INTRODUCTION
Despite a decrease in the occurrence of infective diseases, autoimmune and chronic inflammatory disorders are on the increase in developed countries. Many of these illnesses remain poorly understood due to the intricate network of interactions among genetic, cellular and environmental factors underlying pathogenesis.
Various theories have been developed as to the etiopathogenesis of inflammatory bowel disease (IBD), but so far none of them has led to a therapy with long-term efficacy and free of side effects. The advancement of our knowledge of the biological basis of pathogenesis, combined with recent findings on the regenerative, trophic and immunoregulatory potential of stem cells, have triggered research that could lead to a significant evolution, or revolution, in the treatment of IBD.
Mesenchymal and hematopoietic stem cells (MSCs and HSCs) are catalyzing the attention of IBD investigators, physicians and clinicians. After a number of case reports, and following initial steps within in vitro and in vivo models, cell-based approaches are now moving from the laboratory bench to the patient’s bed. Stem cell transplantation may soon become a therapeutic option for IBD.
Crohn’s disease (CD) and ulcerative colitis (UC), the two main forms of inflammatory bowel diseases, are chronic, relapsing and remitting diseases profoundly affecting the quality of life in an enlarging portion of the population: their incidence and prevalence are growing in Western countries[1]. CD may affect every tract of the digestive system-most commonly the ileal and colonic tracts, and exhibits a histological pattern of transmural inflammation. UC only affects the colon starting from the rectum and moving backward, the inflammation being localized in the mucosal layer. In both CD and UC an alteration of the mucosa leads to the symptoms of the disease. CD can be complicated by the occurrence of fistulas, abscesses and stenosis.
Genetic, environmental and immunological factors contribute to the development of inflammatory bowel disease. The “hygiene hypothesis” proposes a scenario in which a radical change of lifestyle may have led to the shift from infective to chronic inflammatory diseases in Western countries. An immune system exposed to a low number of antigens and struggling against only a few challengers, may be lapsing into an uneducated state. Thus its efforts at eliminating offending agents could then be ineffective or misdirected. The vary environmental modifications that brought humans to this hygienic lifestyle may have contributed to the rise of immune-based disorders.
IBD is thought to be the result of an aberrant immune response to commensal bacteria and luminal antigens in a susceptible host. The “no bacteria, no colitis” paradigm summarizes the evidence obtained from animal models: experimental colitis can only be induced in a conventional, non-germ-free environment. The evidence supporting the existence of a classic infectious agent causing IBD is weak. The enteric flora may trigger an inappropriate, “loss of tolerance”-based immune response, further evolving to chronic inflammation and IBD.
The mucosal tolerogenic state is maintained by dynamic, strictly regulated, physical and immunochemical interactions in a complex crosstalk among the gut microbiome, gut luminal antigens, the intestinal epithelial barrier, lymphocytes, dendritic and mesenchymal cell populations[2]. The circuitry is coordinated so as to obtain a minimal persistent inflammatory state (physiological inflammation) able to control the enteric flora and to cope with non-self antigens. In normal conditions the innate and adaptive immune systems cooperate in order to create this controlled status of inflammation: in CD and UC the balance is lost.
IMMUNE-BASED DISORDERS
Analysis of genetics and immune responses in IBD patients and animal models sheds light on the biological function of the metabolic pathways and cell populations with a central role in the immune tolerance network. Most of the genetic factors that are so far known to contribute to susceptibility towards IBD act as key links in immune recognition and modulation. The existence of a genetic predisposition for IBD has been demonstrated. Genome-wide screening in familial clusters has identified 7 loci with a linkage to IBD (IBD 1-7)[3]. Most linkage regions are associated with both CD and UC: this suggests the existence of common genetic features and mechanisms. The genes residing in the IBD loci (e.g. NOD2/CARD15, TNF-alpha) are implicated in immune function and intestinal permeability[4–7]. Initially, connections between innate immune impairments and IBD emerged. The association between CD and NOD2/CARD15 mutations showed that innate pattern recognition impairments and bacterial sensing may undermine the host/microbe interplay at a critical point. The NOD2/CARD15 protein recognizes peptidoglycans (bacterial muramyl dipeptide, MDP) and is expressed by macrophages and dendritic cells. The mutated gene product fails to bind MDP. The production of alfa-defensins by epithelial Paneth cells, expressing NOD2, is decreased in CD patients when NOD2 is mutated[8]. Innate and adaptive immunity are intimately interconnected, impaired mechanisms may simultaneously affect the two vast pathways of immune response.
Cell populations and mediators of adaptive immunity have been extensively investigated in IBD patients and murine models[9]. A dramatic increase in antibody production has been described both at the mucosal and at the systemic level in IBD patients. An alteration in the relative proportions of the immunoglobulin classes has been found[10]. Autoantibody production has been investigated, but confirmation of their existence is still needed[11].
T lymphocytes are recognized as central effector cells. Their activation in IBD is accompanied multiple alterations in the production of cytokines and soluble mediators with proinflammatory and immunoregulatory significance. Consensus exists on the differing CD4 helper polarization tendency between the two major forms of IBD. In CD the IFN-gamma and IL-2 producing Th1 CD4+ phenotype is predominant, while UC seems to be dominated by atypical Th2 CD4+ T-cells, producing TGF beta and IL-5, and by IL-13 producing CD1d-restricted nonclassical Natural Killer T cells[12]. CD resembles experimental Th1-mediated colitis, whereas the features of UC are recapitulated in models of Th2-mediated colitis[1314]. Observations in patients and murine models suggest that the suppressor activity of CD4+ CD25high T regulatory cells may be abrogated or insufficient to balance the chronic inflammation at the mucosal level[15]. Murine models confirm the ability of CD4+ CD25high T regulatory cells to prevent or ameliorate experimental colitis[16], indicating the positive effect of anergy induction in the inflammatory environment. The suppressor activity of immunoregulatory populations, mediated by cytokines like IL-10 and TGF-beta, may fail because of a disruption of the signaling pathway in activated T cells: Smad7 overexpression antagonizes the TGF-beta dependent inhibition of T proliferation[17].
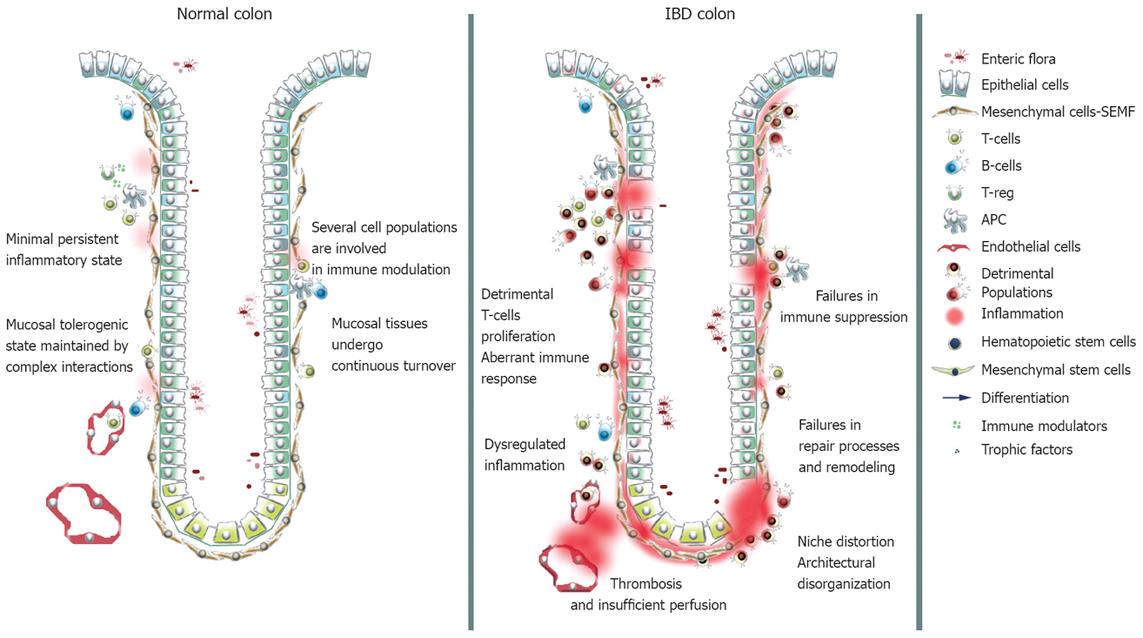
The involvement of cell populations other than the classical immunological ones in immune modulation and IBD pathogenesis is becoming clear. Epithelial, endothelial, mesenchymal cells and platelets cooperate in modulating the immune response, and play a role in the establishment and protraction of inflammatory processes, in recovery and in tissue remodeling (Figure 1). Intestinal epithelial cells (IECs) have been claimed to act as non professional antigen-presenting cells. In the inflamed mucosa, IECs inappropriately express HLA-DR class II and costimulators of the B7 family[18]. In healthy subjects antigen presentation by IECs results in activation of a CD8+ regulatory T cell subset through a non classical MHC classIpathway. IECs interact with the T compartment through members of the CEA family and CD1d, and in normal conditions expand a CD8+ VB5.1+ subset of T cells with regulatory functions. In IBD IECs a defective expression of CEA family members and CD1d occurs, leading to a failure in expansion of the regulatory subset and to a proliferation of CD4+ T cells with the production of inflammatory cytokines[1920]. Moreover, UC IECs fail to express MHC I molecules and CD IECs express HLA-E, MICA and MICB, but not CD1d, failing to expand the regulatory T cell subset[21].
Figure 1 Schematic representation of normal and IBD mucosal tissues.
In normal conditions the innate and the adaptive immune systems cooperate in order to create a controlled status of inflammation: In IBD the balance is lost.
Endothelial cells control the egression of migrating leukocytes in a complex process mediated by cytokines, chemokines and adhesion molecules. A deficit in nitric oxide synthase (NOS) production has been found in IBD intestinal microvascular endothelial cells. Impairment of NO-dependent vasodilation may result in decreased perfusion and poor wound healing[22]. Thrombosis is a frequent complication in IBD patients and platelets are implicated, but interest in these entities has risen following the definition of their immunological properties[23]. Platelets become activated in IBD patients and contribute to exacerbating inflammation through the release of CD40, which triggers a broad spectrum response in the intestinal microvasculature, stroma, and immune cells[24].
The role of mesenchymal cells in mucosal homeostasis and microenvironmental modeling is far more critical than previously thought. Traditionally considered as extracellular matrix (ECM) producers, fibroblasts were thought to be a passive cell population responsive to the chronic inflammatory environment and causing fibrotic complication[25]. Fibroblasts are the main source of matrix metalloproteinases (MMPs); proteolytic enzymes responsible for ECM degradation and ultimately for tissue destruction during inflammation. Interactions with activated T-cells potently stimulate fibroblast production of MMPs and result in tissue injury[2627].
MESENCHYMAL CELLS AND RESTITUTIO AD INTEGRUM
Epithelial ulcerations caused by inflammatory process recover via tissue remodeling in the normal intestine. Resolution of inflammatory activity is associated with repair processes, and mesenchymal cells have a central role in coordinating these events to help remodeling. The repair processes in UC patients are usually able to restore a normal intestinal architecture, but in CD patients an excessive fibrosis frequently leads to the formation of strictures and obstructions. Fibrosis is one of the main complications and a recurrent finding in IBD patients: it is associated with mesenchymal cell persistence and hyperplasia, tissue disorganization and fibrillar collagen deposition.
Isolation and characterization of intestinal fibroblasts has allowed the determination of some peculiar biological features of IBD fibroblasts, for example they display significantly higher proliferation and collagen secretion rates than normal intestinal fibroblasts. Such evidence suggests that a fibroblast subset may be functionally activated in the IBD intestine[2829].
Recent findings suggest that mesenchymal cells derived from bone marrow stem cells may have an important role in repair processes and fibrosis[30]. Intestinal subepithelial myofibroblasts (ISEMFs) are located under the basement membrane, juxtaposed with the base of epithelial cells. These mesenchymal cells regulate a number of epithelial cell functions such as proliferation, differentiation and ECM metabolism, affecting the growth of the basement membrane[31]. They cooperate in pathogen sensing and actively participate in immune responses[32]. Moreover, they exert important functions in tolerance induction[33]. ISEMFs control mucosal repair processes by ECM manipulation, cytokine and growth factor release. Two separate mechanisms mediate the repair operations. Restitution is the first, a response to minor to moderate injury, when the basement membrane remains intact. Here, ISEMFs promote the proliferation and migration of residual epithelial cells over the denuded area by the release of TGF-beta, EGF, aFGF, bFGF and inflammatory cytokines. When the wound is deep and a reconstruction of the subepithelial tissues is needed, mesenchymal cells proliferate and form a new basement membrane, over which epithelial cells will then proliferate and migrate. ISEMFs are therefore responsible for a crucial process in mucosal repair: the maintenance and reconstitution of the basement membrane.
Alpha-SMA positive ISEMFs are increased in IBD mucosa as compared with normal mucosa, and the increase is very marked at the edges of UC ulcerations[34]. A higher ISEMF proliferation rate and growth factor secretion could account for CD fibrogenesis[28]. This cell population, actively participating in ECM metabolism and basement membrane turnover, is a major effector in the tissue remodeling process. Inflammatory cytokines and growth factors like TGF-beta, PDGF-BB, KGF, IGF-1 and EGF control MMP and their tissue inhibitor (TIMP) expression in ISEMFs, and it has been shown that the expression of MMPs and TIMPs is elevated in the inflamed mucosa of IBD patients[35]. ECM metabolism and inflammatory responses are regulated in close association: in colonic SEMFs TNF-alpha, IL-1 beta and IL-17 induce the expression of chemokines like monocyte chemoattractant protein-1 (MCP-1) and interleukin-8 (IL-8) together with MMPs[36]. IL-11, a chemokine with antiinflammatory properties, is able to inhibit the production of IL-1 beta, TNF-alpha and other proinflammatory cytokines from LPS-stimulated macrophages. Data from animal models show that in the gastrointestinal tract IL-11 can prevent or improve acute and chronic inflammation[37]. ISEMFs secrete IL-11 in response to inflammatory cytokines like IL-1 beta, TGF-beta and IL-22. Activated T cells are the main source of IL-22, and this cytokine induces secretion of IL-11 by ISEMF. Mesenchymal cells thus display suppressive immunomodulatory properties[38]. ISEMFs provide support for epithelial cell proliferation and regeneration, having a role in the restitutio ad integrum in IBD. Evidence is mounting as to their role in forming the niche that accommodates epithelial stem cells and in determining epithelial cell fate: pericryptal ISEMFs are the main source of morphogenic signaling[39]. Wnt signaling for example, has specific functions in the intestinal crypt stem cell region. Through its receptor, Fzd, expressed in both ISEMFs and crypt epithelium, Wnt triggers paracrine and autocrine responses. Nuclear localization of beta-catenin/T cell factor (TCF) is confined to epithelial cells at the bottom of the crypts, and upregulated by Wnt signaling. FoxF proteins are key mesenchymal factors that control Wnt expression and ECM deposition, affecting epithelial cell proliferation, polarization and differentiation[40].
MSCS AND HSCS: CONTRIBUTION TO INTESTINAL LINEAGES
Adult bone marrow HSCs possess well-known multipotent capacities and are able to restore the entire haemopoietic compartment after myeloablation. The hypothesis that certain adult stem cells might possess a different, greater potential first came from the observation that in human bone marrow transplants donor cells were subsequently found in several recipient tissues[41]. The attention of cell biologists moved to the bone marrow mesenchymal compartment and evidence regarding the stem potential of stromal cells soon appeared: the plastic-adhering fibroblast-like population displayed a self renewal capacity, high in vitro expansion potential and the ability to differentiate into multiple mesodermal lineages, e.g. osteoblasts, chondrocytes and adipocytes[4243]. These cells, named MSCs and initially isolated from the bone marrow[44], were found to reside in several other tissues. MSCs possess migratory capacity, may be diversely distributed in vivo and may occupy a ubiquitous stem cell niche[4546].
A remarkable amount of evidence exists regarding the multilineage differentiation potential of bone marrow stem cells. Marrow-derived cells have been shown to differentiate towards endodermal[4748], mesodermal[4950] and ectodermal commitments[51]. A unitarian theory on the derivation of the intestinal epithelium from resident stem cells has been developed and widely accepted[52], but a number of recent studies report a bone marrow contribution to gut epithelial and mesenchymal lineages[53–55], even at the single cell level[56]. A repopulation of the gastrointestinal tissues occurs in bone marrow-transplanted patients and donor-derived cells engraft with a high efficiency in the areas of mucosal ulceration undergoing regeneration[57]. Bone marrow sex-mismatched transplantations were performed in mice and a stable engraftment of alpha-SMA+, desmin-, F4/80-, CD34- pericryptal myofibroblasts of donor origin was documented through in situ hybridization for the Y chromosome. The analysis of intestinal biopsies from sex-mismatched BM-transplanted patients with GvHD gave similar results[30]. Inflammatory cytokines and signals may be strong stimuli for the recruitment of marrow stems in the areas of mucosal damage. The engraftment of BM-derived ISEMFs was investigated by using mouse models of chemo-induced acute colitis and IL-10-/- chronic colitis. A significant increase in the number of donor-derived ISEMFs was found in the inflamed areas when compared with the normal adjacent mucosa[58].
MSCS: REGENERATION AND IMMUNE MODULATION
MSCs have been shown to differentiate into a wide range of cell types and to produce a number of growth factors and cytokines that are important for tissue repair and remodeling. Intense studies on the paracrine activity of these cells suggest they are able to participate in tissue healing and long-term repair as trophic mediators[59]. Of fundamental interest for the treatment of chronic inflammatory diseases is the fact that MSCs possess the ability to modulate the immune response and to persist at length in the tissues of allogeneic transplanted recipients[60]. Cells with mesenchymal stem potential can be isolated from several tissues, with differences in yield and in differentiation capacity[466162]. The best characterized MSC population is the one found in the bone marrow where the in vitro expansion protocol is almost standardized[63] but due to the absence of characteristic markers it is still impossible to prospectively isolate MSCs from fresh samples. Differences among laboratories in expansion potential, differentiation capacity, gene expression and phenotype have been ascribed to slightly different in vitro culture and expansion conditions.
Several studies have demonstrated that MSCs possess valuable characteristics for tissue repair or regeneration: these cells have been shown to functionally integrate and remodel bone[6465], cartilage[66] and myocardial tissues[67–69]. MSCs display a further remarkable feature, the ability to migrate and home to the sites of injury[70–73]. Early clinical trials have shown the benefits of allogeneic MSC transplantation for the treatment of graft-versus-host disease (GVHD)[74]. A phase III clinical trial is currently enrolling patients to evaluate the efficacy of ProchymalTM, an allogeneic bone marrow derived MSC preparation, for the treatment of steroid refractory acute GVHD[75]. Human MSCs express intermediate-low levels of HLA classI, low levels of HLA class II and do not activate allogeneic T cells. This ability to escape alloreactive recognition is probably due to a lack in the expression of costimulatory molecules like B7-1, B7-2, CD 40 and CD 40 ligand[7677]. Moreover, MSCs suppress allogeneic T-cell proliferation and do not elicit an immune response after transplantation in immunocompetent recipients[697678]. Several authors have reported the suppression of lymphocyte proliferation in primary mixed leukocyte reactions (MLR) and mitogen responses to phytohemagglutinin (PHA), concanavalin and tuberculin[7980]. Again, MSCs inhibit the T-lymphocyte activation mediated by anti CD3 and CD28 antibodies at primary and in vitro expanded cultures[7681]. The molecular mechanisms that enable MSC to abrogate lymphoproliferation are still unknown but several reports claim the importance of soluble factors: modifications in the cytokine balance, such as an increase in IL-2 and IL-10 levels, are suggested to have lymphosuppressive ability[82–84].
IL-10 is a pleiotropic anti-inflammatory cytokine which potently suppresses antigen presentation by the down-regulation of classical HLA-classIand II, and which inhibits the synthesis of pro-inflammatory cytokines like IFN-gamma, IL-2, IL-3, TNFalpha and GM-CSF[85]. Furthermore, IL-10 induces differentiation of regulatory T cells[86] and the secretion of soluble HLA-G molecules by activated CD14+ peripheral blood monocytes[87]. MSCs have been shown to improve the clinical outcome of autoimmune disease, as a result of suppressive modulation of the pathogenic T-cell autoimmune response[88].
ADVANCES IN CELL THERAPY: HSC AND MSC TRANSPLANTATION
The long-term management of inflammatory bowel disease depends on the intensity, location, endoscopic severity, clinical manifestations and complications of the disease. Many cellular and molecular pathological pathways have been identified as therapy targets[89].
The common progression into an exacerbated form of IBD requires an escalation from antiinflammatory (e.g. 5-aminosalicylates, corticosteroids), to immunosuppressant (e.g. azathioprine, mercaptopurine, cyclosporin) regimens or to biological drugs (e.g. infliximab anti-TNFalfa, vizilizumab anti-CD3), usually with limited success. Non-responding IBD patients will frequently face the decision to undergo necessary invasive surgical procedures, although surgical intervention will not resolve the disease[89]. Since no curative options exist to date, a stem cell-based approach could drive a major change in disease management and treatment.
Impairment in the control of intestinal immune cell function and turnover appears to be of central importance for the dysregulated and protracted inflammatory response in IBD. A high-dose immune ablation regimen could allow detrimental T-lymphocyte repertoires to be eliminated and after HSC transplantation (HSCT) de-novo hematopoiesis would generate naive cells. Patients receiving an autologous HSCT are thought to be subject to an immune system reboot: the genetic defects would not be eliminated but remission could persist in the absence of deleterious environmental triggers. HSC allotransplanted patients probably experience a graft-versus-autoreactive (GVA) response and the immune system gets almost completely replaced. These concepts form the basis of a new therapeutic approach to IBD, and a number of case reports show long-lasting remission of IBD patients undergoing HSC or bone marrow transplantation.
In 1993 the first case of CD regression after autologous HSC transplantation for hematopoietic malignancy was reported[90]. More cases then started to be recorded: in 1996 one UC and two CD patients treated for coincidental malignancies experienced a remission from IBD after high dose chemotherapy and autologous HSCT[91]. In 1998 the case of a 9-year-old patient with CD was reported: after autologous BM transplantation for non-Hodgkin’s lymphoma, a clinical and laboratory CD remission occurred and lasted for at least 7 years[92]. In the same year the clinical outcome of six CD patients undergoing allogeneic transplantation for hematological malignancies was reported. In one patient the inactive CD remained inactive for at least 15 years without immunosuppression and in three patients with active CD the pathology became inactive[93]. In 2000 the case of a 30-year-old patient with a 10-year history of severe CD was reported: after developing Hodgkin’s disease he received an autologous peripheral blood HSCT and remained in remission for both diseases for at least 3 years after transplant[94]. In 2001 a case report described the remission of a 57-year-old UC patient after an autologous peripheral blood HSCT performed for breast cancer chemotherapy[95]. In 2002 a report showed the case of a CD patient, treated with autologous HSCT for acute myeloid leukemia, who remained in clinical remission from both diseases for 5 years[96]. More recently a complete normalization of the Chrohn’s Disease Activity Index (CDAI) was reported after HSC transplantation in two patients with severe, non responsive, infliximab-resistant CD[97]. Two further cases were reported by the same group[98]. A phase I HSCT trial involving 12 patients with refractory CD showed evidence of beneficial effects from autologous HSCT[99]. Peripheral blood stem cells were mobilized with cyclophosphamide plus G-CSF and CD34+ enriched. Eleven of the 12 patients underwent remission with a significant reduction in the CDAI index. A recent phaseI-II study investigated the safety and efficacy of autologous HSCT without CD34+ enrichment in patients with refractory CD: 3 out of 4 patients obtained and maintained clinical and endoscopic remission, despite withdrawal of all drugs[100]. All of these reports encourage further studies on HSC transplantation in IBD. A radical ablation of an aberrant immune system followed by autologous reconstitution may regenerate a naïve, non aberrant immune compartment. The European phase III “ASTIC” trial has been designed to investigate the potential clinical benefit of autologous HSCT after high-dose immune ablation in non-responding patients with severe CD.
Transplant conditioning calls for an aggressive immunosuppression regimen that may play a role in inducing remission, but patients with severely impaired mucosal barrier function undergoing such a regimen may worsen their plight and face even more severe consequences. Nevertheless, the management of CD and UC complications is a major goal of therapy. IBD patients develop non-healing, long-lasting ulcerations which are very resistant to common treatments and to advanced surgical cure. MSCs may allow a therapeutic approach targeting the site of injury, aimed at tissue regeneration and at local immune modulation.
Adipose tissue-derived MSCs[101] have been shown to possess promising potential for ulceration healing in perianal manifestations. A young patient with CD and recurrent rectovaginal fistula was treated with autologous stem cell transplanation using lipoaspirate-derived MSCs. At the time of the stem cell therapy the patient had been treated with different surgical and medical procedures, including infliximab infusion, without effective management of the manifestations. Autologous adipose tissue derived MSCs were isolated, expanded for three or fewer passages and injected into the rectal mucosa; the rectal opening approaching from the posterior vaginal wall was closed with absorbable stitches prior to cell injection. A complete closure of the wound was demonstrated one week after the injection without any adverse events[102].
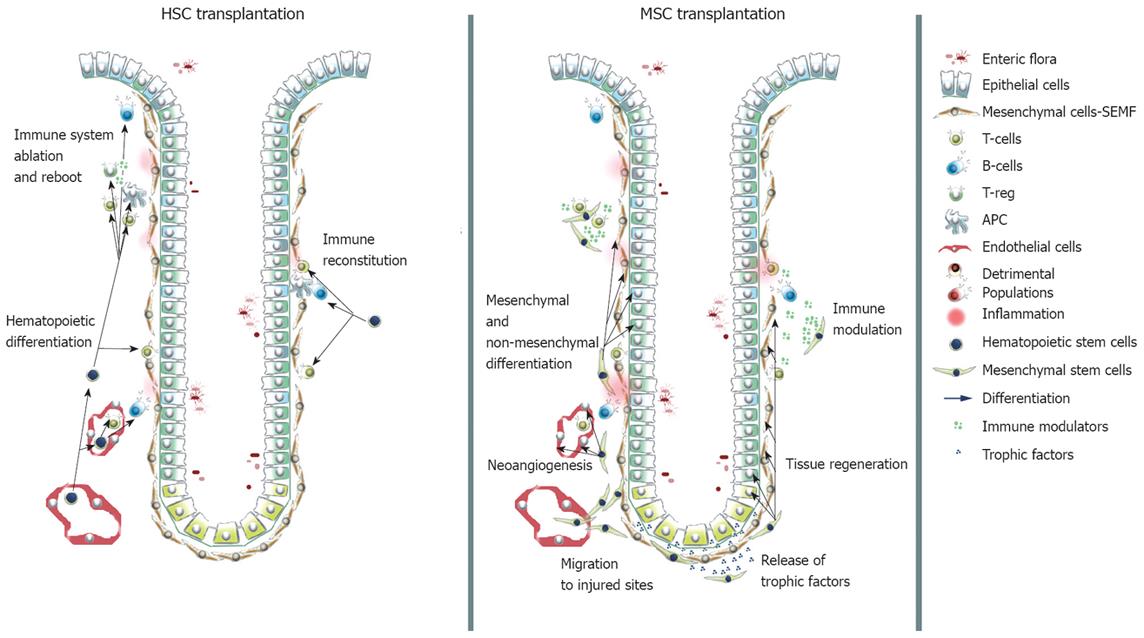
The group designed a prospective PhaseIclinical trial involving 5 CD patients to study the safety and the efficacy of stem cell transplantation in perianal manifestations and complications using autologous adipose tissue-derived stem cells. Three × 106 to 30 × 106 cells were injected directly under each lesion. Healing of the fistula was considered achieved when a total epithelialization of the external opening was evident. They observed complete healing in six of the eight procedures[103]. These reports describe an innovative approach to the local treatment of CD manifestations, based on in situ delivery of autologous MSCs. Whether MSCs contribute to tissue regeneration by direct differentiation, by trophic effect or by immunomodulatory functions has not been investigated yet and could become a matter of concern. ProchymalTM, developed by Osiris Therapeutics Inc.[75], is a preparation for intravenous infusion of bone marrow-derived MSCs obtained from healthy adult donors. The ProchymalTM phase II clinical trial was a prospective, randomized and open label trial. Patients with moderate to severe CD (CDAI > 220), who had previously failed treatment with steroids, infliximab and other immunosuppressive agents, were enrolled and received two infusions of the preparation. Every patient evaluated reported a reduction in CDAI, and a statistically significant decrease in mean CDAI scores, from 341 to 236, occurred by day 28 after the infusions. Improvement was rapidly obtained, with an average CDAI reduction of 62 points by day 7. One-third of the patients reported Inflammatory Bowel Disease Questionnaire (IBDQ) scores of at least 170, indicating they had achieved clinical remission of their disease. The Food and Drugs Administration recently allowed ProchymalTM to advance to a phase III double-blind placebo-controlled trial via Fast Track for the treatment of CD. After GvHD, CD is the second indication for which Osiris received Fast Track status to advance to Phase III. Osiris received orphan drug designation from the FDA and the European Medicine Agency (EMEA) for ProchymalTM. This intravenous preparation of MSCs may have peripheral immunomodulatory functions, leading to abrogation of the pathological inflammation typical of CD. MSCs may contribute to tissue regeneration by direct differentiation toward intestinal lineages[104], but they may also have trophic functions in the healing environment and their immunomodulatory ability may arrest disease protraction (Figure 2).
Figure 2 Contribution of transplanted HSCs and MSCs to the immune compartment and to intestinal lineages.
HSCs are able to restore the immune system to a naive state. MSCs contribute via direct differentiation to mesenchymal and non-mesenchymal lineages, exert trophic functions and modulate in a suppressive fashion the immune response.
CONCLUSION AND FUTURE PROSPECTS
IBD are thought to be the result of an abnormal immune response to commensal bacteria and luminal antigens in a susceptible host. The intermittent and aggressive presentation of the pathology profoundly affects the quality of life of the patients. The inadequacy of conventional therapies and the current understanding of IBD biology are motivating investigators to develop novel approaches to IBD treatment: the advancement in stem cell-based therapies could drive a major change.
Having to cope with exuberant commensal flora and abundant non-self antigens, a number of cell populations cooperate to control intestinal mucosa integrity and to maintain a status of persistent physiological inflammation. Impairments in immunological and regenerative functions at this level lead to IBD. The behavior of classical immune cells appears dysregulated in IBD patients. T-lymphocytes are considered central effector cells, responsible for the release of cytokines implicated in the onset and in the protraction of the inflammation. A lack of suppression is probably the cause of the abnormal and persistent activation of the T-cell compartment. Immunomodulatory activity, impaired in IBD, is carried out by populations of T regulators and by cell populations traditionally considered not involved in immunity. Aberrant epithelial and endothelial cell functions concur in the pathogenesis. Epithelial cells act as non-professional antigen presenting cells: defective expression of molecules implicated in the expansion of immunoregulatory T-cells may cause a failure in the immune inhibition. Deficits in cells of the microvasculature causing inadequate perfusion could account for ineffective ulceration healing. A set of intestinal subepithelial myofibroblasts (ISEMFs), a mesenchymal population juxtaposed to the mucosal epithelial layer exerting trophic and immunomodulatory effects, become activated in IBD and impair correct tissue remodeling processes, so that the restitutio ad integrum ultimately fails (Figure 1). Stem cells residing in the bone marrow have been shown to contribute with their progeny to all of these intestinal lineages and may be of value and interest in clinical settings. Clinical improvements in IBD patients have been reported after allogeneic and autologous transplantation of hematopoietic and MSCs. After immune ablation and reconstitution via HSC transplantation, a reboot to a naive immune system could bring about long-lasting remission. In autologous transplantation genetic defects would persist, but the indulgence may last in the absence of environmental triggers. In allogeneic transplantation the immune replacement would be more radical and potentially more reliable, but the risks associated with graft rejection and complications are probably too high for application in IBD. MSC only received the attention of IBD investigators in recent times. Experimental and clinical data indicate that MSCs have great potential for those clinical applications that require tissue regeneration or repair promotion, owing to their plasticity and their immunomodulatory properties. The biology of this stem population is still largely obscure, but their impressive differentiation potential, combined with exceptional trophic and immunomodulatory capacities, could make MSC an outstanding tool in IBD treatment (Figure 2). Pioneering works have reported impressive results: in mouse models and in IBD patients treated for coincidental malignancies, bone marrow-derived cells of donor origin contribute to tissue repair by differentiating towards the epithelial, myofibroblastic, endothelial and pericytic lineages in the areas of inflammation. Bone marrow transplantation from an unaffected donor is able to ameliorate pathology in a mouse model of chronic genetic-based colitis. Moreover, CD34- bone marrow- and peripheral blood-derived stem cells contributed to mucosal repair via neoangiogenesis in moderate-severe murine colitis and were effective in reducing the pathologic features associated with IBD[104]. Pivotal trials have demonstrated the efficacy of MSC transplantation in patients with CD, though the mechanisms of action underlying the clinical effects of MSCs still need clarifying and the follow-up periods need to be extended. Both HSC and MSC transplantation in IBD are currently being evaluated in Phase III clinical trials.
Ways of recognizing easily accessible and non-controversial new sources of pluripotent stem cells, such as term extraembryonal tissues[62105] as well as improving methods for ex vivo isolation, expansion and delivery may become of central interest. Moreover, MSCs displaying pluripotent, immunomodulatory and trophic potential, and suitable for implanting without manipulation in an allogeneic setting, would have obvious implications and may be promising “off the shelf” therapeutic approaches to IBD. In the future, the possibility of banking cord-blood derived HSC and placenta-derived MSCs[106], could enable these stem cells to be used both in autologous and in allogeneic transplantation settings.