Published online Aug 28, 2008. doi: 10.3748/wjg.14.5051
Revised: August 6, 2008
Accepted: August 15, 2008
Published online: August 28, 2008
AIM: To evaluate the efficacy of colonoscopy follow-up after short-term anti-tuberculosis treatment in patients with nonspecific ulcers on ileocecal areas being suspicious of tuberculous colitis.
METHODS: We prospectively analyzed the colonoscopic findings before and after short term anti-tuberculosis treatment in 18 patients with nonspecific ulcers on the ileocecal area and compared them with 7 patients of confirmed tuberculous colitis by acid-fast bacilli or caseating granuloma on colonic biopsy.
RESULTS: Mean duration for short-term follow-up was 107.3 d with combined chemotherapy containing isoniazid, rifampicin, ethambutol and pyrazinamide. Seven patients with tuberculous colitis showed complete healing of active ulcers after short-term medication. After short-term anti-tuberculosis treatment, follow-up colonoscopy findings divided 18 patients with nonspecific ulcers into two groups by ulcer state. One is the “suspicious tuberculous colitis group” showing healing of ulcers and erosions and another is the “suspicious inflammatory bowel disease group” showing active ulcers with or without aggravation of the lesion. Finally, all 9 of the “suspicious tuberculous colitis group” were diagnosed as tuberculous colitis showing no recurrence of ulcers after termination of 9 mo of anti-tuberculosis medication. Patients of the “suspicious inflammatory bowel disease group” were finally diagnosed as Crohn’s disease or nonspecific colonic ulcers during long-term follow up.
CONCLUSION: Follow-up colonoscopy shows a healing stage ulcer or scarring change without an active ulcer with just 2 mo to 3 mo of medication in patients with tuberculous colitis. Colonoscopy follow-up after short term anti-tuberculosis trial in patients with nonspecific ulcers on the ileocecal area is valuable in making early differential diagnosis of tuberculous colitis.
- Citation: Park YS, Jun DW, Kim SH, Lee HH, Jo YJ, Song MH, Kim NI, Lee JS. Colonoscopy evaluation after short-term anti-tuberculosis treatment in nonspecific ulcers on the ileocecal area. World J Gastroenterol 2008; 14(32): 5051-5058
- URL: https://www.wjgnet.com/1007-9327/full/v14/i32/5051.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.5051
| Group | Tbc colitis (n = 7 ) | Nonspecific ulcers (n =18) | P1 | |
| Suspicious tbc colitis (n = 9 ) | Suspicious IBD (n = 9 ) | |||
| M/F | 4/3 | 2/7 | 4/5 | NS |
| Median age (yr) | 40.1 | 44.1 | 28.1 | 0.03 |
| Chest X-ray (%) | ||||
| Active pulmonary tbc | 5 (71.4) | 3 (33.3) | 0 (0) | |
| Old pulmonary tbc | 0 (0) | 2 (22.2) | 0 (0) | < 0.001 |
| Normal | 2 (28.6) | 4 (44.4) | 9 (100) | |
| Lab finding (%) | ||||
| ESR rise | 7 (100) | 8 (88.9) | 9 (100) | NS |
| pANCA (+/-) | Not check | Not check | 1/8 (11.1/88.9) | |
| Small bowel lesion (%) | ||||
| No lesion | 4 (57.1) | 4 (44.4) | 3 (33.3) | |
| Jejunum | 0 (0) | 0 (0) | 0 (0) | |
| Prox. ileum | 0 (0) | 0 (0) | 1 (11.1) | NS |
| Terminal ileum | 3 (42.9) | 5 (55.5) | 6 (66.7) | |
| Anal & rectal lesion (%) | 0 (0) | 0 (0) | 0 (0) | |
| Group | Tbc colitis (n = 7 ) | Nonspecific ulcers ( n = 18) | P1 | |
| Suspicious tbc colitis (n = 9 ) | Suspicious IBD (n = 9 ) | |||
| Location of lesion (%) | ||||
| Terminal ileum | 3 (42.9) | 5 (55.6) | 6 (66.7) | NS |
| IC valve | 5 (71.4) | 8 (88.9) | 7 (77.8) | NS |
| Cecum | 2 (28.6) | 2 (22.2) | 5 (55.6) | NS |
| Prox. Ascending colon | 6 (85.7) | 6 (66.7) | 6 (66.7) | NS |
| Shape of lesion | ||||
| Geographic ulcer | 3 (42.9) | 2 (22.2) | 2 (22.2) | NS |
| Irregular ulcer | 1 (14.3) | 3 (33.3) | 5 (55.6) | NS |
| Aphthous ulcer | 0 (0) | 2 (22.2) | 6 (66.7) | 0.01 |
| Transverse ulcer | 3 (42.6) | 2 (22.2) | 2 (22.2) | NS |
| Stenosis | 2 (28.6) | 1 (11.1) | 2 (22.2) | NS |
| Pathology (%) | ||||
| Granuloma | 2 (28.6) | 3 (28.0) | 4 (44.4) | NS |
| Caseating granuloma | 4 (57.1) | 0 (0) | 0 (0) | 0.01 |
| Acid fast bacilli+ | 3 (21.7) | 0 (0) | 0 (0) | 0.01 |
| Non-specific inflammation | 0 (0) | 6 (66.7) | 5 (55.6) | 0.02 |
| Group | Tbc colitis (n = 7 ) | Nonspecific ulcers ( n = 18) | P1 | |
| Suspicious tbc colitis (n = 9 ) | Suspicious IBD (n = 9 ) | |||
| Active ulcer | 0/7 | 0/9 | 9/9 | < 0.001 |
| Improvement of stenosis | 2/2 | 0/1 | 0/2 | NS |
| No. of inflammatory polyps | Increase | Increase | Increase | NS |
| Extent of lesion | Decrease | Decrease | Increase | 0.01 |
| Feasibility to drug (%) | 0.01 | |||
| Very good | 5 (71.4) | 4 (44.4) | 0 (0) | |
| Good | 2 (28.6) | 5 (55.6) | 5 (55.6) | |
| Poor | 0 (0) | 0 (0) | 4 (44.4) | |
| Patient | Response | Other manifestation during follow-up | Final Dx |
| #1 F/32 | Response to mesalazine | CD | |
| #2 F/15 | Response to steroid | perianal abscess after 2 years | CD |
| #3 F/20 | Response to mesalazine | CD | |
| #4 F/39 | No response to mesalazine, active ulcer and symptoms | hemicolectomy | Non-specific ulcers |
| #5 M/24 | Response to mesalazine | CD | |
| #6 M/42 | Response to steroid | CD | |
| #7 M/16 | Response to mesalazine | Ileocecectomy due to ileal perforation after 2 years | CD |
| #8 F/27 | Response to mesalazine | perianal fistula after 2 years | CD |
| #9 M/26 | Response to steroid | perianal abscess after 1 year | CD |
Worldwide incidence of abdominal tuberculosis has been steadily increasing for the past 20 years[1-4], and 2%-3% of reported patients with abdominal tuberculosis have isolated colonic involvement[5]. Particularly in Asia, pulmonary and extrapulmonary tuberculosis have been rare until now. Although both the incidence and prevalence rates of inflammatory bowel disease (IBD) are still relatively low compared to Europe and North America, they are increasing rapidly in many Asian countries, including Korea[6].
Difficult colonoscopic differentiation between tuberculous colitis and Crohn’s colitis is caused when both entities can present themselves with mucosal ulcerations and nodularity, aphthous ulcers, edematous mucosal folds, strictures, pseudopolyps and luminal narrowing on the ileocecal area[7].
Although caseating granulomas and acid-fast bacilli make it easy to differentiate the disease entity, many nonspecific ulcers on the ileocecal area in patients with chronic diarrhea and right lower quadrant pain do not provide confirmative diagnosis between tuberculous colitis and other inflammatory bowel diseases.
Therapeutic trial of anti-tuberculosis treatment has been accepted in the case of high clinical suspicion of tuberculosis, which should be continued if there is a good clinical response[8]. Sometimes clinical response is not correlated with disease itself. Generally 9 mo to 12 mo of anti-tuberculous medication is necessary for tuberculous colitis. In patients with IBD, disease will be more aggravated during that long-term therapeutic trial. The clinician needs a diagnostic tool confirming tuberculous colitis as early as possible. The precise colonoscopic features after 2 mo to 3 mo of short-term medication in patients with either tuberculous colitis or suspicious tuberculous colitis have not been documented. Thus, we prospectively evaluated the colonoscopic findings after short-term anti-tuberculosis treatment both in patients with nonspecific ulcers on the ileocecal area and in patients with confirmed tuberculous colitis.
This prospective case-control study was conducted at the Medical Center of Eulji University, from March 2002 to October 2004. Eighteen patients (6 males and 12 females) with chronic diarrhea or right lower abdominal discomfort and showing nonspecific ulcers on the ileocecal area on initial colonoscopy were enrolled. The definition of nonspecific ulcer in this study is colonic ulcer with chronic active inflammation without caseating granuloma on biopsy. As a control, we evaluated colonoscopic features of 7 patients with tuberculous colitis who had either acid-fast bacilli or caseating granuloma on colonic biopsy combined with active pulmonary tuberculosis.
Patients with less than 3 wk of symptoms combined with perianal lesion or skipped lesion on either the left side of the colon or proximal small bowel were excluded. Stool exam and culture studies were performed to exclude any parasites and bacterial infection. The anti-tuberculosis medication trial proceeded after full and informed consent was granted by each patient.
One expert colonoscopist, with more than 10 years experience, performed the colonoscopies throughout this study. Colonoscopy was performed with videocolonoscopes (CF240L, Olympus, Japan) after 4 L polyethylene glycol preparation. The whole colon from rectum to cecal base and terminal ileum was photographed for comparison. Chest X-ray and small bowel double-contrast barium study were performed before medication for evaluation of small bowel lesion and pulmonary lesion. During anti-tuberculous medication, liver function test results, clinical symptoms and follow-up chest X-ray were regularly evaluated.
To determine the patient’s susceptibility to drug, we regularly evaluated the tolerance, upper GI symptoms and allergic symptoms relating to medication on outpatient care. “Very good” meant “no complaints during medication; no side effects”, “good” meant “some complaints after taking medication”, “poor” meant “difficulty in taking medication” due to GI trouble or side effects.
The regimen for tuberculosis was combined chemotherapy containing isoniazid, rifampicin, ethambutol and pyrazinamide.
The follow-up colonoscopy was performed after 2-3 mo of anti-tuberculosis medication on all enrolled patients by the aforementioned colonoscopist, and some of the features evaluated were ulcer shape, number and location. Compared with colonoscopic features of patients with tuberculous colitis, we subdivided the patients with nonspecific ulcers into either a “suspicious tuberculous colitis group” or “suspicious inflammatory bowel disease (IBD) group”. If there were healing of active ulcers similar to tuberculous colitis on follow-up colonoscopy findings, the patients were classified as “suspicious tuberculous colitis group”, and if there were still active ulcers or extension of the lesion, classified as “suspicious IBD group”. Finally, we confirmed tuberculous colitis by complete resolution of the whole lesion and clinical symptoms after 10 mo of anti-tuberculosis treatment in the “suspicious tuberculous colitis group”. For the “suspicious IBD group”, we stopped anti-tuberculosis treatment after a short-term trial and reevaluated.
The human subject committee of our hospital approved this study.
Kruskal-Wallis tests (three groups) were used for intergroup comparison of continuous variables, whereas Fisher exact tests were used to compare the categorical variables. Continuous variables are summarized as median ± SD, whereas categorical variables are summar-ized as counts or percentages. Statistical analysis was performed with SPSS 11.0 software.
All enrolled patients were performed follow-up colonoscopy after short-term anti-tuberculosis treatment. The mean duration of the trial was 107.3 (62-120) d.
Clinical features for enrolled patients are summarized in Table 1. The median age of patients in the “suspicious IBD group” was younger than patients in the “tuberculous colitis group” or “suspicious tuberculous colitis group”. Active lesion or old pulmonary lesion on chest X-ray was significant in helping the diagnosis of tuberculous colitis (P < 0.001). On laboratory findings, there are no specific differences between groups. Serologic results of patients with nonspecific ulcers are not so helpful in diagnosis. On small bowel study, there are no significant differences between groups. All of the enrolled patients had no ano-rectal symptoms or any other Upper GI tract lesion.
Initial Colonoscopy findings before anti-tuberculosis medication are summarized in Table 2. Ulcers were located at the ileocecal valve, cecum, proximal ascending colon and terminal ileum in both groups. There were no rectal and perianal lesion in enrolled patients initially. Ulcers showed geographic, irregular shape and sometimes-transverse array. Significantly, aphthous ulcers were common in the “suspicious IBD group” (P = 0.01). About 30% of patients in each group showed non-caseating granuloma on biopsy. Acid-fast bacilli were noted in only 3 patients of tuberculous colitis even though this is definite evidence of tuberculosis infection.
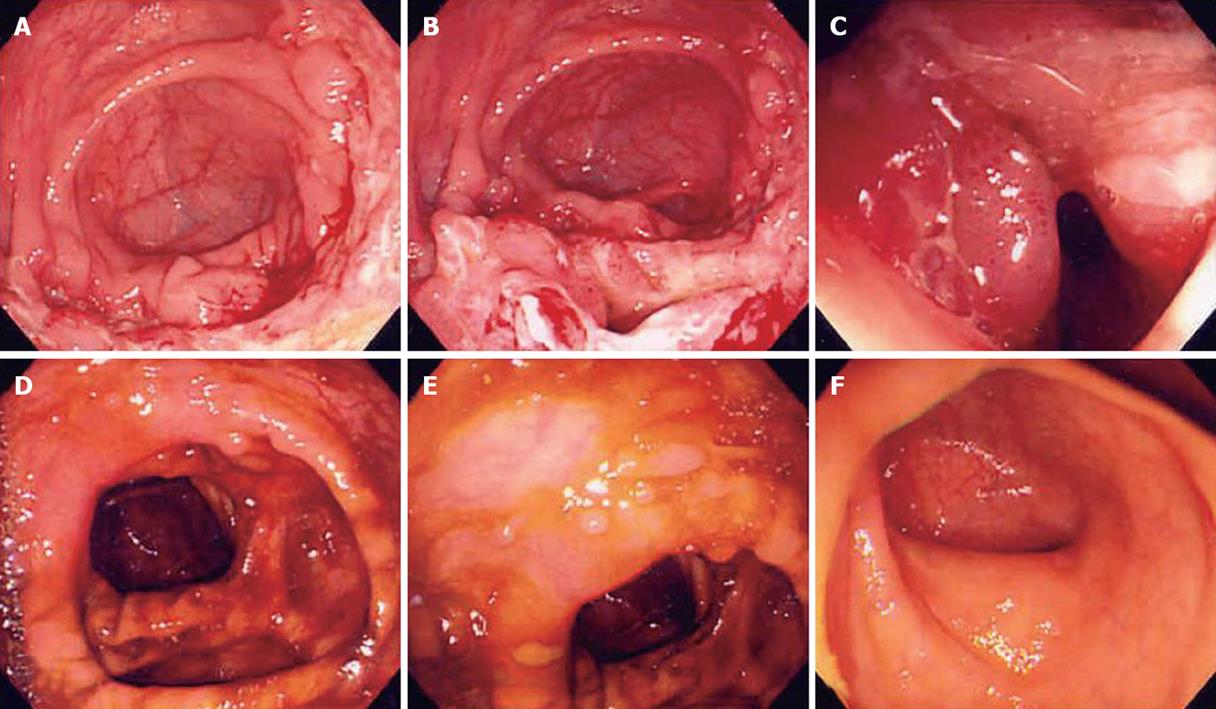
The follow-up colonoscopy features are summarized in Table 3. After short-term anti-tuberculosis medication, there are only scarring and inflammatory polyps remained in patients with tuberculous colitis (Figure 1).
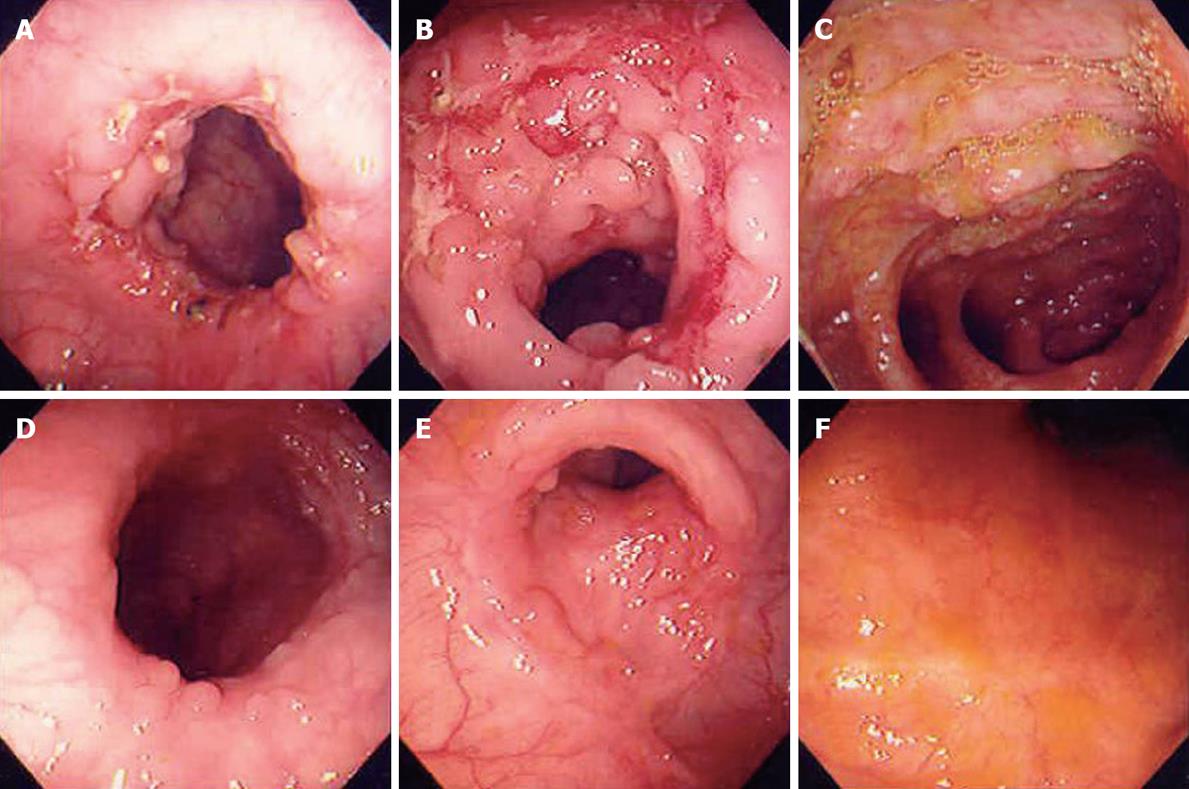
Nine of 18 patients with nonspecific ulcers also showed dramatic response after short-term trial similar to patients with tuberculous colitis presenting no active ulcer or erosions, irrespective of initial lesion (Figure 2, P < 0.001). We regarded them as the “suspicious tuberculous colitis group” and maintained anti-tuberculosis treatment for 10 mo.
The extent of lesion and stenosis in “tuberculous colitis” and “suspicious” groups were also improved, except in one patient of the “suspicious tuberculous colitis group” who underwent ileocecectomy due to obstruction during short-term anti-tuberculosis treatment.
Another 9 patients of nonspecific ulcers who showed active ulcers remaining and sometimes more aggravated after trial (P < 0.001) were regarded as the “suspicious IBD group”.
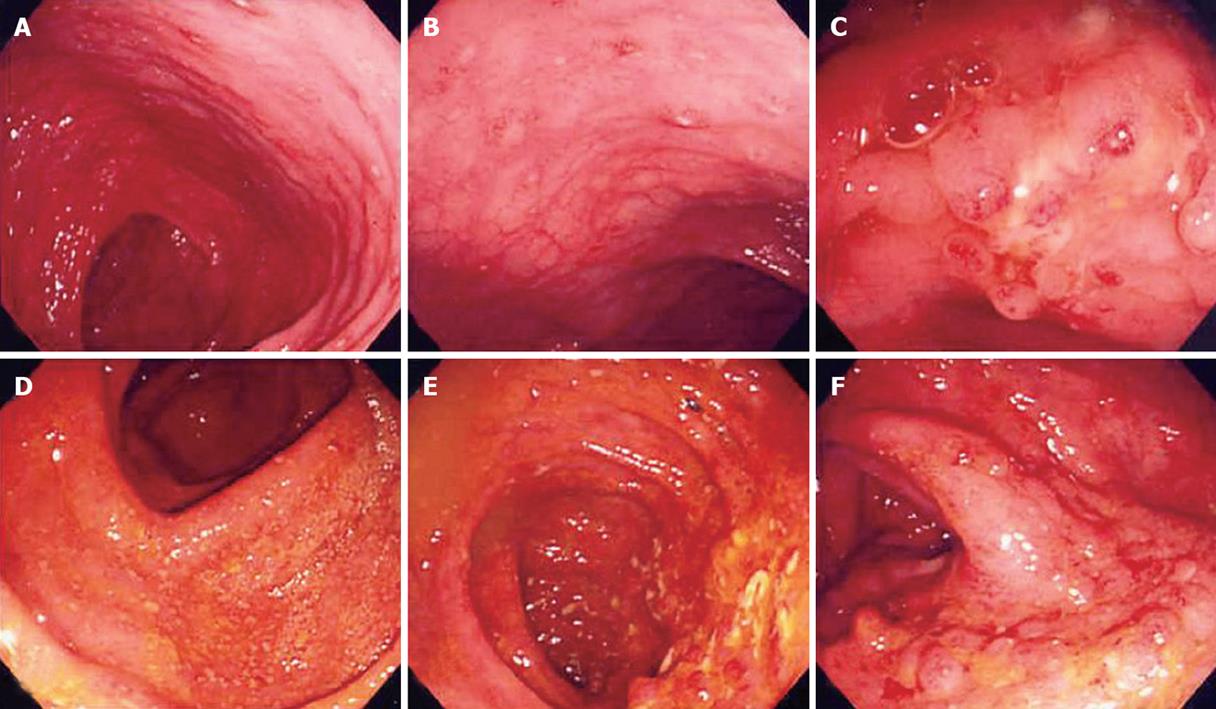
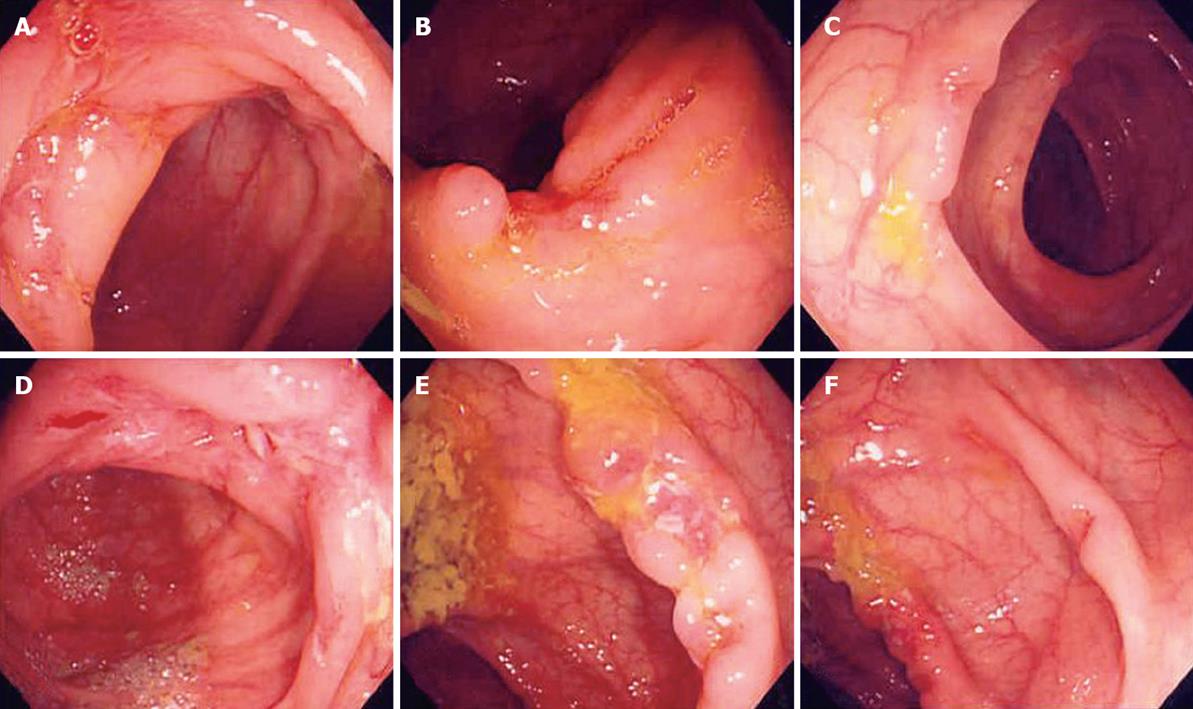
Some patients of the “suspicious IBD group” showed an expansion of the extent of involved colon with aggravation of stenosis (Figures 3 and 4).
Most of the patients in the “suspicious tuberculous colitis group” showed good tolerance of anti-tuberculosis treatment during the trial, some of the patients in the “suspicious IBD group” showed poor tolerance of anti-tuberculosis treatment (P = 0.01, Table 3). But more than half of the “suspicious IBD group” also showed good toleration and mild symptomatic improvement in spite of aggravating colonoscopic findings.
The 9 patients of the “suspicious tuberculous colitis group” showed weight gain and general improvement in well being along with complete resolution of the whole lesion and GI symptoms after 10-mo of anti-tuberculosis treatment. They were therefore finally confirmed as tuberculous colitis.
The 9 patients in the “suspicious IBD group” who had active ulcers on follow-up colonoscopy were reevaluated; a second biopsy was performed and withdrawn from the anti-tuberculosis treatment trial. They were switched to mesalazine and/or corticosteroid for inflammatory bowel disease. The new medication was well-tolerated and showed slow clinical improvement by 8 of the “suspicious IBD group”. However, 1 patient of the “suspicious IBD group” complained of sustained symptoms and weight loss and his 3rd colonoscopy showed more aggravation of lesions, chronic inflammation was found on repeated biopsy. After a 6-mo trial of mesalazine which did not show improvement, it was decided to perform a right hemicolectomy for confirmative diagnosis and treatment (Figure 4). The surgical specimen showed diffuse chronic ulcers on the ileocecal valve and ascending colon with neither granuloma nor any other malignant cells. After operation, the patient was observed for 2 years without further GI problems. During the 2 years of follow-up, 8 patients of the “suspicious IBD group” were improved in diarrhea, abdominal pain and colonic lesion, but 3 patients developed anal problems, and 1 patient underwent an operation due to ileal perforation. Finally 8 patients of the “suspicious IBD group” were diagnosed as Crohn’s disease, one patient as nonspecific simple colonic ulcers after a right hemicolectomy (Table 4).
The Asian Pacific region was previously designated as a low incidence area of IBD, but this could be due to lack of solid population-based studies, low diagnostic awareness, and high incidence of intestinal infections. Current literature and recent information leave no doubt that a true increase of IBD is occurring throughout this region[9]. By contrast, the incidence of tuberculosis is rising, both in the United States of America as well as in the United Kingdom[3,10,11]. Following the resurgence of tuberculosis, two series of patients with abdominal tuberculosis were recently published in the United States[12,13].
Thus, each side of the world is having to face the task of differentiating between the two diseases, Crohn’s disease and tuberculous colitis, which are similar in both clinical features and colonoscopy findings. In spite of similar clinical findings, the ultimate natural history of the two diseases is different. Appropriate anti-tuberculosis treatment leads in most cases of tuberculous ileocolitis to complete cure, whereas Crohn’s disease is a progressive relapsing illness unaffected by anti-tuberculosis treatment.
In tuberculous colitis, the cecum, Ileocecal valve and terminal ileum are commonly affected sites[14-17], and are also the most common sites of Crohn’s disease. In all areas of the world where Tuberculosis is endemic high-risk patients in developed countries need differentiation of the two diseases as early as possible.
Typical colonoscopy features described in patients with tuberculous colitis are transverse or linear ulcers, nodules, a deformed ileocecal valve and cecum, presence of inflammatory polyps, and multiple fibrous bands arranged in a haphazard fashion[14-16,18]. By contrast, typical Crohn’s disease shows segmental longitudinal ulcers with a cobble stone appearance, stricture, perianal lesion and pseudo polyps[19]. In clinical practice, however, nonspecific ulcers on ileocecal valve and cecum without typical features are often seen.
Biopsy during colonoscopy helps in the diagnosis of nonspecific ulcers in the ileocecal area. Caseating granulomas and/or acid-fast bacilli are present only in a small proportion of patients with tuberculous colitis[20]. Granulomas, with or without caseation, are usually seen in less than 50% of patients with tuberculous colitis[3,15,16], while clusters of epithelioid cells without well formed granulomas have been reported to occur in 20%-30% of the biopsies obtained[15,16]. Our study shows non-caseating granuloma is not significant to the differential diagnosis of tuberculous colitis from Crohn’s colitis.
Acid-fast bacilli have been reported in 50%-100% of specimens from patients with tuberculous colitis[21-24], but there are several reports where acid-fast bacilli could not be detected on histological examination of the biopsy material[14-16,18,25]. Acid-fast bacilli were seen in only 3 specimens (21.7%) in our cases. Thus, in a considerable number of tuberculous colitis the biopsies have features of chronic inflammation but no granulomas, caseation or clusters of epithelioid cells[15,16]. Culture of the biopsy material may increase the diagnostic yield[3]. However, disappointing results with 0% detection of acid-fast bacilli have also been reported[16]. Polymerase chain action analyses of biopsy specimens obtained endoscopically has been shown to be more sensitive than culture and acid-fast stains in diagnosing tuberculous colitis[26]. Other studies have suggested that an enzyme-linked immunosorbent assay using mycobacterial saline-extracted antigen may increase the yield of correct diagnosis of colonic tuberculosis[27]. The symptoms of tuberculous colitis such as chronic diarrhea, RLQ discomfort, and malaise are non-specific. But chest radiographs showing evidence of active or healed pulmonary infection may be a clue to diagnosis[15,16,28], in our cases, 70% of tuberculous colitis and 33% of suspicious tuberculous colitis cases showed active pulmonary tuberculosis and old scar in 22% of suspicious cases. By contrast, none of the patients of the “suspicious IBD group” showed abnormal chest radiographs (P < 0.001).
In clinical practice, therefore, nonspecific ulcers on the ileocecal valve and cecum without any other clue for specific diagnosis are often seen. Efforts to exclude other infection including amoeboma, Yersinia infection, GI histoplasmosis, and periappendiceal abscess must be considered[3]. If the clinical and colonoscopy features are suggestive of tuberculous colitis and multiple target biopsies do not show evidence of any other disease, then a therapeutic trial of anti-tuberculosis treatment can safely be given to these patients[29]. Clinical response to the anti-tuberculosis treatment is usually dramatic and less than 1-year treatment is sufficient for patients with tuberculous colitis compared to life long treatment for inflammatory bowel disease.
The recommended regimen for pulmonary tuberculosis is combined chemotherapy containing isoniazid, rifampicin, ethambutol and pyrazinamide during 9 mo[30]. But the exact duration of anti-tuberculosis treatment for tuberculous colitis is not yet established due to difficulties in bacteriological diagnosis and the assessment of response to therapy. As a result, extra-pulmonary tuberculosis has been treated for 12 mo to 24 mo on the basis of warnings of disease recurrence without supportive data. Recent study has shown tuberculosis enterocolitis can be treated with 9 mo of chemotherapy without disease recurrence[29]. Our study showed that 10 mo of 4 regimens of chemotherapy were adequate for patients with tuberculous colitis combined with or without pulmonary tuberculosis.
How long a period will be adequate for an empirical trial of undetermined patients? Generally, 2 mo to 3 mo are accepted as adequate for trials based on clinical response. However, the colonoscopy findings are not reported after a 2-mo to 3-mo trial. As our study shows, active ulcers and erosions are completely healed after 2 mo to 3 mo of medication and only leave scarring and inflammatory polyps in all patients with tuberculous colitis and suspicious tuberculous colitis. That means a 2-mo to 3-mo trial of anti-tuberculosis treatment and colonoscopy follow-up are very useful for differential diagnosis of tuberculous colitis with other inflammatory bowel diseases. It is an exciting result that some patients with suspicious tuberculous colitis, who showed no active ulcer and significant resolution on follow-up colonoscopy without any other specific clue for tuberculosis, were also confirmed as tuberculous colitis after 10-mo medication. Likewise, every patient of the “suspicious IBD group” who showed no response to a short-term trial was ultimately confirmed as Crohn’s disease or non-specific ulcer. This study also showed that, in patients with suspicious inflammatory bowel disease, a 2-mo to 3-mo trial was sufficient to exclude tuberculous colitis and adequate medication for inflammatory bowel disease could be started based on the trial.
Clinical response of symptoms and tolerance to anti-tuberculosis treatment was also important during the trial. In our study, most patients of the “suspicious tuberculous colitis group” tolerated medication well. About 45% of patients in the “suspicious IBD group” showed poor tolerance. But half also showed good tolerance to anti-tuberculosis treatment and mild clinical response during the trial too. Thus, during the anti-tuberculosis treatment trial, the physician’s concern for a patient’s tolerance to drug and clinical improvement is helpful but not definitive to decide maintaining anti-tuberculous treatment. We conclude that in cases of a therapeutic trial on nonspecific ulcers of the ileocecal area, 2 mo to 3 mo of anti-tuberculous medication and colonoscopy follow-up are valuable for making early confirmative diagnosis.
Differentiation between tuberculous colitis and Crohn's colitis can be difficult due to nonspecific GI symptoms and similar colonoscopic findings. In Asia, pulmonary and extrapulmonary tuberculosis are not rare until now. Also, the incidence and prevalence rates of inflammatory bowel disease (IBD) are increasing rapidly in many Asian countries, including Korea.
There are efforts to differentiate two disease entities through colonoscopic findings. But some of them still could not be confirmed by initial colonoscopic findings. Short-term trial of anti-tuberculosis treatment is necessary before confirmative diagnosis of Crohn’s colitis in the endemic area of tuberculosis.
The precise colonoscopic features after short-term medication in patients with either tuberculous colitis or suspicious tuberculous colitis have not been documented. This study was performed prospectively and showed that active ulcers and erosions were completely healed after 2-mo to 3-mo of medication and only leave scarring and inflammatory polyps in all patients with tuberculous colitis and suspicious tuberculous colitis. This study showed that, in patients with suspicious inflammatory bowel disease, a 2-mo to 3-mo trial and colonoscopic evaluation would be sufficient to exclude tuberculous colitis and adequate medication for inflammatory bowel disease to be started.
We conclude that in cases of therapeutic trial on nonspecific ulcers on the ileocecal area, especially the endemic area of tuberculosis, 2 mo to 3 mo of anti-tuberculous medication and colonoscopy follow-up are valuable for making early confirmative diagnosis. Duration of the therapeutic trial will be shortened after further clinical trial.
This study provides colonoscopic features after short term anti-tuberculosis medication in patients with tuberculosis colitis and other chronic inflammatory bowel disease.
Peer reviewer: Otto Schiueh-Tzang Lin, MD, C3-Gas, Gastroenterology Section, Virginia Mason Medical Center, 1100 Ninth Avenue, Seattle, WA 98101, United States
S- Editor Zhong XY L- Editor Alpini GD E- Editor Lin YP
| 1. | Guth AA, Kim U. The reappearance of abdominal tuberculosis. Surg Gynecol Obstet. 1991;172:432-436. |
| 3. | Marshall JB. Tuberculosis of the gastrointestinal tract and peritoneum. Am J Gastroenterol. 1993;88:989-999. |
| 4. | Snider DE Jr, Roper WL. The new tuberculosis. N Engl J Med. 1992;326:703-705. |
| 5. | Palmer KR, Patil DH, Basran GS, Riordan JF, Silk DB. Abdominal tuberculosis in urban Britain--a common disease. Gut. 1985;26:1296-1305. |
| 6. | Loftus EV Jr. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504-1517. |
| 7. | Ferentzi CV, Sieck JO, Ali MA. Colonoscopic diagnosis and medical treatment of ten patients with colonic tuberculosis. Endoscopy. 1988;20:62-65. |
| 8. | Misra SP, Misra V, Dwivedi M, Gupta SC. Colonic tuberculosis: clinical features, endoscopic appearance and management. J Gastroenterol Hepatol. 1999;14:723-729. |
| 9. | Ouyang Q, Tandon R, Goh KL, Ooi CJ, Ogata H, Fiocchi C. The emergence of inflammatory bowel disease in the Asian Pacific region. Curr Opin Gastroenterol. 2005;21:408-413. |
| 10. | Ouyang Q; OPCS. Registrar General's Weekly Return for England and Wales. London: HMSO 1994; . |
| 11. | Hayward AC, Watson JM. Tuberculosis in England and Wales 1982-1993: notifications exceeded predictions. Commun Dis Rep CDR Rev. 1995;5:R29-R33. |
| 12. | Guth AA, Kim U. The reappearance of abdominal tuberculosis. Surg Gynecol Obstet. 1991;172:432-436. |
| 13. | Rosengart TK, Coppa GF. Abdominal mycobacterial infections in immunocompromised patients. Am J Surg. 1990;159:125-131. |
| 14. | Bhargava DK, Tandon HD, Chawla TC, Shriniwas , Tandon BN, Kapur BM. Diagnosis of ileocecal and colonic tuberculosis by colonoscopy. Gastrointest Endosc. 1985;31:68-70. |
| 15. | Shah S, Thomas V, Mathan M, Chacko A, Chandy G, Ramakrishna BS, Rolston DD. Colonoscopic study of 50 patients with colonic tuberculosis. Gut. 1992;33:347-351. |
| 16. | Singh V, Kumar P, Kamal J, Prakash V, Vaiphei K, Singh K. Clinicocolonoscopic profile of colonic tuberculosis. Am J Gastroenterol. 1996;91:565-568. |
| 17. | Chen WS, Leu SY, Hsu H, Lin JK, Lin TC. Trend of large bowel tuberculosis and the relation with pulmonary tuberculosis. Dis Colon Rectum. 1992;35:189-192. |
| 18. | Aoki G, Nagasako K, Nakae Y, Suzuki H, Endo M, Takemoto T. The fiber colonoscopy diagnosis of tuberculous colitis. Endoscopy. 1975;7:113-121. |
| 19. | D’Haens G, Rutgeerts P. Endoscopic evaluation. Crohn’s disease. 1st ed. New York: Marcel Dekker, Inc 1996; 113-123. |
| 20. | Pulimood AB, Ramakrishna BS, Kurian G, Peter S, Patra S, Mathan VI, Mathan MM. Endoscopic mucosal biopsies are useful in distinguishing granulomatous colitis due to Crohn's disease from tuberculosis. Gut. 1999;45:537-541. |
| 21. | Pettengell KE, Pirie D, Simjee AE. Colonoscopic features of early intestinal tuberculosis. Report of 11 cases. S Afr Med J. 1991;79:279-280. |
| 23. | Wig KL, Chitkara NL, Gupta SP, Kishore K, Manchanda RL. Ileocecal tuberculosis with particular reference to isolation of Mycobacterium tuberculosis. With a note on its relation to regional ileitis (Crohn's disease). Am Rev Respir Dis. 1961;84:169-178. |
| 24. | Sakai Y. Colonoscopic diagnosis of the intestinal tuberculosis. Mater Med Pol. 1979;11:275-278. |
| 25. | Radhakrishnan S, Al Nakib B, Shaikh H, Menon NK. The value of colonoscopy in schistosomal, tuberculous, and amebic colitis. Two-year experience. Dis Colon Rectum. 1986;29:891-895. |
| 26. | Anand BS, Schneider FE, El-Zaatari FA, Shawar RM, Clarridge JE, Graham DY. Diagnosis of intestinal tuberculosis by polymerase chain reaction on endoscopic biopsy specimens. Am J Gastroenterol. 1994;89:2248-2249. |
| 27. | Bhargava DK, Dasarathy S, Shriniwas MD, Kushwaha AK, Duphare H, Kapur BM. Evaluation of enzyme-linked immunosorbent assay using mycobacterial saline-extracted antigen for the serodiagnosis of abdominal tuberculosis. Am J Gastroenterol. 1992;87:105-108. |
| 28. | al Karawi MA, Mohamed AE, Yasawy MI, Graham DY, Shariq S, Ahmed AM, al Jumah A, Ghandour Z. Protean manifestation of gastrointestinal tuberculosis: report on 130 patients. J Clin Gastroenterol. 1995;20:225-232. |
| 29. | Horvath KD, Whelan RL. Intestinal tuberculosis: return of an old disease. Am J Gastroenterol. 1998;93:692-696. |
| 30. | Kim SG, Kim JS, Jung HC, Song IS. Is a 9-month treatment sufficient in tuberculous enterocolitis? A prospective, randomized, single-centre study. Aliment Pharmacol Ther. 2003;18:85-91. |