Published online Aug 7, 2008. doi: 10.3748/wjg.14.4705
Revised: April 10, 2008
Accepted: April 17, 2008
Published online: August 7, 2008
Tubulovillous adenomas are common in the colon and rectum, but are rare in the common bile duct. Biliary adenomas may produce obstructive jaundice, which can be easily confused with a malignant neoplasm or stone. We report a case of a carcinoma in situ arising in a tubulovillous adenoma of the distal common bile duct causing obstructive jaundice. A 55-year-old male presented with a 10-d history of pruritus and progressive jaundice. Abdominal sonography and computed tomography showed a mass in the distal common bile duct. Endoscopic retrograde cholangiopancreatography showed luminal narrowing of the bile duct due to a polypoid mass. Positron emission tomography demonstrated no abnormal uptake. It was thought that this mass was a malignant tumor, thus a pylorus-preserving panceaticoduodenectomy was performed. The final pathology showed a tubulovillous adenoma with carcinoma in situ of the distal common bile duct. At follow-up 8 mo later, endoscopy showed multiple polyps in the rectum, colon and stomach. The polyps were removed by endoscopic mucosal resection and shown to be tubular adenomas with high grade dysplasia. Biliary adenomas require careful follow-up for early detection of recurrence and malignant transformation.
-
Citation: Kim BS, Joo SH, Joo KR. Carcinoma
in situ arising in a tubulovillous adenoma of the distal common bile duct: A case report. World J Gastroenterol 2008; 14(29): 4705-4708 - URL: https://www.wjgnet.com/1007-9327/full/v14/i29/4705.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.4705
Biliary adenomas are very rare tumors, which may pose diagnostic dilemmas preoperatively. Due to recent advances in diagnostic techniques and early diagnosis, biliary adenomas are often detected as high grade dysplasias or carcinomas in situ. The optimal therapeutic strategy for adenomas of the distal common bile duct (CBD) or ampullary region has not been established. We report herein a case of a bile duct tubulovillous adenoma with carcinoma in situ presenting painless jaundice in a man who was treated with a pylorus-preserving pancreaticoduodenectomy (PPPD) and presented 8 mo later with a tubular adenoma in the gastrointestinal tract with high grade dysplasia. Our patient did not exhibit any clinical signs of familial adenomatous polyposis[1] or Gardner’s syndrome[2]. We also reviewed the literature about the common bile duct adenoma found in the English literature[3–22] (Table 1).
| Author | Sex | Age(yr) | Presentation | Treatment | Histology |
| Hulten, 1970[3] | M (n = 2) | 61, 80 | Bilary colic (n = 1) and jaundice (n = 2) | Local excision (n =2) | Papilloma (n = 2) with moderate atypia |
| Styne, 1986[4] | F (n = 1) | 59 | Recurrent cholangitis | Local excision | Papilloma |
| Saxe, 1988[5] | M (n = 1) | 64 | Painful jaundice and pruritus | Whipple | Villous adenoma |
| Harshfield, 1990[6] | M (n = 1) | 78 | Chronic right upper quadrant pain | Local excision | Villous adenoma |
| Sturgis, 1992[7] | F (n = 1) | 81 | Right upper quadrant pain | Endoscopic excision | Tubulovillous adenoma |
| Hanafy, 1993[8] | M (n = 1) | 76 | Mild jaundice and abdominal mass | Local excision | Villous adenoma |
| Buckley, 1993[9] | M (n = 1) | 34 | Chronic jaundice and abdominal pain | Whipple | Villous adenoma with malignant foci |
| Blot, 1996[10] | M (n = 1) | 84 | Febrile jaundice | Local excision | Villous adenoma |
| Kawakatsu, 1997[11] | F (n = 3) | 60.6 | Febrile jaundice | Whipple (n = 2), local excision (n = 3) | Villous adenoma with mild dysplasia (n = 1), malignant foci (n = 4) |
| M (n = 2) | (mean) | ||||
| Chae, 1999[12] | M (n = 1) | 77 | Painless jaundice and pruritus | Local excision | Villous adenoma with malignant foci |
| Inagaki, 1999[13] | M (n = 1) | 73 | Epigastric pain and jaundice | Whipple | Papilloma |
| Chang, 2001[14] | M (n = 1) | 51 | Febrile jaundice, abdominal mass | Operation refused | Papilloma with focal dysplasia |
| Oshikiri, 2002[15] | F (n = 1) | 69 | Jaundice | Whipple | Papilloma |
| Ariche, 2002[16] | F (n = 1) | 77 | Abdominal pain | Local excision | Villous adenoma with adenocarcinoma |
| Aggarwal, 2003[17] | M (n = 1) | 55 | Abdominal pain | Whipple | Adenoma with moderate dysplasia |
| Jao, 2003[18] | M (n = 1) | 60 | Abdominal screening ultrasound | Endoscopic excision | Tubulovillous adenoma |
| Lou, 2003[19] | M (n = 1) | 47 | Fever, abdominal pain | Local excision | Tubular adenoma with moderate dysplasia |
| Fletcher, 2004[20] | M (n = 1) | 74 | Painless jaundice and pruritus | Whipple | Papilloma |
| Katsinelos, 2006[21] | M (n = 1) | 58 | Painful jaundice | Whipple | Villous adenoma with atypia |
| Xu, 2008[22] | F (n = 1) | 27 | Painless jaundice and pruritus | Whipple | Villous adenoma with mild dyplasia |
| Present case | M (n = 1) | 55 | Painless jaundice and pruritus | PPPD | Tubulovillous adenoma with carcinoma in situ |
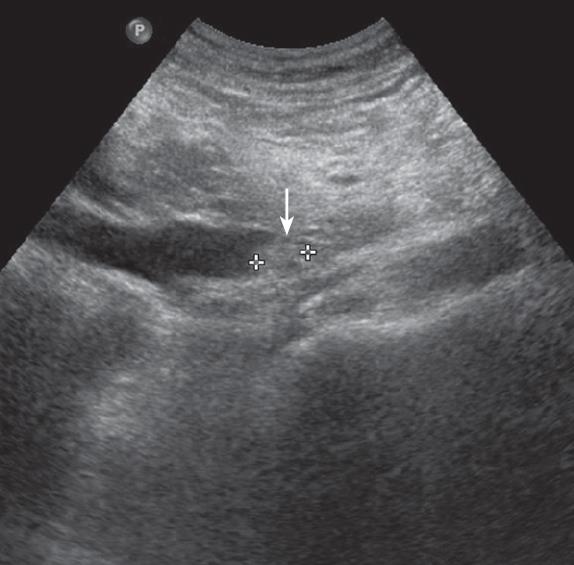
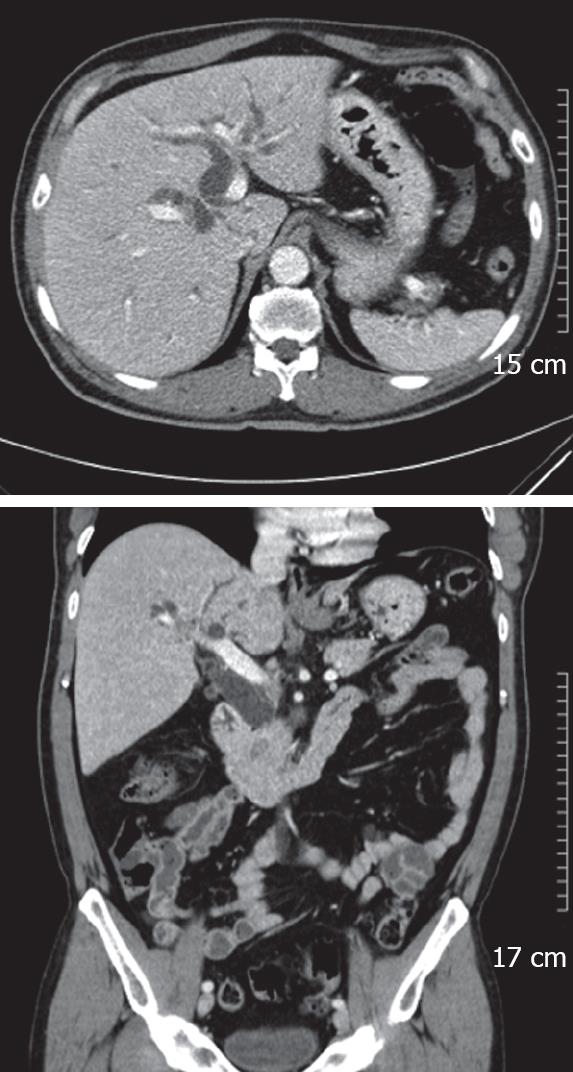
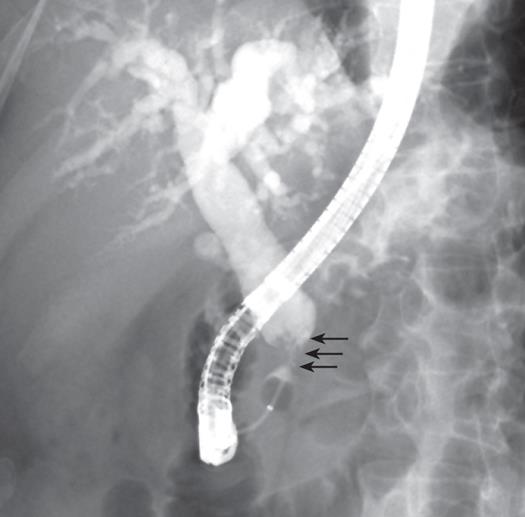
A 55-year-old man was admitted to our hospital with a 10-d history of painless obstructive jaundice and pruritus. He had diabetes mellitus which had been controlled by an oral hypoglycemic agent. He had no family history of colorectal cancer or polyposis. On physical examination, he had icteric sclerae. Abdominal examination revealed no palpable mass or tenderness. Laboratory tests showed elevations of total bilirubin (8.2 mg/dL), direct bilirubin (6.3 mg/dL), alkaline phosphatase (302 IU/L), alanine transaminase (27 IU/L) and gamma glutamyl transpeptidase (321 IU/L). The serum amylase was within the normal range. The carbohydrate antigen 19-9 level was 131.6 U/mL (normal range, ≤ 27 U/mL) and the carcinoembryonic antigen level was 1.5 ng/mL (normal range, 5 ng/mL). The hepatitis serologic markers were all negative. On abdominal ultrasonography, the CBD was dilated with a distal non-shadowing polypoid mass, however, there was no pancreatic duct dilatation (Figure 1). The CT findings were similar to the ultrasonographic findings and therefore a distal CBD tumor was suspected (Figure 2). Endoscopic retrograde cholangiopancreatography showed a 2cm polypoid mass and stricture in the distal CBD (Figure 3). Bile cytology revealed no malignancy. Positron emission tomography (PET) showed no hypermetabolic lesions. Based on the preoperative diagnosis of a distal tumor, a PPPD was performed.
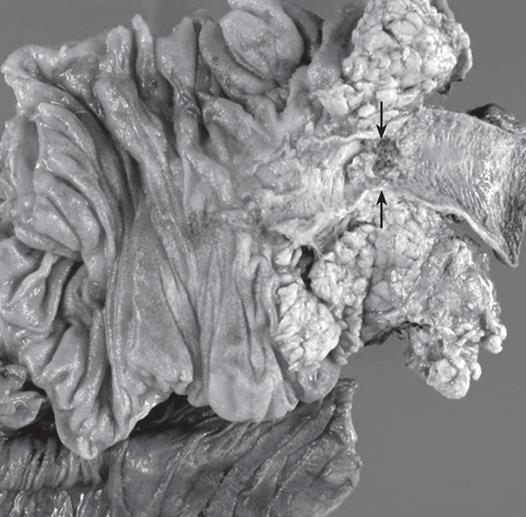
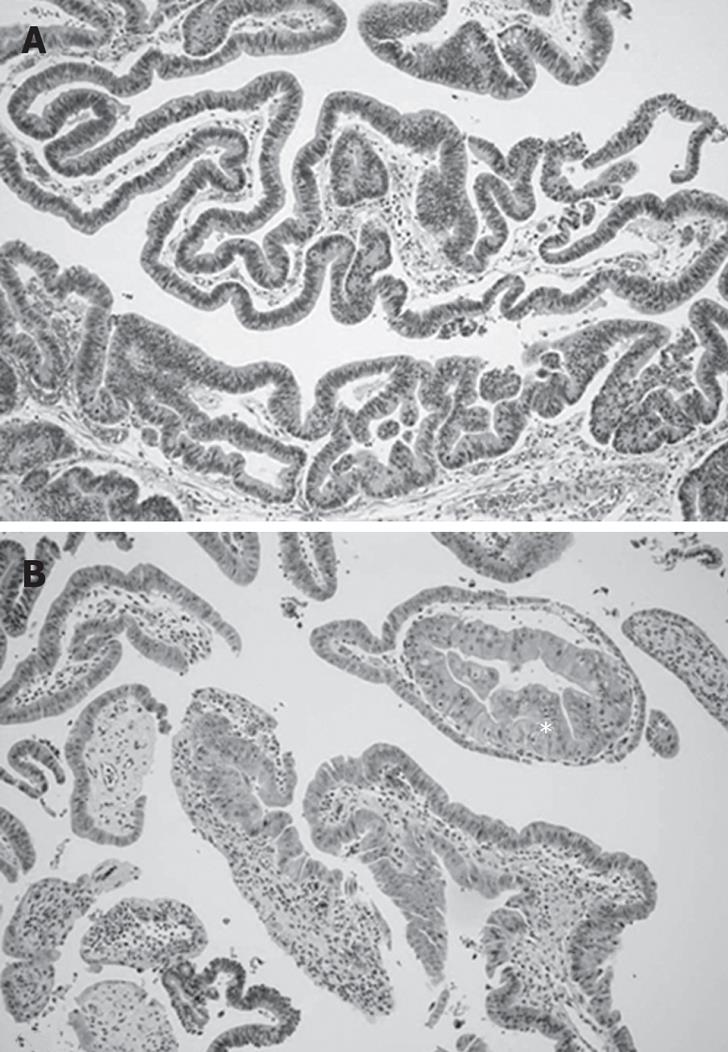
The resected specimen revealed a 2 cm × 1.5 cm polypoid mass in the distal CBD (Figure 4). The final pathology showed a tubulovillous adenoma with carcinoma in situ in the distal CBD (Figure 5). There was no lymph node metastasis. The patient recovered uneventfully. Eight months later, he developed multiple polyps (two in rectum, three in the colon and two in the stomach). An endoscopic mucosal resection was performed, which revealed a tubular adenoma with high grade dysplasia.
Tubulovillous adenomas are usually encountered in the gastrointestinal tract, but as a primary site, the CBD is rare. Adenomas arising from the CBD are summarized in Table 1. Saxe et al[5] was first to report a case of a villous adenoma in the CBD. The clinical manifestations of a biliary adenoma include jaundice, right upper quadrant abdominal pain, dyspepsia, nausea and vomiting in a fashion similar to ampullary tumors. Villous adenomas are benign tumors, but are considered to be premalignant. It is possible that the adenoma-to-carcinoma sequence occurs in biliary tumors[23–26]. Considering the similarity of the histologic and biologic characteristics of adenomas in the other segments of the GI tract, such an adenoma-to-carcinoma carcinogenic process involving the rectum, ampulla, gallbladder, and biliary duct occurs within the biliary tract[2728]. Therefore, complete resection of the lesion makes it possible to avoid development of carcinoma[29]. It is difficult to differentiate biliary adenomas from other malignant lesions with radiologic imaging[23]. Predicting the presence of malignant foci preoperatively is difficult. However, suspicion of malignancy could be made by an experienced biliary endoscopist.
Appropriate management of these lesions in the distal CBD has not been clearly defined. In 1992, Sturgis et al[7] first reported that high-risk patients with tubulovillous adenomas of the CBD were best treated by endoscopic resection but the risk of recurrence is high. Other treatment options, such as local resection, are performed in high-risk patients thought preoperatively to have benign tumors[10]. Ariche et al[16] proposed that resection with free margins of the CBD with lymph node dissection of the hepatoduodenal ligament for tumors in the mid part of the CBD is an appropriate treatment option. If the remaining duct length is inadequate, local resection is impossible and PD should be considered mandatory in cases involving cancer of the distal CBD. If malignancy is suspected or the size is larger than approximately 2 cm, radical resection is needed. We considered the other treatment option, duodenum preserving pancreatic head resection(DPPHR) which was first introduced by Beger et al[27] for chronic pancreatitis and has been increasingly used in neoplastic lesions, cystadenoma, borderline lesions, and carcinoma in situ[28–30]. However, we suspected that this tumor would be malignant and performed PPPD considering the size, site and clinical findings. Compared to Whipple-type resection, duodenum preserving pancreatic head resection has benefits in regard to postoperative morbidity and mortality, maintenance of glucose metabolism, absence of delay of gastric emptying, shorter hospital stay offers better quality of life of patients. However, duodenum preserving pancreatic resection has two major problems, incomplete lymph node dissection and ischemia of duodenum and has not been used yet as a surgical option of adenoma of common bile duct. Maeda et al[31] reported that duodenum preserving pancreatic resection in the treatment of pancreatic metastasis from renal cell carcinoma should be considered as radical lymph node dissection is not necessary. And then, if the nature and extent of common bile duct adenoma is suggestive of benign tumor and lymph node enlargement is absent, preoperatively, DPPHR should be considered in the treatment of common bile duct adenoma.
In this case, colonoscopy has not been performed and gastroscopy revealed no tumors in the stomach and duodenum preoperatively. At the time of follow-up 8 mo postoperatively, colonoscopy and gastroscopy revealed multiple polyps in the rectum, sigmoid colon, and stomach. They were removed by endoscopic mucosal resection and confirmed as tubular adenomas with high grade dysplasia. In view of the risk of recurrence of adenomas, careful follow-up is in order.
Järvinen et al[32] have reported biliary involvement in familial adenomatous coli patients. The present report is the first case of a tubulovillous adenoma with carcinoma in situ of the distal CBD and several tubular adenomas with high grade dysplasia of the GI tract confirmed 8 mo apart. We think that although adenoma of the biliary tract and GI tract did not exist concurrently, tubulovillous adenoma of the distal CBD may have developed by a similar mechanism to that of the GI tract. In conclusion, this case suggests that adenomas arising from the distal CBD can transform into carcinoma and support the existence of an adenoma-to-carcinoma sequence given that carcinoma in situ with an adenomatous lesion of the distal CBD and tubular adenoma of the GI tract adenoma ultimately developed.
| 1. | Spigelman AD, Farmer KC, James M, Richman PI, Phillips RK. Tumours of the liver, bile ducts, pancreas and duodenum in a single patient with familial adenomatous polyposis. Br J Surg. 1991;78:979-980. |
| 2. | Erwald R. Gardner’s syndrome with adenoma of the common bile duct. A case report. Acta Chir Scand Suppl. 1984;520:63-68. |
| 3. | Hulten J, Johansson H, Olding L. Adenomas of the gallbladder and extrahepatic bile ducts. Acta Chir Scand. 1970;136:203-207. |
| 4. | Styne P, Warren GH, Kumpe DA, Halgrimson C, Kern F Jr. Obstructive cholangitis secondary to mucus secreted by a solitary papillary bile duct tumor. Gastroenterology. 1986;90:748-753. |
| 5. | Saxe J, Lucas C, Ledgerwood AM, Sugawa C. Villous adenoma of the common bile duct. Arch Surg. 1988;123:96. |
| 6. | Harshfield DL, Teplick SK, Stanton M, Tunuguntla K, Diner WC, Read RC. Obstructing villous adenoma and papillary adenomatosis of the bile ducts. AJR Am J Roentgenol. 1990;154:1217-1218. |
| 7. | Sturgis TM, Fromkes JJ, Marsh W Jr. Adenoma of the common bile duct: endoscopic diagnosis and resection. Gastrointest Endosc. 1992;38:504-506. |
| 8. | Hanafy M, McDonald P. Villous adenoma of the common bile duct. J R Soc Med. 1993;86:603-604. |
| 9. | Buckley JG, Salimi Z. Villous adenoma of the common bile duct. Abdom Imaging. 1993;18:245-246. |
| 10. | Blot E, Heron F, Cardot F, Kerleau JM, Metayer J, Michot F, Bourreille J. Villous adenoma of the common bile duct. J Clin Gastroenterol. 1996;22:77-79. |
| 11. | Kawakatsu M, Vilgrain V, Zins M, Vullierme M, Belghiti J, Menu Y. Radiologic features of papillary adenoma and papillomatosis of the biliary tract. Abdom Imaging. 1997;22:87-90. |
| 12. | Chae BW, Chung JP, Park YN, Yoon DS, Yu JS, Lee SJ, Lee KS, Chung JB, Lee SI, Moon YM. Villous adenoma of the bile ducts: a case report and a review of the reported cases in Korea. Yonsei Med J. 1999;40:84-89. |
| 13. | Inagaki M, Ishizaki A, Kino S, Onodera K, Matsumoto K, Yokoyama K, Makino I, Ojima H, Tokusashi Y, Miyokawa N. Papillary adenoma of the distal common bile duct. J Gastroenterol. 1999;34:535-539. |
| 14. | Chang YT, Wang HP, Sun CT, Chang MC, Chang YC, Wu MS, Lin JT. Papillary adenoma of the bile duct. Gastrointest Endosc. 2001;53:777. |
| 15. | Oshikiri T, Kashimura N, Katanuma A, Maguchi H, Shinohara T, Shimizu M, Kondo S, Katoh H. Mucin-secreting bile duct adenoma--clinicopathological resemblance to intraductal papillary mucinous tumor of the pancreas. Dig Surg. 2002;19:324-327. |
| 16. | Ariche A, Shelef I, Hilzenrat N, Dreznik Z. Villous adenoma of the common bile duct transforming into a cholangiocarcinoma. Isr Med Assoc J. 2002;4:1149-1150. |
| 17. | Aggarwal S, Kumar S, Kumar A, Bhasin R, Garg PK, Bandhu S. Extra-hepatic bile duct adenoma in a patient with a choledochal cyst. J Gastroenterol Hepatol. 2003;18:351-352. |
| 18. | Jao YT, Tseng LJ, Wu CJ, Young TM, Mo LR, Wang CH, Wey KC. Villous adenoma of common bile duct. Gastrointest Endosc. 2003;57:561-562. |
| 19. | Lou HY, Chang CC, Chen SH, Fang CL, Shih YH, Liu JD, Pan S. Acute cholangitis secondary to a common bile duct adenoma. Hepatogastroenterology. 2003;50:949-951. |
| 20. | Fletcher ND, Wise PE, Sharp KW. Common bile duct papillary adenoma causing obstructive jaundice: case report and review of the literature. Am Surg. 2004;70:448-452. |
| 21. | Katsinelos P, Basdanis G, Chatzimavroudis G, Karagianno-ulou G, Katsinelos T, Paroutoglou G, Papaziogas B, Paraskevas G. Pancreatitis complicating mucin-hyperse-creting common bile duct adenoma. World J Gastroenterol. 2006;12:4927-4929. |
| 22. | Xu HX, Chen LD. Villous adenoma of extrahepatic bile duct: contrast-enhanced sonography findings. J Clin Ultrasound. 2008;36:39-41. |
| 23. | Buckley JG, Salimi Z. Villous adenoma of the common bile duct. Abdom Imaging. 1993;18:245-246. |
| 24. | Hasebe T, Sakamoto M, Mukai K, Kawano N, Konishi M, Ryu M, Fukamachi S, Hirohashi S. Cholangiocarcinoma arising in bile duct adenoma with focal area of bile duct hamartoma. Virchows Arch. 1995;426:209-213. |
| 25. | Serafini FM, Carey LC. Adenoma of the ampulla of Vater: a genetic condition? HPB Surg. 1999;11:191-193. |
| 26. | Sato T, Konishi K, Kimura H, Maeda K, Yabushita K, Tsuji M, Miwa A. Adenoma and tiny carcinoma in adenoma of the papilla of Vater--p53 and PCNA. Hepatogastroenterology. 1999;46:1959-1962. |
| 27. | Beger HG, Krautzberger W, Bittner R, Buchler M, Limmer J. Duodenum-preserving resection of the head of the pancreas in patients with severe chronic pancreatitis. Surgery. 1985;97:467-473. |
| 28. | Tanaka M, Chari S, Adsay V, Fernandez-del Castillo C, Falconi M, Shimizu M, Yamaguchi K, Yamao K, Matsuno S. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17-32. |
| 29. | Beger HG, Gansauge F, Siech M, Schwarz M, Poch B. Duodenum-preserving total pancreatic head resection for cystic neoplastic lesions in the head of the pancreas. J Hepatobiliary Pancreat Surg. 2008;15:149-156. |
| 30. | Ahn YJ, Kim SW, Park YC, Jang JY, Yoon YS, Park YH. Duodenal-preserving resection of the head of the pancreas and pancreatic head resection with second-portion duodenectomy for benign lesions, low-grade malignancies, and early carcinoma involving the periampullary region. Arch Surg. 2003;138:162-168; discussion 168. |
| 31. | Maeda H, Okabayashi T, Nishimori I, Kobayashi M, Sugimoto T, Kohsaki T, Onishi S, Hanazaki K. Duodenum-preserving pancreatic head resection for pancreatic metastasis from renal cell carcinoma: a case report. Langenbecks Arch Surg. 2007;392:649-652. |
| 32. | Jarvinen HJ, Nyberg M, Peltokallio P. Biliary involvement in familial adenomatosis coli. Dis Colon Rectum. 1983;26:525-528. |