Published online Aug 28, 2008. doi: 10.3748/wjg.14.4995
Revised: August 10, 2008
Accepted: August 17, 2008
Published online: August 28, 2008
Giant duodenal ulcers (GDUs) are a subset of duodenal ulcers that have historically resulted in greater morbidity than usual duodenal ulcers. Until recently, few cases had been successfully treated with medical therapy. However, the widespread use of endoscopy, the introduction of H-2 receptor blockers and proton pump inhibitors, and the improvement in surgical techniques all have revolutionized the diagnosis, treatment and outcome of this condition. Nevertheless, GDUs are still associated with high rates of morbidity, mortality and complications. Thus, surgical evaluation of a patient with a GDU should remain an integral part of patient care. These giant variants, while usually benign, can frequently harbor malignancy. A careful review of the literature highlights the important differences when comparing GDUs to classical peptic ulcers and why they must be thought of differently than their more common counterpart.
- Citation: Newton EB, Versland MR, Sepe TE. Giant duodenal ulcers. World J Gastroenterol 2008; 14(32): 4995-4999
- URL: https://www.wjgnet.com/1007-9327/full/v14/i32/4995.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.4995
The history of giant duodenal ulcers (GDUs) dates back to 1931 when Brdiczka first described these exceptionally large ulcers[1]. Initially, GDUs were notable for being difficult to diagnose with barium roentgenogram[2,3] and their high morbidity and mortality[4-6]. Prompt and correct diagnosis was often delayed, and for decades the only curative intervention was invasive, surgical procedures fraught with technical difficulties. Until the early 1980’s, few cases were ever described within medical literature in which patients with GDUs were successfully treated with medical therapy. Since the late 1970’s and early 80’s, technological and pharmacological advancements have markedly changed the manner in which physicians diagnose, treat and manage patients with GDUs. The widespread use of endoscopy, the introduction of H-2 receptor blockers and proton pump inhibitors, and the improvement in surgical techniques have all contributed to this evolution. Despite this, we suggest that GDUs remain an under recognized entity, and are often assumed to be identical to standard sized ulcers. A careful review of the literature highlights the important differences when comparing GDUs to classical peptic ulcers and why they must be thought of differently than their more common counterpart.
Brdiczka is credited with being the first to describe GDUs and their radiographic appearance[1]. Most early case reports and small series emphasized the common problem of missed diagnosis on barium studies[7-10]. This was due to the fact that the ulcer crater was so large it would often be mistaken for a normal or slightly deformed duodenal cap. Kirsh and Brendel best illustrated this in their 1968 publication[4]. They examined 42 cases of GDU and found that only 24 were correctly diagnosed with a barium meal. They also established criteria for the diagnosis of GDUs. Their criteria included: an ulcerative crater greater than 2 cm, performance of the roentgen examination before surgical or pathological demonstration of ulcer, proof that the lesion was benign and confirmation at surgery or post-mortem examination. Over the years, with the advent and technologic advancements of endoscopic evaluation, the criteria have unofficially evolved. Today, endoscopy has essentially replaced barium contrast studies for visualization of the upper gastrointestinal tract and there is little difficulty with the diagnosis of these lesions. GDUs are generally now defined simply as a benign, full thickness ulcer at least 2 cm in diameter, and usually involving a large portion of the duodenal bulb[11].
Peptic ulcer disease remains a common medical problem worldwide, with the lifetime prevalence ranging from approximately 11% to 20% for men, and 8% to 11% for women[12]. Given this substantial lifetime prevalence, there are major economic loses and health care expenditures due to this problem. GDUs comprise approximately 1%-2% of all duodenal ulcers[13] and 5% of peptic ulcers requiring surgical intervention[14]. In most epidemiologic studies, the male to female ratio of standard sized ulcer disease is 2.3 to 1[15], and the ratio in those with GDUs is approximately 3 to 1[11].
The etiology of standard sized and GDUs has been associated with two major contributing causes: recent usage of nonsteroidal anti-inflammatory drugs (NSAIDs), and Helicobacter pylori (H pylori) infection. Worldwide, infection with H pylori continues to be widespread despite decreasing prevalence in select countries such as the United States. In addition, NSAID use has remained high, and the point prevalence of standard sized duodenal ulcers can reach 19% in chronic NSAID users[16]. Given these statistics, the clinician’s awareness and familiarity with GDUs will be necessary, as patients will assuredly continue to develop these dangerous variants of peptic ulcer disease.
As stated above, GDUs have been most commonly associated with recent NSAID use and H pylori infection. The mechanism by which each of these factors causes duodenal ulcers is markedly different. Simply stated, H pylori infection has been shown to lead to a dysregu-lation of acid secretion, and an antral predominant gastritis. This leads to a high duodenal acid load and promotes gastric metaplasia within the duodenal bulb. This in turn favors H pylori colonization in the duodenum within these islands of gastric metaplasia. Through multiple different mechanisms, the bacteria cause inflammation within the duodenum, which is exacerbated by the increased acid load, and ultimately leads to the formation of an ulcer[17]. Meanwhile, NSAIDs lead to ulcer formation primarily through the inhibition of prostaglandins and direct mucosal injury[17].
The true incidence of H pylori in standard sized duodenal ulcer formation is not known, but recent data suggests that it exceeds 85%[18,19]. However, two recent studies suggest that the percentage of GDUs caused by H pylori is less than when compared to standard sized ulcers, and that NSAID use may play a more prominent role[20,21]. In 1999, Fischer et al reviewed 28 cases of GDU. In their study, only 39% of patients tested were found to have H pylori infection. In addition, Colleen et al[20] evaluated 184 patients with duodenal ulcers, and compared patients with standard sized ulcers to those with GDUs. They found that 53% of patients with GDUs had used daily NSAIDs in the month prior to presentation versus only 8% in the standard sized duodenal ulcer group. This same study also evaluated the basal acid output of patients with GDUs as compared to those with standard size ulcers; however, no significant difference existed[20].
This collection of data suggests that daily NSAID use plays a more prominent role in the formation of GDUs than in standard sized duodenal ulcers. And while H pylori infection likely plays a role in the formation of GDUs, it is not as prevalent as it is with the formation of standard size duodenal ulcers.
Unfortunately, little data exists to explain the pathophysiologic differences that lead certain patients to develop these giant variants. Plausible explanations include genetic predisposition, dietary or environmental factors, microbial influence, variations in immunologic response or any combination of these factors[22,23]. Clearly, investigation and research are necessary to help provide further insight into these heretofore unexplained mechanisms.
The presenting symptoms of most patients reflect the anatomical changes and the histopathology of the disease process. The most common of these symptoms is abdominal pain. Most patients describe the pain as involving the epigastric region, and some experience involvement of the right hypochondrium and/or radiation into the back. This requires the physician to include biliary and pancreatic pathology in their differential diagnosis. The pain of these ulcers has been described as more persistent than classically described with smaller duodenal ulcers. In addition, patients with GDUs are not provided relief from food or alkali[6,24].
The majority of GDUs will present with hemorrh-age[6,25]. This may manifest with melena, hematochezia, hematemesis, or any combination of the above. Anemia typically occurs in the setting of the bleeding ulcers. Surgical and endoscopic evaluation has often revealed the gastroduodenal artery within the ulcer bed. The size of the ulcer and the surrounding inflammation may cause gastric outlet obstruction. This often causes nausea and vomiting. Obstruction may be evident endoscopically by the presence of a large volume of gastric contents.
Additionally, the inflammatory mass can produce significant constitutional symptoms such as weight loss, cachexia, malnutrition and chronic abdominal pain. This constellation of symptoms can often mislead the clinician to suspect malignancy as the most likely diagnosis.
Other important historical features are the personal history of ulcer disease and the recent use of NSAIDs. Some case series note that over 40% of patients will have a prior history of a peptic ulcer[11]. In 1994, Colleen et al[20] revealed that patients with daily NSAID use greater than 1 mo prior to presentation had a markedly increased risk of forming GDUs.
It is well known that there is a high rate of complications with GDUs, and these complications directly lead to the high morbidity and mortality associated with this entity[5,6,25]. Common complications encountered with GDUs include bleeding and, on occasion, massive hemorrhage. One study showed that a GDU with adherent clot or a visible vessel on index EGD is a marker of an ulcer that is more likely to require surgical intervention[21]. Another feared complication is perforation. In previous case series, the perforation rate has varied between 0% and 7%[20,21,25,26]. Other complications include obstruction of the affected portion of the duodenum or proximal pylorus due to the massive inflammatory response, fistula formation, adhesions to or erosions into surrounding organs, and stricture formation in the biliary tree, pancreatic duct or the small bowel itself. These inflammatory changes are also one of the reasons that make a surgical approach fraught with technical difficulties[11,25,26].
As discussed above, the difficulty in diagnosis of GDUs has been one of the hallmarks of this disease entity since first described. Making the radiographic diagnosis by barium meal has been difficult[2,3]. The size of the ulcer often causes replacement of the duodenal bulb. As a result, GDUs may be altogether missed or misinterpreted as a deformed bulb, diverticulum or pseudodiverticulum during a barium study. The radiographic criteria to make the diagnosis of GDU were summarized by Klamer and Mahr in 1978[25]. Despite increased awareness of these criteria by clinicians, the successful diagnosis based solely on upper GI series remained unacceptably low. As a result, the entity of GDUs was likely under-diagnosed and frequently missed[4].
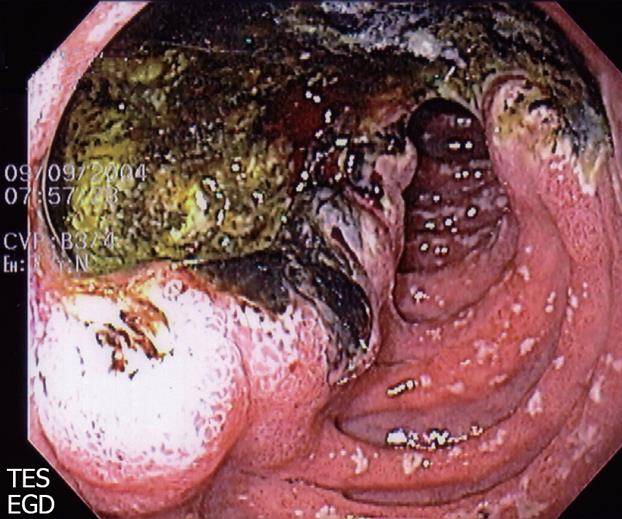
The advent and widespread use of endoscopy has markedly improved clinician’s ability to detect GDUs with greater accuracy[27]. Jaszewski et al highlighted this in 1983. In their series, seven cases of GDU were initially evaluated by upper GI barium meal. EGD then followed. GDUs were successfully diagnosed with roentgenography in only three of the seven cases. However, endoscopy confirmed the presence in all seven cases. This highlighted the importance of endoscopy in patients with symptoms suggestive of giant or regular duodenal ulcers. Subsequent case series have since used only endoscopy with measurements as diagnostic criteria[13,21]. Indeed, barium studies are now used far less frequently, typically when endoscopy is contraindicated or incapable of passing through a stricture. As a result, many clinicians may now encounter a GDU during an endoscopy without the expectation of finding one, and may be inclined to misdiagnose a GDU as a simple peptic ulcer. Please see Figure 1 for an example of a GDU appearance during endoscopy.
The other obvious benefit of endoscopy is the ability to biopsy. This allows the clinician to exclude a neoplastic source as the cause of ulcer formation. While routine biopsy of standard sized duodenal ulcers is generally not recommended, this practice is worthwhile in the setting of GDUs, particularly those with nodularity at the edge. These giant variants, while usually benign, can frequently harbor malignancy. This was supported by a recent review of 52 cases of duodenal ulcers larger than 2 cm which were biopsied by Rathi et al. They found a malignancy rate of approximately 19% (primary duodenal carcinoma in 15%, lymphoma and tuberculosis in 2% each)[13]. Thus, we recommend multiple biopsies from the ulcer edges in all cases of GDUs.
Prior to endoscopy, the differential diagnosis of benign GDUs had to include entities that mimicked its appearance on upper GI series. This included carcinoma of the duodenum, lymphosarcoma, duodenal diverticulum, pseudodiverticulum, regional enteritis, MALT lymphoma and tuberculosis. However, with the widespread use of endoscopy as detailed above, the differential diagnosis is somewhat narrowed. Frequently, the endoscopist cannot differentiate a benign giant ulcer from a malignant one macroscopically. As described above, patients may present with insidious symptoms such as weight loss, anorexia, and malnutrition raising concern for a malignant etiology. Therefore, again, biopsy and histopathologic examination is a necessity. Gastrinoma must also be included in the differential diagnosis, particularly in patients with evidence of acid hypersecretion, multiple ulcers extending beyond the second portion of the duodenum, diarrhea or a personal or family history of multiple endocrine neoplasia type 1 (MEN1).
Prior to the introduction of H2 receptor blockers in the late 1970’s, GDUs were managed primarily with surgery. This was reflected in the literature, as before 1982, there were very few cases published detailing successful medical management of GDUs[10-12]. Lumsden was able to document one patient who was a long-term (> 6 mo), asymptomatic survivor of a GDU after medical treatment only[6].
Initially, the mortality rate associated with surgical management of GDUs was extremely high, reaching greater than 40% in early case series[24,25]. Those patients who had prompt and accurate pre-operative diagnosis had the lowest mortality. The inflammatory qualities of GDUs that make them distinct in size and nature from standard ulcers also make them more difficult to approach surgically[11]. Classically, the surgical technique most commonly recommended is truncal vagotomy and subtotal gastrectomy[21]. However, technical variations of the surgical approach have been debated. For example, management of the duodenal stump has been controversial[11,28].
In subsequent decades, the mortality rate has fallen markedly due to numerous factors including improved radiographic technique, the advent of endoscopy and improved surgical and anesthetic techniques[11,21]. Most recently, since the 1970s, the advent of new acid suppression medication, the discovery of H pylori and its role in ulcer formation, and the importance of eradication therapy has led to the possibility of successful medical management of GDUs[21,27,29].
In 1983, Jaszewski et al[27] reviewed 14 consecutive cases of GDUs between 1980 and 1982. Nine patients qualified for a trial of extended conservative medical treatment with close follow up. They were treated with cimetidine 1200 mg daily and antacids every 2 h while awake. Eight of the nine patients were successfully treated with the medical regimen and were asymptomatic for a mean of 14.9 mo. One patient failed medical treatment and had a perforation on day 23. This study suggested that medical treatment of uncomplicated GDUs could be accomplished with medical management, close observation and follow up with the assistance of endoscopic monitoring. In a Letter to the Editor responding to this article, Porro et al retrospectively reviewed 23 cases of GDUs, nine of which were eligible for similar treatment with cimetidine. Their letter agreed that H2 receptor blockers could be used as short-term medical treatment of GDUs[30].
Both of these reviews were performed before the release of proton pump inhibitors. In 1999, Fischer et al[21] published a prospective study of 28 patients with GDUs. One patient met criteria for immediate surgical referral, and the remaining 27 were placed on omeprazole 40 mg by mouth daily. Of these 27 patients, 7 required secondary surgical treatment for GDU complications or failure of medical therapy (4 emergent operations for re-bleeding, 3 elective operations for gastric outlet obstruction). Of these twenty remaining patients, fifteen of them had complete documented healing on endoscopy. Of the eight patients requiring surgery, seven had a visible vessel or adherent clot on index EGD. Lastly, only 39% of patients had evidence of H pylori infection, and these patients were less likely to require surgery than those who were H pylori negative. The authors concluded that omeprazole appears to be a safe first-line treatment in stable patients, and should decrease the eventual need for operative intervention.
Several studies have confirmed the superiority of proton pump inhibitors (PPIs) versus H2 receptor blockers in the treatment of standard sized gastric and duodenal ulcers[31-34]. Thus, attempts at medical treatment of GDUs should consist of proton pump inhibitors.
Despite the marked improvement delivered by the administration of proton pump inhibitors, GDUs are still associated with high rates of morbidity, mortality and complications. Thus, surgical evaluation of a patient with a GDU should remain an integral part of patient care. Indeed, there are indications for emergent and elective surgical intervention. The most common emergent indications include uncontrolled hemorrhage and perforation whereas unresolving obstruction, intractable or recurrent bleeding, and fistula formation are some of the elective indications[14]. Based on the data above, PPIs should be administered as a medical adjunct to the operation.
Lastly, discontinuation of NSAIDs and antimicrobial treatment of H pylori infection are recommended in the presence of these risk factors. Eradication of H pylori has been well documented to improve healing of peptic ulcers[29,35].
Historically, the two hallmark features of GDU disease were the difficulties in prompt diagnosis and the failures of medical management. A review of the literature suggests the changing nature of both of these features. The advent and widespread use of endoscopy has made a prompt diagnosis of GDU more accurate and easy to obtain. Once a diagnosis has been made, initiation of therapy can begin. Uncontrolled hemorrhage, perforation and unstable patients still should be cared for with immediate surgical evaluation and management. Recent data examining the use of H2-receptor blockers and proton pump inhibitors suggest that stable patients may be safely treated initially with medication, close observation and repeat endoscopic evaluation[21,27,30]. In addition, endoscopic biopsies allow a clinician to test for malignant etiologies of giant ulcers, which may be more common than suspected[13]. For this reason, we recommend that biopsies should be performed on all duodenal ulcers > 2 cm in diameter, particularly those with nodular appearing edges.
Due to evolving endoscopic and medical therapies, the management of GDUs has changed. What was once a disease that was difficult to diagnose, and managed solely with surgical intervention has become one easily diagnosed and potentially treated medically. It is of utmost importance that physicians recognize GDUs as being different than their standard sized counterparts, and that we continue to further our understanding of this entity.
Peer reviewer: Vasiliy I Reshetnyak, MD, PhD, Professor, Scientist Secretary of the Scientific Research Institute of General Reanimatology, 25-2, Petrovka str., Moscow 107031, Russia
S- Editor Li DL L- Editor Li M E- Editor Lin YP
| 1. | Brdiczka JG. Das Grosse ulcus duodeni in rontgenbild. Fortschr Geb Rontgenstr. 1931;44:177-181. |
| 2. | Freedman E, Goehring HD. Diagnostic errors in ulcerative lesions of the stomach and duodenum. Am J Roentgenol Radium Ther. 1940;44:48-58. |
| 3. | Elkin WP. Diagnostic problems in cases of large or giant duodenal ulcer. Radiology. 1941;37:748-750. |
| 5. | Carmichael JL, Tidwell OK. Giant duodenal ulcer: a case report and brief review of the literature. Ala J Med Sci. 1970;7:385-387. |
| 8. | Bullock WK, Snyder EN. Benign giant duodenal ulcer. Gastroenterology. 1952;20:330-336. |
| 10. | Pinck RL, Held BT. Giant ulcers or walled-off perforations of the duodenum. N Engl J Med. 1961;264:541-543. |
| 12. | Kurata JH, Nogawa AN, Abbey DE, Petersen F. A prospective study of risk for peptic ulcer disease in Seventh-Day Adventists. Gastroenterology. 1992;102:902-909. |
| 13. | Rathi P, Parikh S, Kalro RH. Giant duodenal ulcer: a new look at a variant of a common illness. Indian J Gastroenterol. 1996;15:33-34. |
| 14. | Gustavsson S, Kelly KA, Hench VS, Melton LJ 3rd. Giant gastric and duodenal ulcers: a population-based study with a comparison to nongiant ulcers. World J Surg. 1987;11:333-338. |
| 15. | Watanabe Y, Kurata JH, Kawamoto K, Kawai K. Epidemiological study of peptic ulcer disease among Japanese and Koreans in Japan. J Clin Gastroenterol. 1992;15:68-74. |
| 16. | Loeb DS, Talley NJ, Ahlquist DA, Carpenter HA, Zinsmeister AR. Long-term nonsteroidal anti-inflammatory drug use and gastroduodenal injury: the role of Helicobacter pylori. Gastroenterology. 1992;102:1899-1905. |
| 17. | Shiotani A, Graham DY. Pathogenesis and therapy of gastric and duodenal ulcer disease. Med Clin North Am. 2002;86:1447-1466, viii. |
| 18. | Ciociola AA, McSorley DJ, Turner K, Sykes D, Palmer JB. Helicobacter pylori infection rates in duodenal ulcer patients in the United States may be lower than previously estimated. Am J Gastroenterol. 1999;94:1834-1840. |
| 19. | Bytzer P, Teglbjaerg PS. Helicobacter pylori-negative duodenal ulcers: prevalence, clinical characteristics, and prognosis--results from a randomized trial with 2-year follow-up. Am J Gastroenterol. 2001;96:1409-1416. |
| 20. | Collen MJ, Santoro MJ, Chen YK. Giant duodenal ulcer. Evaluation of basal acid output, nonsteroidal antiinflammatory drug use, and ulcer complications. Dig Dis Sci. 1994;39:1113-1116. |
| 21. | Fischer DR, Nussbaum MS, Pritts TA, Gilinsky NH, Weesner RE, Martin SP, Giannella RA. Use of omeprazole in the management of giant duodenal ulcer: results of a prospective study. Surgery. 1999;126:643-648; discussion 648-649. |
| 22. | Arkkila PE, Kokkola A, Seppala K, Sipponen P. Size of the peptic ulcer in Helicobacter pylori-positive patients: association with the clinical and histological characteristics. Scand J Gastroenterol. 2007;42:695-701. |
| 23. | Karpouza A, Samouilidou E, Karagiannis S, Kostopoulou V, Sotiropoulou M, Roma E, Petraki K, Michopoulos S. Patients with duodenal ulcer have lower levels of serum cholesterol compared to other dyspeptic patients independently of Helicobacter pylori status. Scand J Gastroenterol. 2008;43:922-928. |
| 24. | Mistilis SP, Wiot JF, Nedelman SH. Giant duodenal ulcer. Ann Intern Med. 1963;59:155-164. |
| 25. | Klamer TW, Mahr MM. Giant duodenal ulcer: a dangerous variant of a common illness. Am J Surg. 1978;135:760-762. |
| 26. | Morrow CE, Mulholland MW, Dunn DH, Schwartz ML, Sutherland DE, Goodale RL, Humphrey E, Najarian JS. Giant duodenal ulcer. Am J Surg. 1982;144:330-331. |
| 27. | Jaszewski R, Crane SA, Cid AA. Giant duodenal ulcers. Successful healing with medical therapy. Dig Dis Sci. 1983;28:486-489. |
| 28. | Wu X, Zen D, Xu S, Zhang L, Wang P. A modified surgical technique for the emergent treatment of giant ulcers concomitant with hemorrhage in the posterior wall of the duodenal bulb. Am J Surg. 2002;184:41-44. |
| 29. | Arkkila PE, Seppala K, Kosunen TU, Sipponen P, Makinen J, Rautelin H, Farkkila M. Helicobacter pylori eradication as the sole treatment for gastric and duodenal ulcers. Eur J Gastroenterol Hepatol. 2005;17:93-101. |
| 30. | Bianchi Porro G, Lazzaroni M, Petrillo M. Giant duodenal ulcers. Dig Dis Sci. 1984;29:781. |
| 31. | Walan A, Bader JP, Classen M, Lamers CB, Piper DW, Rutgersson K, Eriksson S. Effect of omeprazole and ranitidine on ulcer healing and relapse rates in patients with benign gastric ulcer. N Engl J Med. 1989;320:69-75. |
| 32. | Bader JP, Delchier JC. Clinical efficacy of pantoprazole compared with ranitidine. Aliment Pharmacol Ther. 1994;8 Suppl 1:47-52. |
| 33. | Yeomans ND, Tulassay Z, Juhasz L, Racz I, Howard JM, van Rensburg CJ, Swannell AJ, Hawkey CJ. A comparison of omeprazole with ranitidine for ulcers associated with nonsteroidal antiinflammatory drugs. Acid Suppression Trial: Ranitidine versus Omeprazole for NSAID-associated Ulcer Treatment (ASTRONAUT) Study Group. N Engl J Med. 1998;338:719-726. |
| 34. | Agrawal NM, Campbell DR, Safdi MA, Lukasik NL, Huang B, Haber MM. Superiority of lansoprazole vs ranitidine in healing nonsteroidal anti-inflammatory drug-associated gastric ulcers: results of a double-blind, randomized, multicenter study. NSAID-Associated Gastric Ulcer Study Group. Arch Intern Med. 2000;160:1455-1461. |
| 35. | Arkkila PE, Seppala K, Kosunen TU, Haapiainen R, Kivilaakso E, Sipponen P, Makinen J, Nuutinen H, Rautelin H, Farkkila MA. Eradication of Helicobacter pylori improves the healing rate and reduces the relapse rate of nonbleeding ulcers in patients with bleeding peptic ulcer. Am J Gastroenterol. 2003;98:2149-2156. |