INTRODUCTION
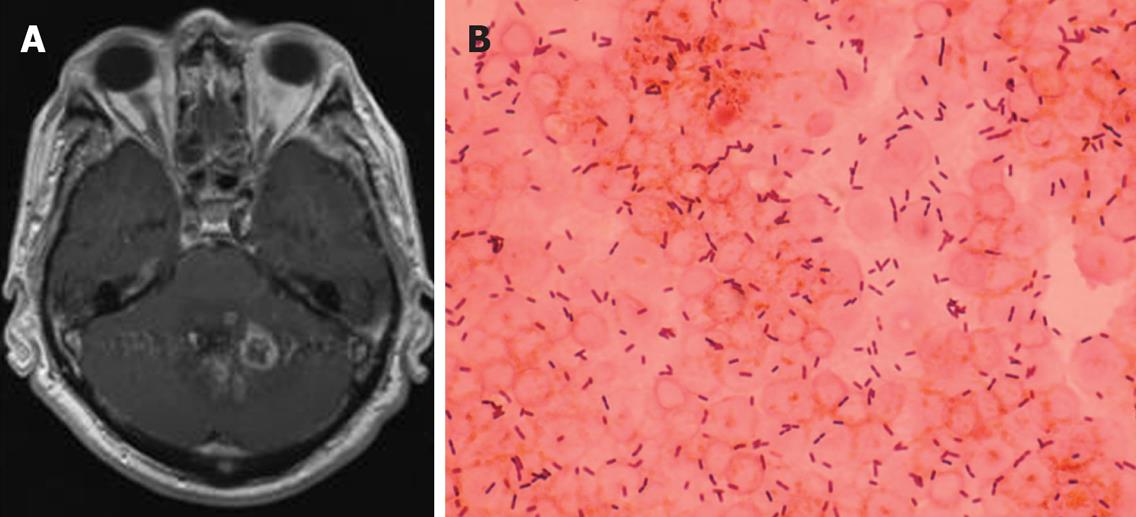
Figure 1 A: Gadolinium enhanced brain MRI demonstrating multiple ring-enhancing lesions, compatible with abscesses, located in the cerebellum; B: Gram stain of the spinal fluid of the same patient showing gram-positive rods.
Listeria monocytogenes was isolated in spinal fluid culture.
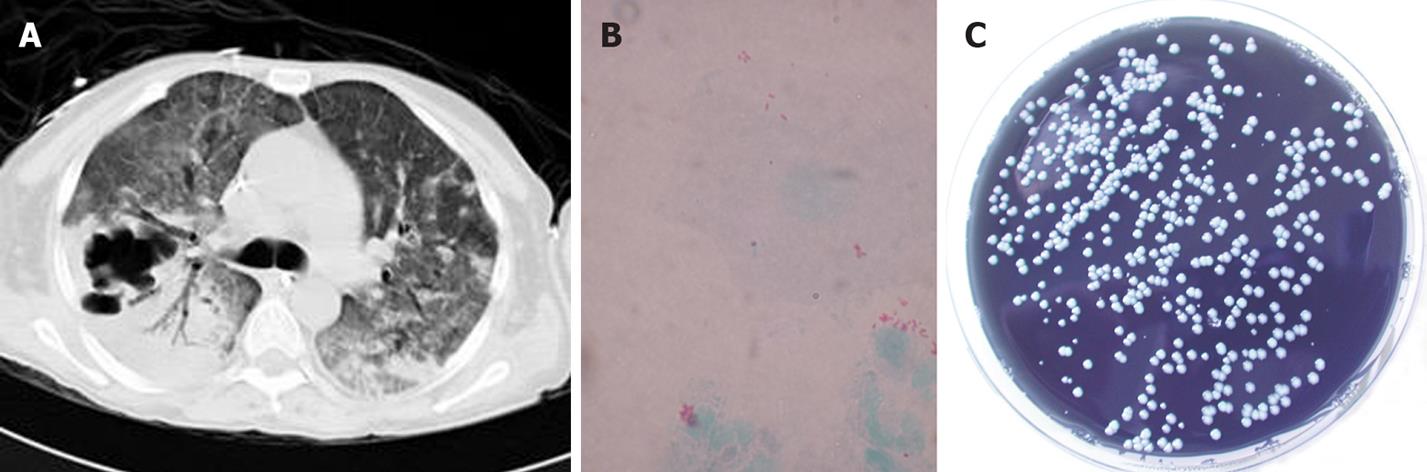
Figure 2 A: Chest CT scan showing multilobar pneumonic consolidation with cavitary lesions; B: Gimenez stain of a bronchoalveolar lavage specimen of the same patient showing small red coccobacilli; C: Legionella pneumophila was cultured on selective buffered charcoal yeast extract agar.
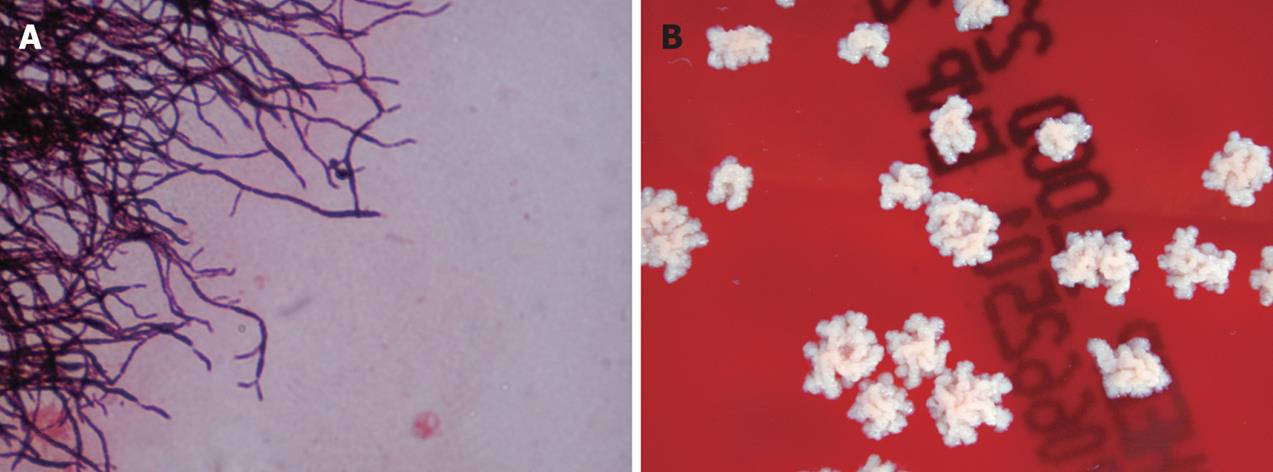
Figure 3 A: Gram stained smear of a subcutaneous lesion aspirate demonstrating branching and beaded filamentous appearance gram-positive rods; B: Nocardia asteroides was isolated in culture.
Since the first report in 1963, liver transplantation has become an established treatment for properly selected patients with end-stage liver disease, primary and some secondary hepatic malignancies and some rare metabolic liver-based disorders. During the past decade, survival rates after liver transplantation have steadily improved, with one-year survival exceeding 90% and approaching 70% at 8 years[1]. Increasingly, potent immunosuppressive agent use has dramatically reduced the incidence of rejection in the transplanted population, while increasing patient susceptibility to opportunistic infections and cancer[2]. However, recent advances in effective antimicrobial prophylactic strategies have led to a decline in the incidence of opportunistic infections in liver transplant recipients. Nevertheless, morbidity and mortality due to infectious complications remain as major problems in this selected population.
Infection and rejection remain major causes of morbidity after a liver transplant, accounting for up to 85% of deaths in some studies[3]. Infections were the most common cause of death after liver transplantation, and were present in 64% of a total of 321 cases in a study[4]. Two-thirds of all infections occurred during the first 100 d post transplantation in this study. Overall, the infections were bacterial in 48% of the cases, fungal in 22%, and viral in 12%[4]. The ratio of infectious to non-infectious causes of death did not change significantly during the 15-year study period, and the relative percentages of bacterial, fungal, and viral infections showed relatively little discrepancy on a year-to-year basis in this study[4]. In one other study, 83% of a liver transplant population had 1 or more episodes of infection and 67% had severe infections[5]. 70% of severe infections occurred within the first 2 mo after transplantation. The most frequent severe infections occurred were abdominal abscesses, bacterial pneumonia, invasive candidiasis, Pneumocystis jiroveci pneumonia, and symptomatic cytomegalovirus infection[5]. In a 10-year Swiss single-centre study[6], 80% of patients developed an infection following transplantation. Almost half of these were bacterial with an infection rate that peaked during the first month following transplantion. The temporal relation between graft rejection and the occurrence of infection showed that the incidences of both viral and bacterial infections were increased by immunosuppressive supplementation. The overall incidence of bacterial infections was much higher during the first month following transplantation than after 1 mo in this study; 188.2 vs 5.8 episodes per 1000 patient-days[6].
Patterns of opportunistic bacterial infections after transplantation have been altered by routine antimicrobial prophylaxis for Pneumocystis jirovecii (trimethoprim-sulfamethoxazole prophylaxis for as little as 3 mo or for as long as a lifetime), and by infections due to organisms with antimicrobial resistance. Infections occur in a generally predictable pattern after solid-organ transplantation and most can be grouped into three major periods: the early period posttransplant (up to 6 wk), an intermediate period (1 to 6 mo); and a late period (more than 6 mo)[2].
Infections occurring immediately post-transplant-ation are similar to those seen in post-operative immuno-competent hosts. Bacterial infections predominate; they usually have a nosocomial source, such as central vascular access sites, external drainage catheters or are related to foreign bodies, necrotic tissue, or prolonged endobronchial intubation. Abdominal abscesses and peritonitis, intrahepatic abscesses (often associated with hepatic artery thrombosis), cholangitis, wound infections, nosocomial pneumonias (in patients who require prolonged ventilation) are common during this time frame. Patients may become infected with nosocomial, antimicrobial-resistant bacteria, including methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus faecalis, Clostridium difficile, and antimicrobial-resistant gram-negative bacteria.
During the intermediate period, the nature of common infections changes and patients are most at risk for the development of opportunistic infections due to the cumulative effect of relatively high-dose immunosuppression, although residual problems from the perioperative period can persist. Viral pathogens and allograft rejection are responsible for the majority of febrile episodes that occur during the period from 1 to 6 mo after transplantation[2].
Opportunistic infections are uncommon after 6 mo in patients receiving stable and reduced maintenance doses of immunosuppression with good graft function, and little is documented about them in the literature. Potential etiologies of infection in these patients are diverse, including common, community-acquired bacterial infections seen in the general population, and uncommon opportunistic infections of clinical significance only in immunocompromised hosts. Respiratory infections due to capsulated pathogens, such as Streptococcus pneumoniae and Haemophilus influenza, can be life-threatening if not promptly treated in this population. In addition, transplant recipients may be more susceptible to certain bacterial pathogens, such as the Nocardia species, Legionella species, Listeria monocytogenes, Mycoplasma species, Salmonella species Bartonella species, or Rhodococcus equi. These late bacterial infections may be very difficult to recognize and treat in this population. Little information is available on the opportunistic late bacterial infections in liver transplant recipients. This review will focus on the risk, recognition and management of specific late bacterial infections, which are anticipated to occur more than 6 mo after liver transplantation.
SPECIAL INFECTION CHARACTERISTICS OF THE LIVER TRANSPLANT PATIENT
In general, it is more difficult to recognize infection in transplant recipients than it is in patients with normal immune function. Inflammatory responses associated with infection may be impaired by immunosuppressive therapy, and signs and symptoms of infection may often be diminished. Furthermore, noninfectious causes of fever, such as allograft rejection, may develop in these patients. Because of the immunosuppressed state of this population, they are susceptible to a broad range of opportunistic infections, which frequently present with multi-organ system involvement and progress rapidly. In addition, altered anatomy following transplant surgery may alter the physical signs of infection.
Cytomegalovirus infection can be documented in more than half of patient populations after organ transplantation[7]; with viral replication persisting long term. It is known that cytomegalovirus can affect the capacity of the host to mount a defense against complicating infections. Cytomegalovirus infection was associated with a 2.45-fold higher incidence of major infections between day 30 and 180 after orthotopic liver transplantation in a study, with most of these infections caused by gram-positive cocci[8].
Serologic testing is not generally useful for diagnosis since seroconversion is often delayed in these patients (antigen-based tests or nucleic acid-based molecular assays are recommended for diagnosing in this population). In the same way, tissue biopsies with histopathology and microbiology are often needed to make an early and specific microbiologic diagnosis.
The choice of antimicrobial regimens is often more complex than in other patients due to increased antimicrobial resistance, urgency of therapy and the high frequency of drug related toxicities and interactions with immunosuppressant drugs.
SPECIFIC LATE BACTERIAL INFECTIONS
Listeria monocytogenes infection in the liver transplant patient
Listeriosis is a clinical condition occurring with an annual incidence of 4.4/million individuals in the US and an associated mortality of approximately 20%[9]. Listeria monocytogenes has long been known as a pathogen of immunocompromised hosts (Figure 1). However, Listeria infections have only rarely been reported following orthotopic liver transplantation. The low incidence may be due, in part, to the prophylactic use of trimethoprim-sulfamethoxazole for Pneumocystis jiroveci pneumonia for up to 1 year following liver transplantation. Listeriosis occurs rarely after this 1 year period, so that further use of prophylactic antibiotics against this infection seems unjustified according to some authors[10]. The route of infection is usually through the intestinal tract following ingestion of contaminated food products, as well as from mother to child either transplacentally or during childbirth. The importance of dietary and other environmental exposures cannot be underestimated[11]. Most reported cases of listeriosis occurred months to years following liver transplantation, but it may also occur in the early postoperative period[12]. Its principal manifestations include bacteremia[10,13] and meningitis[14,15], although endocarditis[16], peritonitis[17], hepatitis[18,19], epididymitis or orchitis[20] have been reported. Listeria meningitis can be associated with profound changes in mental status or focal neurologic signs from involvement of the brain stem (rhombencephalitis) or even presence of a brain abscess[15]. Clinically, bacteremia is almost always present and is usually instrumental in getting the diagnosis. Treatment with antibiotics including ampicillin or trimethoprim-sulfamethoxazole with or without an aminoglycoside has proven successful in the majority of the cases.
Five cases of listeriosis following liver transplantation have been reported[14], four within 3 wk and one within 4 mo following transplantation. Rettally et al[10] reported a case of a patient presenting with L. monocytogenes bacteremia at 32 mo following orthotopic liver transplantation. The patient was successfully treated with 3 wk of intravenous treatment with ampicillin and vancomycin. In the case reported by Avery et al[16], the patient was receiving trimethoprim-sulfamethoxazole prophylaxis for P. jiroveci pneumonia until 7 mo post-transplant, at which time aerosolized pentamidine was substituted because of the concern about possible drug hepatotoxicity. Three months later, the patient was diagnosed with a tricuspid valve L. monocytogenes endocarditis. The patient was successfully treated with a total of 6 wk of ampicillin followed by penicillin therapy, with gentamicin administered for the first 4 wk of this course. Doses of her immunosuppressive medications were decreased. Bourgeois et al[18] reported the case of a liver transplant recipient who was diagnosed with L. monocytogenes acute hepatitis 8 mo after grafting. The patient made a full clinical recovery after therapy with ampicillin and gentamicin intravenously for 4 wk.
Legionella species infection in the liver transplant patient
Legionella species account for approximately 5% of cases of pneumonia in the general US population. Although more than 30 species have been described in the Legionellae family, 70% to 90% of infections are caused by L. pneumophila, particularly serogroups 1 and 6, followed by L. micdadei[21]. Aquatic habitats are considered the environmental reservoir for community and nosocomial acquired pneumonia[22]. Susceptible hosts include the elderly, cigarette smokers, individuals receiving immunosuppressive therapy, and organ transplant recipients[23]. Although community acquisition occurs, nosocomial L. pneumonia is more commonly reported in transplant patients (Figure 2). In solid organ recipients, infection often occurs early in the post-transplantation period or coincidentally with corticosteroid therapy for allograft rejection[22]. The radiographic findings of L. pneumonia in immunosuppressed patients can vary and include unilateral or bilateral dense, patchy, or nodular pulmonary infiltrates that can progress to cavitation.
The first case of infection with Legionella species in a liver transplant recipient was reported by Tokunaga et al[24] in 1992. L. pneumophila, serogroup 1, was identified by direct immunofluorescence in the lung and liver graft from a 2-mo-old infant who underwent orthotopic liver transplantation because of fulminant hepatic failure secondary to neonatal hepatitis. The patient died of respiratory failure due to this infection 22 d after transplantation despite treatment with erythromycin. Singh et al[25] described 3 adult liver transplant patients with evidence for Legionellosis acquired within 2 mo of surgical transplantation, including one case of L. bozemanii pneumonia, one case of L. pneumophila pericarditis, and one case of L. pneumophila pneumonia. In addition, the third case also had L. micdadei isolated from pleural fluid. All three cases responded to a combination of erythromycin and ciprofloxacin. A L. pneumonia nosocomial outbreak after liver transplantation has been described[26]. Patients presented with fever, nonspecific constitutional symptoms, and either unilateral or bilateral infiltrates developing 3 wk to 12 wk after transplantation. Treatment with either erythromycin (four cases) or ciprofloxacin (one case) was successful in three of five patients. A hospital-acquired pneumonia due to L. parisiensis was reported by Presti et al[27] in 1997. The diagnosis was made on the basis of clinical and radiological signs of pneumopathy in a liver transplant patient and was confirmed by microbiological data. The patient was successfully treated with erythromycin for 3 wk, allowing resolution of the pneumonia. Ernst et al[28] reported a liver transplant recipient with community acquired L. micdadei pneumonia 8 years after transplantation. The patient was successfully treated with a 3-wk course of ciprofloxacin. According to the authors, the predisposition in this case might in part be attributed to the need for a course of heightened immunosuppressive agents to treat an episode of acute organ rejection. Fraser et al[29] reported a liver transplant recipient who presented with cavitary pneumonia caused by L. pneumophila 7 years after transplantation. The patient required surgical resection and a 21-d course of therapy with levofloxacin for diagnosis and cure.
Legionella pneumonia is a rare complication of orthotopic liver transplantation in adults, and it remains a significant cause of morbidity and mortality in the susceptible host. Definitive diagnosis of Legionella pneumonia can be particularly elusive. As the urinary Legionella antigen is negative when other species or serogroups different than L. pneumophila serogroup 1 are implicated, examination of respiratory secretions in combination with immunofluorescent staining or specialized culture is required to establish the diagnosis. Erythromycin, rifampin, trimethoprim-sulfamethoxazole, doxycycline, clindamycin, and quinolones, alone or in combination, for 3 wk are effective treatments. Due to the risk of undesirable drug interaction of erythromycin with tacrolimus or cyclosporine, fluoroquinolone agents such as levofloxacin or ciprofloxacin may be the preferred treatment for legionellosis in transplant recipients.
Nocardia species infection in the liver transplant patient
Nocardia species are ubiquitous environmental saprophytes, living in soil, organic matter and water[30]. There are at least 12 species with N. asteroides complex (N. asteroides sensu strictu, N. farcinica and N. nova), with N. brasiliensis, N. otitidiscaviarum and N. transvaliensis[30] being the most important ones. Nocardia species are Gram-positive, variably acid-fast, actinomycetes with branching rods (Figure 3). The frequency of nocardial infections in solid organ transplant recipients varies between 0.7% and 3% and has mostly been reported in heart, kidney and liver transplant recipients, and less frequently in lung transplantation[31]. In a recent study involving 1840 liver transplant recipients, only 2 (0.1%) had a Nocardia infection and only 1 of them had a disseminated infection consisting of bacteremia[31]. Disseminated nocardiosis is more frequent in cellular immunocompromised patients, and it is often a potentially life-threatening infection. It is endogenous (i.e., secondary to bloodstream spread) from a primary pulmonary infection[30]. However, it may result very rarely from a primary nonpulmonary (e.g. cutaneous, gastrointestinal) infection. The brain is the most frequent nonpulmonary site involved in disseminated nocardiosis[32]. A N. farcinica disseminated (subcutaneous and neural) infection has been described in a liver transplant recipient[33]. The patient presented in postoperative mo 3 with fever and pain in the lower leg. Ten days after admission, the patient developed neurological symptoms and 3 brain abscesses were detected in an MRI.
A case of N. asteroides osteomyelitis of the tibia accompanied by brain abscesses in a liver transplant recipient was recently described[34]. The majority of bone cases are presumed to be due to direct extension from local soft tissue infection. Imaging of the brain should be performed in all cases of nocardiosis affecting a liver transplant recipient, since central nervous system involvement may be present even in the absence of neurological findings. Pulmonary involvement should also be excluded.
An optimal therapeutic approach has not been well established, especially in the disseminated forms. It is debated whether invasive resection or aspiration of the CNS lesions are necessary[2,35,36]. Some authors recommend an early biopsy of the lesions to achieve specific identification, even in cases where an extra-cranial focus of infection is found[37]. However, there are some reports that suggest that brain surgery is not always needed[35,36]. Sulfonamides are the drugs of choice for treatment of nocardiosis; however, there are an increasing number of reports of resistance to these agents[38]. The length of therapy in the treatment of nocardial infections in the transplant patient is debated. Some authors suggest extending it for 9 mo to 12 mo if there is CNS involvement[37]. The clinical use of other drugs should be supported by susceptibility testing. Linezolid is an oxazolidinone with activity against all of the clinically relevant species of Nocardia[39], which has been shown to be an effective alternative for the treatment of nocardiosis, especially for the CNS forms[34]. Lewis et al described a case in which minocycline was used for 12 mo with a good response. Doxycycline and minocycline[34,40] may be attractive options as rescue therapy in those patients in which other treatments failed.
Rhodococcus equi infection in the liver transplant patient
Rhodococcus equi is an intracellular gram-positive, aerobic, nonmotile, non-spore-forming bacillus that is an increasingly important opportunistic respiratory pathogen in immunocompromised hosts. Impairment of cell-mediated immunity is the most important risk factor for infection with R. equi in humans[41]. Several cases have been described in patients with immunosuppression due to either human immunodeficiency infection, therapy for neoplastic disorders, or kidney and heart transplantation. 15 patients with a R. equi infection following solid-organ transplantation have been described in the literature[42-45]. In all patients, rhodococcal infection presented late after transplantation (median, 4 years). R. equi resides in the soil and humans acquire the infection through the respiratory tract. Exposure to farm animals was reported in only two cases; in four cases contact with contaminated soil or manure was considered a likely infectious source. Thirteen of 15 patients had pulmonary and/or pleural involvement. Soft-tissue infections were common in this population. Most patients received erythromycin and/or ciprofloxacin in combination therapy, which was generally associated with a successful outcome. Recurrent disease was observed after initial treatment with imipenem and tobramycin and in patients receiving monotherapy.
Sabater et al[43] reported the first case of R. equi infection in a liver transplant recipient. The patient was diagnosed with two subcutaneous abscesses on his left arm and an asymptomatic necrotizing bilateral pneumonia 28 mo following transplantation. The patient had been intensively immunosuppressed and working with calf manure. He was successfully treated with a combination of erythromycin, ciprofloxacin, and rifampicin. A case of vertebral osteomyelitis due to R. equi in a liver transplant recipient 7 mo after transplantation was reported by Fischer et al[42]. The patient was initially diagnosed with a recurrent pneumonia and a pleura-based lung abscess and subsequently developed osteomyelitis of the lower thoracic spine. Drainage of the paraspinal abscess and removal of the infected bone was performed while he was being treated with erythromycin and imipenem. Postoperatively, the patient received intravenous imipenem, vancomycin, and erythromycin for 14 d and then oral clarithromycin and rifabutin. Schilz et al[44] reported a case of a liver transplant patient with a pulmonary nodule caused by R. equi that followed a benign clinical course and resolved spontaneously.
Although solid-organ transplant recipients are rarely affected, R. equi must be included in the differential diagnosis of recurrent pneumonia or lung abscess in liver transplant recipients. Metastatic spread of infection is common, and diagnosis of pulmonary rhodococcal infection should prompt a search for additional involvement of extra-pulmonary sites. Monotherapy must be considered insufficient for transplant patients and surgery may be helpful in some cases.
Other late bacterial infections in the liver transplant patient
Bartonella henselae, the etiologic agent of cat-scratch disease, may cause a spectrum of illness in both immunocompetent and immunocompromised hosts. Disseminated infections caused by Bartonella species have been reported infrequently in solid organ transplant recipients. Humar et al[46] reported a case of a liver transplant recipient who presented 4 years later with fever of unknown origin, and was found to have a granulomatous hepatitis of the transplant allograft with a Bartonella species. Diagnosis was performed by serology (immunofluorescent antibody test). The patient completed a successful 4 wk course of azithromycin.
Apalsch et al[47] described a case of a disseminated cat-scratch disease in a 12-year-old liver transplant recipient with fever and lymphadenopathy. Granulomatous inflammation was shown in liver and lymph node biopsy specimens.
Because this organism may be difficult to grow in culture, serological and molecular tests are important for diagnosis. Antimicrobial therapy can result in prompt resolution of illness and usually consists of a macrolide or tetracycline derivative. However, the optimal antibiotic and duration of therapy for disseminated disease in immunocompromised patients is unknown.
Three cases of Mycoplasma hominis infections have been reported in the literature. Jacobs et al[48] reported the case of a recipient of liver transplantation with a postsurgical infection and Vogel et al[49] reported two patients with extragenital infections.
Clostridium difficile remains a significant complication in liver transplant recipients. In this patient population, C. difficile diarrhea may be more difficult to diagnose and may have a more complicated clinical course than in non-immunocompromised patients. Although the prevalence of C. difficile infection is usually the highest during the early post-transplant period, when immunosuppression is the highest, Albright et al[50] described 3 cases of late onset C. difficile infection (2, 3 and 5 years after transplantation). Nevertheless, the prognosis is good in most cases with timely diagnosis and treatment.