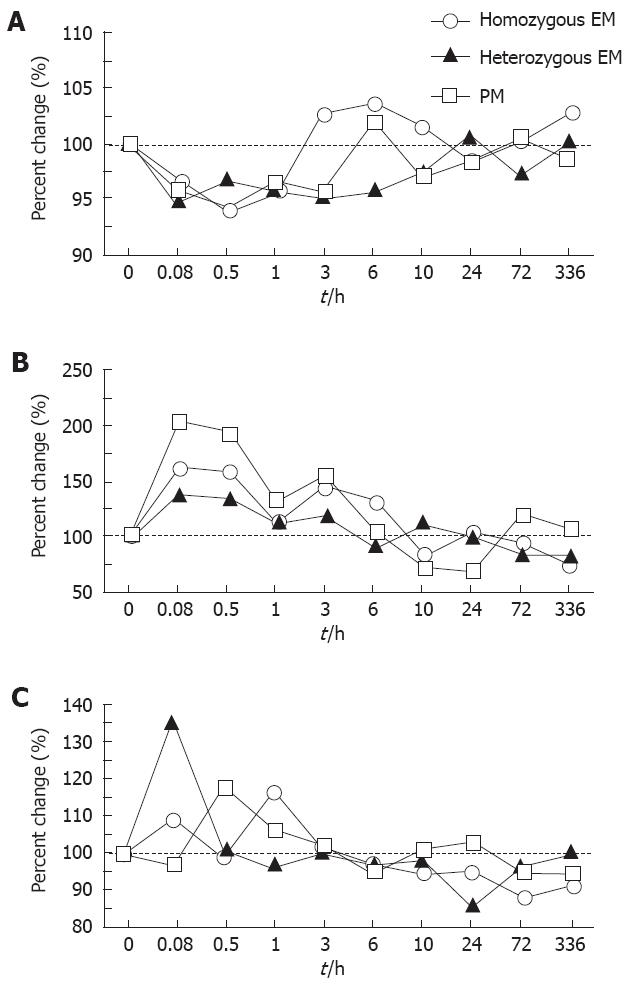
Percent changes in the critical flicker fusion test, postural sway test, and eye movement test after an intravenous administration of 5 mg of diazepam as a function of CYP2C19 genotype status are shown in Figure 1. Sedative effects with diazepam (5 mg) assessed by those methods did not significantly differ among the different CYP2C19 genotype groups throughout the 336 h postinfusion period (P > 0.05) (Figure 1). For this reasoning, the data derived from the different CYP2C19 genotypes were combined together in the following analyses.
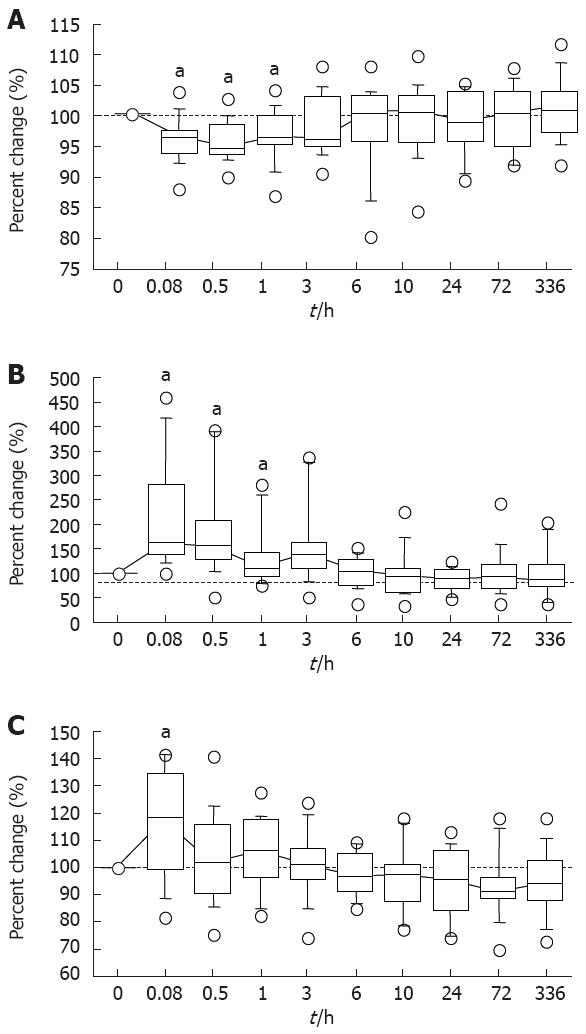
In a critical flicker fusion test, the median percent changes from the baseline values (before diazepam infusion) and the postinfusion values at 5 and 30 min, and at 1 h significantly differed (P = 0.0038, 0.0018 and 0.0090, respectively) (Figure 2A). The median percent changes from the baseline values still appeared increased for longer than 3 h postinfusion, but not significantly (Figure 1A).
In the moving radial area of a postural sway test, the median values at 5 and 30 min, and 1 h and 3 h postinfusion were 167.9% (range; 100.3%-456.7%), and 153.2% (52.3%-391.0%), and 111.2% (75.6%-281.8%) and 130.8% (51.0%-337.1%), which were significantly greater than the baseline value (P = 0.0007, and 0.0018, and 0.0409 and 0.0125, respectively) (Figure 2B). These objective sedative assessment values required 3 h to 6 h postinfusion to return to the respective baseline levels (Figure 2).
In the saccadic latency of an eye moving test, the median percent change from the baseline at 5 min postinfusion was 115.9% (81.9%-141.1%) (P = 0.0171) (Figure 2C).
The overall results indicated that the objective sedative effects of diazepam assessed by the three methods continued for up to 3 h postinfusion.
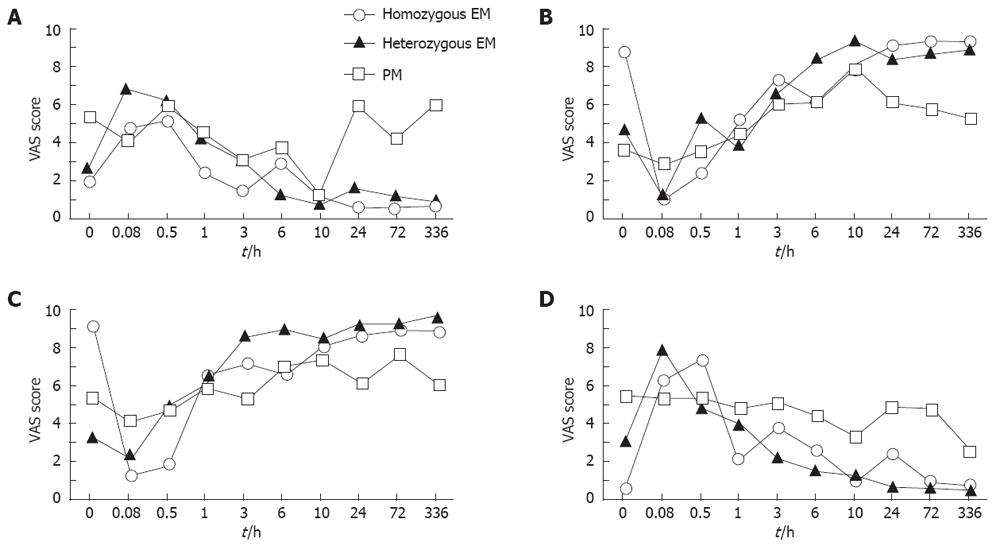
The sedative effects assessed by VAS method were fairly similar among the different CYP2C19 genotype groups throughout the 336-h postinfusion period (P > 0.05) (Figure 3). The data of VAS method obtained from all of the subjects with different CYP2C19 genotypes were, therefore, combined as mentioned above.
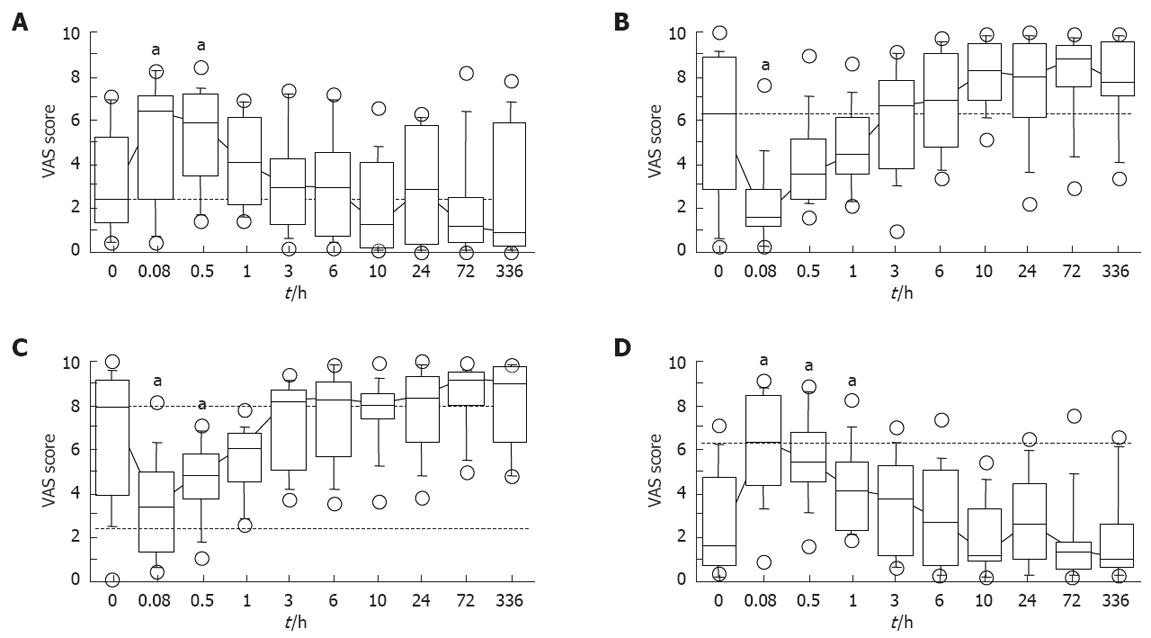
The median values by “alert to drowsy” of mental sedation parameter at 5 and 30 min postinfusion were significantly decreased compared with the baseline value (P = 0.0098 and 0.0047, respectively) (Figure 4A). This value returned to the level not different from the baseline of 1 h later (Figure 4A). In the “fuzzy to clear headed” of mental parameter, the median value at 5 min later was significantly lower (P = 0.076), but returned to the baseline at 30 min postinfusion (Figure 4B).
The median values of the “well-coordinated to clumsy” physical sedation parameter at 5 min and 30 min and 1 h after diazepam infusion were significantly increased compared with the baseline value (P = 0.0031, and 0.0038 and 0.0468, respectively) (Figure 4C). In the “lethargic to energetic” physical parameter, the median values at 5 min and 30 min postinfusion were significantly lower (P = 0.0119 and 0.0468, respectively), but returned to baseline level by 1 h postinfusion (Figure 4D).
These results assessed by the VAS symptom assessment method indicated that the subjective sedative effects of diazepam (5 mg) continued for less than 1 h postinfusion, although the objective sedative effects continued longer (for 1 h to 3 h postinfusion).
The development of an optimal infusion regimen of a premedication (e.g. diazepam) and an appropriate manual check at the endoscopy unit is necessary for a comfortable and safe gastrointestinal endoscopy. Although the proper premedication for gastrointestinal endoscopy should have a shorter onset of action, short elimination half-life, and faster time to recovery, diazepam is one of the first-line sedative drugs used as a premedication for gastrointestinal endoscopy in Japan. In this study, we demonstrated that the objective sedative effects by an intravenous infusion of diazepam (5 mg) continued for no less than 3 h with psychological tests, whereas the subjective sedative effects continued for no more than 1 h. Although the plasma diazepam concentrations of individuals with CYP2C19 PMs have been reported to be greater than those with homozygous EMs[5-9], we did not find any significantly different sedative effects of diazepam among the different CYP2C19 genotype groups. Based upon these observations, we thought that patients who undergo an intravenous infusion of diazepam (5 mg) should be cared for at least 3 h postinfusion in the hospital irrespective of CYP2C19 genotype status for the prevention of adverse events.
In the present study, the values of VAS method used for assessing the subjective symptom parameters demonstrated continuous sedative effects only during 30 min to 60 min after the infusion of diazepam (5 mg). After 1 h had passed from administration, the major parameters of the VAS assessment method returned to the respective baseline levels. However, the objective assessment by a critical flicker fusion test and a postural sway test revealed that the sedative effects of diazepam remained for approximately 3 h to 6 h postinfusion, indicating that a discrepancy exists between the subjective and objective sedative symptoms from 1 h to 6 h after the infusion of diazepam. Therefore, even if the patients feel improved or recovered from the sedation by diazepam at 1 h postinfusion, they may be at risk for some adverse effects, such as falling down and/or having driving errors. Then, we anticipate that the patients who undergo an intravenous infusion of diazepam (5 mg) should be checked for the prevention of possible sedative adverse effects during a 6 h postinfusion period at the endoscopy unit.
The sedative effects of diazepam are mediated viaα1-GABAA receptors in the brain[22,23], and the minimum plasma concentration of diazepam to yield sedative effects is more than 300 ng/mL to 400 ng/mL[18,19]. The pharmacokinetics of diazepam are affected by age[24,25], gender[26], obesity[26], liver disease[27] and CYP2C19 genotype status[5-9]. The mean plasma elimination half-life of diazepam in PMs of CYP2C19 has been reported to be much longer than that in homozygous EMs: those values after a single oral dose of diazepam were 84.0 ± 13.7 h for PMs, 62.9 ± 9.8 h for heterozygous EMs and 20.0 ± 10.8 h for homozygous EMs[28]. However, plasma diazepam levels do not always correlate with the sedative effects[29,30]. Moreover, Kosuge et al[16] reported that influences of diazepam on psychomotor functions did not differ among the 3 different CYP2C19 genotype groups, as observed in this study. Although we cannot offer appropriate explanations for the lack of difference in the pharmacodynamics of diazepam among the different CYP2C19 genotype groups, several possible mechanisms can be raised as follows: first, the acute tolerance to diazepam may be developed, which is mediated by the dysfunction of the cortical GABA transmitter system, such as the decrease of glutaminic acid decarboxylase[31,32], reelin[33] and GABA membrane transporter[34], GABAA receptor up-regulation[35] and the decrease of dendritic spines[36]. Second, diazepam as well as its metabolites such as temazepam and N-desmethyldiazepam, have sedative effects[3,4]. Therefore, although the metabolic disposition of diazepam differs among the different CYP2C19 genotype groups, the total amounts of diazepam plus its active metabolites would not differ among the different CYP2C19 genotype groups, resulting in no statistical difference in the pharmacodynamics of diazepam. Third, our pharmacodynamic assessment methods may not have a sufficient power for the limited sample size (n = 15) in the psychomotor function status. Nevertheless, in a cimetidine-diazepam interaction study, only minimal changes were observed in the pharmacodynamic effects despite an increase in plasma diazepam concentration by about 60% during treatment with cimetidine[37]. Similarly, in a fluoxetine-diazepam interaction study[38], despite that the significant increment of plasma diazepam concentration occurred by about 50% with the co-administration of fluoxetine, no psychopharmacological changes were detected by the pharmacodynamic assessment methods similar to those we used. With the limitation of absent data on plasma concentrations of diazepam and its metabolites (e.g. desmethyldiazepam and temazepam) in this study, we are tempted to assume that the sedative effects assessed by the pharmacodynamic assessment methods we used would not differ among the different CYP2C19 genotype groups when given an intravenous 5-mg infusion of diazepam as noted above.
Recently, when gastrointestinal endoscopy is performed for patients, midazolam and propofol are often used as a sedative drug. However, midazolam and propofol act quickly and potently, and therefore, advanced effects such as respiratory trouble occurs sometimes. However, the proper premedication for gastrointestinal endoscopy should have a shorter onset of action, short elimination half-life, and faster time to recovery. Therefore, we hope to evaluate the sedative effects of those drugs by using the psychopharmacological tests.
In conclusion, this study suggests that the psycho-pharmacological tests appear to be a useful tool for determining the optimal treatment with a benzodiazepine, such as diazepam, for gastrointestinal endoscopy. If patients undergo gastrointestinal endoscopy for sedation by an intravenous infusion of diazepam (5 mg), the sedative effects of patients should be monitored for preventing the possible adverse effects up to at least a 3-h postinfusion period in the endoscopy unit of the hospital, although patients appear to subjectively feel unimpaired or recovered.
Benzodiazepine is commonly used as a premedication for gastrointestinal endoscopy. However, use of benzodiazepine sometimes causes undesirable events, which leads to some accidents. For the prevention of such undesirable accidents, therefore, the dose of premedication should be minimized or optimized. For benzodiazepine, the pharmacokinetics of diazepam depends significantly on cytochrome P450 (CYP) 2C19 genotype status. However, whether the pharmacodynamic effects of an intravenous infusion would differ between the CYP2C19 EMs and PMs remain unknown. Moreover, there was no optimal protocol of diazepam use in the endoscopy unit.
When patients receive gastrointestinal endoscopy, the development of optimal infusion regimens of sedative drug and care protocol in the endoscopy unit are required to prevent advanced effects in relation to CYP2C19 genotypes.
There are many reports, which investigate the pharmacokinetics and pharmacodynamics of diazepam in pharmacological studies. The innovation of this study is to demonstrate the recommendation and attention of gastrointestinal endoscopy with diazepam (5 mg/body) as follows: An up to 3 h clinical care appears to be required after the infusion of diazepam irrespective of CYP2C19 genotype status, although patients feel subjectively improved.
The physical sedative effects assessed by the critical flicker test continued for 1 h and those by the moving radial area of a postural sway test continued for 3 h, which significantly changed compared with the baseline level before infusion (P < 0.05). On the other hand, the mental sedative effects by the VAS method improved within 1 h. The CYP2C19 genotype-dependent differences in the postinfusion sedative effects were not observed in any of the four psychomotor function tests. Therefore, up to 3-h of clinical care is required after the infusion of diazepam (5 mg) irrespective of CYP2C19 genotype status, although patients feel markedly improved. Recently, many drugs, such as midazolam and propofol, were used at endoscopy as sedation drugs. Additional research, which compares with diazepam and other sedation drugs in efficacy and care time using the psychopharmacological tests, is required.
The critical flicker fusion test: the measurement of the discrimination of fused flickering red light. The value used for the fusion time was flickers per second. The results of an eye movement test: the saccadic latency (per second), which is time from the displacement of red light signal to the response of eye movement. The subjects responded to and followed a signal for 30 s as quickly as possible. The postural sway test: the measurement of moving radial area (cm2) for the 60 s period with eyes closed. The radial area was determined by calculating the radial distance to the center of pressure at each sampling interval from the geometric center of the stance.
Authors examined whether the sedative effects assessed by psychomotor tests would depend on the CYP2C19 genotypes after an infusion regimen of diazepam commonly used for gastrointestinal endoscopy in Japan. It’s an interesting study, and the methodology is sound, and is a good objective assessment.
Peer reviewer: William Dickey, Altnagelvin Hospital, Londonderry, Northern Ireland BT47 6SB, United Kingdom
S- Editor Li DL L- Editor Alpini GD E- Editor Lin YP
| 1. | Bell GD. Review article: premedication and intravenous sedation for upper gastrointestinal endoscopy. Aliment Pharmacol Ther. 1990;4:103-122. |
| 2. | Nagengast FM. Sedation and monitoring in gastrointestinal endoscopy. Scand J Gastroenterol Suppl. 1993;200:28-32. |
| 3. | Yasumori T, Nagata K, Yang SK, Chen LS, Murayama N, Yamazoe Y, Kato R. Cytochrome P450 mediated metabolism of diazepam in human and rat: involvement of human CYP2C in N-demethylation in the substrate concentration-dependent manner. Pharmacogenetics. 1993;3:291-301. |
| 4. | Andersson T, Miners JO, Veronese ME, Birkett DJ. Diazepam metabolism by human liver microsomes is mediated by both S-mephenytoin hydroxylase and CYP3A isoforms. Br J Clin Pharmacol. 1994;38:131-137. |
| 5. | Andersson T, Cederberg C, Edvardsson G, Heggelund A, Lundborg P. Effect of omeprazole treatment on diazepam plasma levels in slow versus normal rapid metabolizers of omeprazole. Clin Pharmacol Ther. 1990;47:79-85. |
| 6. | Ishizaki T, Chiba K, Manabe K, Koyama E, Hayashi M, Yasuda S, Horai Y, Tomono Y, Yamato C, Toyoki T. Comparison of the interaction potential of a new proton pump inhibitor, E3810, versus omeprazole with diazepam in extensive and poor metabolizers of S-mephenytoin 4'-hydroxylation. Clin Pharmacol Ther. 1995;58:155-164. |
| 7. | Sohn DR, Kusaka M, Ishizaki T, Shin SG, Jang IJ, Shin JG, Chiba K. Incidence of S-mephenytoin hydroxylation deficiency in a Korean population and the interphenotypic differences in diazepam pharmacokinetics. Clin Pharmacol Ther. 1992;52:160-169. |
| 8. | Wan J, Xia H, He N, Lu YQ, Zhou HH. The elimination of diazepam in Chinese subjects is dependent on the mephenytoin oxidation phenotype. Br J Clin Pharmacol. 1996;42:471-474. |
| 9. | Schmider J, Greenblatt DJ, von Moltke LL, Shader RI. Relationship of in vitro data on drug metabolism to in vivo pharmacokinetics and drug interactions: implications for diazepam disposition in humans. J Clin Psychopharmacol. 1996;16:267-272. |
| 10. | Sugimoto M, Furuta T, Shirai N, Kajimura M, Hishida A, Sakurai M, Ohashi K, Ishizaki T. Different dosage regimens of rabeprazole for nocturnal gastric acid inhibition in relation to cytochrome P450 2C19 genotype status. Clin Pharmacol Ther. 2004;76:290-301. |
| 11. | Shirai N, Furuta T, Moriyama Y, Okochi H, Kobayashi K, Takashima M, Xiao F, Kosuge K, Nakagawa K, Hanai H. Effects of CYP2C19 genotypic differences in the metabolism of omeprazole and rabeprazole on intragastric pH. Aliment Pharmacol Ther. 2001;15:1929-1937. |
| 12. | Chang M, Dahl ML, Tybring G, Gotharson E, Bertilsson L. Use of omeprazole as a probe drug for CYP2C19 phenotype in Swedish Caucasians: comparison with S-mephenytoin hydroxylation phenotype and CYP2C19 genotype. Pharmacogenetics. 1995;5:358-363. |
| 13. | Chang M, Tybring G, Dahl ML, Gotharson E, Sagar M, Seensalu R, Bertilsson L. Interphenotype differences in disposition and effect on gastrin levels of omeprazole--suitability of omeprazole as a probe for CYP2C19. Br J Clin Pharmacol. 1995;39:511-518. |
| 14. | Ishizaki T, Horai Y. Review article: cytochrome P450 and the metabolism of proton pump inhibitors--emphasis on rabeprazole. Aliment Pharmacol Ther. 1999;13 Suppl 3:27-36. |
| 15. | Norris H. The action of sedatives on brain stem oculomotor systems in man. Neuropharmacology. 1971;10:181-191. |
| 16. | Kosuge K, Jun Y, Watanabe H, Kimura M, Nishimoto M, Ishizaki T, Ohashi K. Effects of CYP3A4 inhibition by diltiazem on pharmacokinetics and dynamics of diazepam in relation to CYP2C19 genotype status. Drug Metab Dispos. 2001;29:1284-1289. |
| 17. | Sundstrom I, Ashbrook D, Backstrom T. Reduced benzodiazepine sensitivity in patients with premenstrual syndrome: a pilot study. Psychoneuroendocrinology. 1997;22:25-38. |
| 18. | Bowden CL, Fisher JG. Relationship of diazepam serum level to antianxiety effects. J Clin Psychopharmacol. 1982;2:110-114. |
| 19. | Dasberg HH, van der Kleijn E, Guelen JP, van Praag HM. Plasma concentrations of diazepam and of its metabolite N-desmethyldiazepam in relation to anxiolytic effect. Clin Pharmacol Ther. 1974;15:473-483. |
| 20. | de Morais SM, Wilkinson GR, Blaisdell J, Meyer UA, Nakamura K, Goldstein JA. Identification of a new genetic defect responsible for the polymorphism of (S)-mephenytoin metabolism in Japanese. Mol Pharmacol. 1994;46:594-598. |
| 21. | de Morais SM, Wilkinson GR, Blaisdell J, Nakamura K, Meyer UA, Goldstein JA. The major genetic defect responsible for the polymorphism of S-mephenytoin metabolism in humans. J Biol Chem. 1994;269:15419-15422. |
| 22. | Tauber M, Calame-Droz E, Prut L, Rudolph U, Crestani F. alpha2-gamma-Aminobutyric acid (GABA)A receptors are the molecular substrates mediating precipitation of narcosis but not of sedation by the combined use of diazepam and alcohol in vivo. Eur J Neurosci. 2003;18:2599-2604. |
| 23. | van Rijnsoever C, Tauber M, Choulli MK, Keist R, Rudolph U, Mohler H, Fritschy JM, Crestani F. Requirement of alpha5-GABAA receptors for the development of tolerance to the sedative action of diazepam in mice. J Neurosci. 2004;24:6785-6790. |
| 24. | Herman RJ, Wilkinson GR. Disposition of diazepam in young and elderly subjects after acute and chronic dosing. Br J Clin Pharmacol. 1996;42:147-155. |
| 25. | Divoll M, Greenblatt DJ, Ochs HR, Shader RI. Absolute bioavailability of oral and intramuscular diazepam: effects of age and sex. Anesth Analg. 1983;62:1-8. |
| 26. | Greenblatt DJ, Abernethy DR, Locniskar A, Harmatz JS, Limjuco RA, Shader RI. Effect of age, gender, and obesity on midazolam kinetics. Anesthesiology. 1984;61:27-35. |
| 27. | Branch RA, Morgan MH, James J, Read AE. Intravenous administration of diazepam in patients with chronic liver disease. Gut. 1976;17:975-983. |
| 28. | Qin XP, Xie HG, Wang W, He N, Huang SL, Xu ZH, Ou-Yang DS, Wang YJ, Zhou HH. Effect of the gene dosage of CgammaP2C19 on diazepam metabolism in Chinese subjects. Clin Pharmacol Ther. 1999;66:642-646. |
| 29. | Marks IM, Viswanathan R, Lipsedge MS, Gardner R. Enhanced relief of phobias by flooding during waning diazepam effect. Br J Psychiatry. 1972;121:493-505. |
| 30. | Tansella M, Siciliani O, Burti L, Schiavon M, Zimmermann C, Gerna M, Tognoni G, Morselli PL. N-desmethyldiazepam and amylobarbitone sodium as hypnotics in anxious patients. Plasma levels, clinical efficacy and residual effects. Psychopharmacologia. 1975;41:81-85. |
| 31. | Benes FM, Vincent SL, Alsterberg G, Bird ED, SanGiovanni JP. Increased GABAA receptor binding in superficial layers of cingulate cortex in schizophrenics. J Neurosci. 1992;12:924-929. |
| 32. | Veldic M, Guidotti A, Maloku E, Davis JM, Costa E. In psychosis, cortical interneurons overexpress DNA-methyltransferase 1. Proc Natl Acad Sci USA. 2005;102:2152-2157. |
| 33. | Guidotti A, Auta J, Davis JM, Di-Giorgi-Gerevini V, Dwivedi Y, Grayson DR, Impagnatiello F, Pandey G, Pesold C, Sharma R. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch Gen Psychiatry. 2000;57:1061-1069. |
| 34. | Woo TU, Whitehead RE, Melchitzky DS, Lewis DA. A subclass of prefrontal gamma-aminobutyric acid axon terminals are selectively altered in schizophrenia. Proc Natl Acad Sci USA. 1998;95:5341-5346. |
| 35. | Lewis DA, Volk DW, Hashimoto T. Selective alterations in prefrontal cortical GABA neurotransmission in schizophrenia: a novel target for the treatment of working memory dysfunction. Psychopharmacology (Berl). 2004;174:143-150. |
| 36. | Rosoklija G, Toomayan G, Ellis SP, Keilp J, Mann JJ, Latov N, Hays AP, Dwork AJ. Structural abnormalities of subicular dendrites in subjects with schizophrenia and mood disorders: preliminary findings. Arch Gen Psychiatry. 2000;57:349-356. |
| 37. | Greenblatt DJ, Abernethy DR, Morse DS, Harmatz JS, Shader RI. Clinical importance of the interaction of diazepam and cimetidine. N Engl J Med. 1984;310:1639-1643. |
| 38. | Lemberger L, Rowe H, Bosomworth JC, Tenbarge JB, Bergstrom RF. The effect of fluoxetine on the pharmacokinetics and psychomotor responses of diazepam. Clin Pharmacol Ther. 1988;43:412-419. |