Published online Jul 28, 2008. doi: 10.3748/wjg.14.4576
Revised: June 6, 2008
Accepted: June 13, 2008
Published online: July 28, 2008
The risk of thromboembolism is increased in inflammatory bowel disease and its symptoms may be overlooked. Furthermore, its treatment can be complex and is not without complications. We describe a case of an adolescent boy who developed a cerebral sinus venous thrombosis during a relapse of his ulcerative colitis and who, while on treatment with heparin, developed heparin-induced thrombocytopenia (HIT). The treatment was then switched to fondaparinux, a synthetic and selective inhibitor of activated factor X.
- Citation: Thorsteinsson GS, Magnussson M, Hallberg LM, Wahlgren NG, Lindgren F, Malmborg P, Casswall TH. Cerebral venous thrombosis and heparin-induced thrombocytopenia in an 18-year old male with severe ulcerative colitis. World J Gastroenterol 2008; 14(28): 4576-4579
- URL: https://www.wjgnet.com/1007-9327/full/v14/i28/4576.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.4576
Ulcerative colitis (UC) is a chronic inflammatory bowel disease (IBD) with unknown aetiology, which is localized in the colon. It affects both adults and children, and approximately 10% of the patients are diagnosed during childhood[1]. Drugs used to induce its remission consist mainly of 5-aminosalicylic acid (5-ASA) and steroids, and 5-ASA as its maintenance therapy[2] is often used. In more severe or steroid refractory cases, immune modulating therapy with azathioprine or 6-mercaptopurine, may be used, although the evidence for this is less convincing as compared to the treatment of Crohn’s disease (CD)[3]. Despite intensive pharmacological treatment, relapse is not uncommon. Extra intestinal manifestations are reported to occur in about 40% of adult patients with UC[4]. Figures for children are lower.
There are several risk factors for cerebral venous thrombosis (CVT), such as hormones (e.g. contraceptives and pregnancy), different kinds of hereditary thrombophilia and local factors including tumors[5]. In childhood, other risk factors such as local head/neck infections, sepsis and dehydration due to systemic illness have been described[6]. CVT is associated with a significant morbidity and mortality in children, and antithrombotic therapy with heparin in the acute phase followed by warfarin is recommended both in children and in adults[57]. However, CVT in children and adolescents with IBD is, only sparsely described. Hence, we describe a case of an adolescent boy who developed a cerebral sinus venous thrombosis during a relapse of his UC, and who, while on treatment with heparin, developed heparin-induced thrombocytopenia (HIT).
Written consent was received from the patient.
The patient was an 18-year old male who presented with extensive ulcerative colitis at the age of 12.5 years. He was initially treated with steroids and olsalazine. He went into remission within 3 mo. During the following years, it was complicated with several relapses, which were treated with repeated courses of prednisolone. Thus, two years after diagnosis, azathioprine (AZA) was started at a dosage of 1 mg/kg. His clinical condition was quite stable until the age of 17 years when an upper respiratory infection induced another relapse with worsening of the patient’s general condition and a pronounced weight loss. A high dose of prednisolone (i.e., 40 mg daily) was restarted and the AZA dose was further increased (approximate 2 mg/kg). One month after the introduction of steroids, the patient developed unilateral peritonsillitis and was admitted to hospital for incision and intravenous penicillin G treatment. The dose of prednisolone was by then tapered to 10 mg daily. The pharyngeal symptoms resolved and the patient was discharged on the third day. However, 5 d after discharge, he was readmitted due to a three-day history of severe headache, accompanied with nausea and vomiting. His colitis was clinically improved, apart from continuing daily episodes of loose stools.
The vital signs were normal. Neurological examination did not show any abnormalities or clinical signs of meningitis. During the first days after admission, the patient’s headache deteriorated. A CT-scan of the head was performed and interpreted as normal. Hence, a lumbar puncture performed surprisingly revealed an intralumbar pressure of 49 cmH2O (< 20). The spinal fluid analyses were otherwise normal. An eye fundoscopy revealed bilateral papilloedema and a diagnosis of pseudotumor cerebri induced by the steroid treatment was suspected. In order to reduce the cerebrospinal pressure, 10 mL of cerebrospinal liquid was withdrawn. After an asymptomatic period of about 14 h, the patient’s general appearance deteriorated with recurring severe headache.
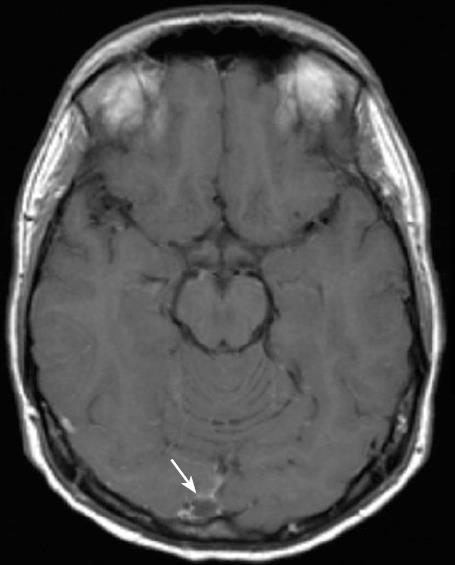
Repeated neurological examinations did not reveal any focal symptoms. A magnetic resonance imaging of the brain (MRI) showed a CVT in the right sinus transversum and confluence area (Figure 1). D-dimer was clearly elevated. Treatment with intravenous heparin infusion was initiated with a loading dose of 5000 IU, followed by a continuous heparin infusion adjusted according to aPTT (approximately 27 IU/kg per hour was required to maintain aPTT 2-3 times the baseline value). Despite an ongoing active colitis, anticoagulant therapy was considered safe.
In the following 7 d, the patient’s general condition improved, although his appetite was poor and the blood-stained diarrhoea continued. Mild iron deficiency anaemia was noted, but vitamin B12 and homocysteine levels were normal. Parenteral nutrition was given as a nutritional support.
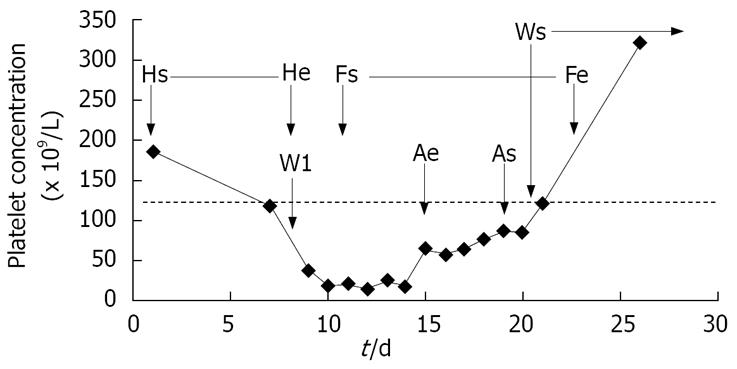
On day 7, warfarin was introduced under concomitant continuous heparin infusion. A rapid platelet count fall of 80% in 48 h was noted. Hence, azathioprine was temporarily stopped due to its known risk of thrombocytopenia. Heparin-induced thrombocytopenia (HIT) was suspected, warfarin was stopped and heparin infusion was switched to fondaparinux (Arixtra®, GlaxoSmithKline). The starting dose of fondaparinux was 7.5 mg, which was tapered to 5 mg after 3 d. The diagnosis was later confirmed by the detection of IgG-antibodies against heparin with consistent criteria for HIT[8]. The patient received a total of 6 units of platelet transfusion over a period of 4 d when the platelet count was < 18 × 109/L, due to the high expected risk of bleeding in this patient with colitis. Despite this, he had blood in his stools on several occasions during this period. The platelet count started to rise 7 d after heparin was stopped. When the platelet count reached > 80 × 109/L, azathioprine and warfarin were reintroduced and after 2 d of therapeutic INR between 2 and 3, fondaparinux was stopped after 9 d of treatment. The course of thrombocytopenia and medical intervention are presented in Figure 2.
During the course of CVT and HIT, a low dose of prednisolone and olsalazine was continued.
A follow-up CT scan 6 mo after the CVT diagnosis showed normal blood flow in all cerebral venous sini with no clinical neurological sequels.
A thorough work-up 7 mo after discharge showed that antithrombin, fibrinogen, lipoprotein (a), factor V gene mutation, prothrombin gene mutation, PAI-I, antiphospholipid antibodies, homocysteine, were all normal. Warfarin treatment was stopped after 8 mo of treatment. Thereafter, protein S, protein C, lupus anticoagulants, and FVIII were tested normal.
The colitis eventually went into clinical and biochemical remission. The patient was hesitant to undergo another colonoscopy until 14 mo later, when the biopsies showed a mild diffuse inflammatory activity and some architectural mucosal changes.
The increased risk of thromboembolism (TE) in patients with active IBD is well established[9–13] with a 6.5% incidence of thrombosis or a 3-4 fold higher risk than in the general population[14]. TE can also occur in the pediatric age group[15]. The potential mechanisms underlying the prothrombotic state in IBD are hypercoagulation (elevated FVIII, fibrinogen, decrease in antithrombin, protein S and protein C ), hypofibrinolysis [elevated PAI-1 and lipoprotein (a)], platelet abnormalities, endothelial dysfunction (increased von Willebrand factor) and immunological abnormalities (antiphosphlipid antibodies)[1416]. The common genetic risk factors for TE factor V mutation (Factor VLeiden) and prothrombin mutation (G20210A) are not overrepresented in patients with TE and IBD compared to other patients with TE[16].
It has been suggested that hyperhomocysteinemia is a risk factor for vascular disease and a mediator of TE in adults and occurs more commonly in both pediatric and adult IBD patients than in healthy controls[1718], which probably reflects the nutritional status with depleted levels of folate, cobalamin (B12) and pyridoxine (B6)[19]. The increased risk of TE in IBD associated with hyperhomocysteinemia is, however, questioned[19]. Other acquired risk factors for TE that can affect patients with IBD are steroid treatment, surgery, immobilisation, dehydration, central venous catheters[16] iron deficiency and infections[6].
In our patient, the risk factors for the development of CVT were relapse of the disease, treatment with high doses of steroids and development of peritonsillitis, surgical incision, dehydration, and iron deficiency anemia. Despite the risk factors, the diagnosis of CVT was delayed mainly due to this complication which is rather rare. It is important to raise the question about sinus thrombosis in the request form to the radiology department since not only unenhanced CT, but also contrast CT needs to be performed for most cases.
Cerebral venous thrombosis is a rare, but serious complication of IBD and seems to be more common in UC than in CD patients[13]. Its prognosis is variable with a high risk of residual symptoms in affected children and adolescents. Even death has been reported[13]. The most frequent symptom is headache, which occurs in 75%-96% of patients. The headache is often severe and diffuse, usually preceding the appearance of neurological deficits. A combination of focal deficits, headache, seizures and altered consciousness is very suggestive of CVT[5]. Apart from supportive care, anticoagulation therapy with heparin is the first line therapy for mild to moderate cases. Thrombolysis using recombinant tissue-type plasminogen activator (rt-PA) or urokinase has also been tried with various successes[20]. Due to the potential risks, expert guidelines recommend that local thrombolysis using urokinase[20] or (rt-PA)[5] should be restricted to comatose patients or patients who deteriorate despite anticoagulant therapy.
HIT is defined as an immune-mediated side effect of heparin therapy, which causes a drop in platelet count of ≥ 50%[21]. HIT usually occurs after 5-10 d of heparin treatment, but a faster drop may be seen if heparin has been given earlier. Apart from thrombocytopenia, venous or arterial thrombosis can occur, even before thrombocytopenia. Other symptoms observed in children with HIT are acute thoracic pain, respiratory distress, anaphylactic shock and prolonged fever[8]. An auto-immune response to platelet surface factor 4 (PF4) in complex with heparin is the major pathophysiological factor[22]. These complexes lead to cellular activation and development of thrombocytopenia or thrombosis[22]. In children, this is probably as common as in adults. In a recent review by Risch and co-workers, the incidence of HIT in children is estimated to be 0%-2.3%[21]. Approximately 90 pediatric HIT cases have been reported in the literature until now[21]. Children or neonates treated in intensive care units after cardiac surgery and adolescents treated with unfractionated heparin after thromboembolism constitute the highest risk of developing HIT in the pediatric population[21]. Although a rapid increase in platelet levels after cessation of heparin is observed, stopping heparin, as a sole treatment, is not recommended since unfavourable outcome has been reported in over 40% of patients due to the risk of thrombosis. Hence, alternative anticoagulant therapy is recommended. Danaparoid (heparinoid) inhibits mainly factor Xa, and to a lesser extent prothrombin, lepirudin (thrombin inhibitor), and argatroban (thrombin inhibitor) are the recommended drugs for the treatment of HIT. Treatment of HIT with danaparoid has a risk of cross reactivity. Leptirudin and argatroban are expensive, administered as continuous intravenous infusion, and require frequent aPTT testing. However, for our patient, fondaparinux was used as anti-coagulant therapy. Fondaparinux is a synthetic and selective inhibitor of activated factor X (Xa), administered as subcutaneous injection once daily. The antithrombotic effect is achieved by a selective binding of fondaparinux to antithrombin, which increases 300-fold the endogenic neutralisation by antithrombin on factor Xa. Hence, it inhibits the production of thrombin and the development of thrombosis[23]. Fondparinux does not appear to interact with HIT-related antibodies to induce platelet activity and aggregation according to in vitro tests and small clinical trails[24].
For our patient, all potential future surgical procedures and longer periods of immobilization should be accompanied with anti-embolic prophylactic treatment. However, due to his episode of HIT, heparin and low molecular weight heparin (LMWH) is contraindicated and alternative anticoagulant treatment must be used.
Patients with severe IBD are frequently treated with AZA, which may cause a problem during anticoagulant therapy for TE. Scarce reports are available on the AZA treatment which may interact with warfarin by diminishing its effect. Hence, the dose of warfarin may have to be increased 3-4 fold in order to achieve an optimal PK INR level[25].
Thromboembolic complications of IBD are not uncommon. If headache occurs during severe relapse of thromboembolism, CVT must be excluded. A CT scan with and without contrast is recommended as the first initial investigation. Platelet levels should be closely monitored during heparin treatment. When heparin is used, LMWH should be considered due to its lower risk of HIT than unfractionated heparin[26]. However, heparin has advantages in patients with a high risk of bleeding due to shorter T½. More studies concerning fondaparinux in treatment of patients with HIT are needed. It is necessary to evaluate the screening methods for identification of IBD patients with a high risk of thromboembolic complications. Furthermore, the efficacy and safety of prophylactic antithrombotic treatment of children with severe IBD must be evaluated in controlled clinical trials.
| 1. | Sawczenko A, Sandhu BK, Logan RF, Jenkins H, Taylor CJ, Mian S, Lynn R. Prospective survey of childhood inflammatory bowel disease in the British Isles. Lancet. 2001;357:1093-1094. |
| 2. | Sutherland L, Macdonald JK. Oral 5-aminosalicylic acid for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2006;CD000543. |
| 3. | Sands BE. Immunosuppressive drugs in ulcerative colitis: twisting facts to suit theories? Gut. 2006;55:437-441. |
| 4. | Ozdil S, Akyuz F, Pinarbasi B, Demir K, Karaca C, Boztas G, Kaymakoglu S, Mungan Z, Besisik F, Cakaloglu Y. Ulcerative colitis: analyses of 116 cases (do extraintestinal manifestations effect the time to catch remission?). Hepatogastroenterology. 2004;51:768-770. |
| 5. | Masuhr F, Mehraein S. Cerebral venous and sinus thrombosis: patients with a fatal outcome during intravenous dose-adjusted heparin treatment. Neurocrit Care. 2004;1:355-361. |
| 6. | Barnes C, Deveber G. Prothrombotic abnormalities in childhood ischaemic stroke. Thromb Res. 2006;118:67-74. |
| 8. | Risch L, Fischer JE, Herklotz R, Huber AR. Heparin-induced thrombocytopenia in paediatrics: clinical characteristics, therapy and outcomes. Intensive Care Med. 2004;30:1615-1624. |
| 9. | Derdeyn CP, Powers WJ. Isolated cortical venous thrombosis and ulcerative colitis. AJNR Am J Neuroradiol. 1998;19:488-490. |
| 10. | Hasegawa H, Yokomori H, Tsuji T, Hirose R. Hemorrhagic cerebral sinus thrombosis in a case of controlled ulcerative colitis. Intern Med. 2005;44:155. |
| 11. | Musio F, Older SA, Jenkins T, Gregorie EM. Case report: cerebral venous thrombosis as a manifestation of acute ulcerative colitis. Am J Med Sci. 1993;305:28-35. |
| 12. | Tsujikawa T, Urabe M, Bamba H, Andoh A, Sasaki M, Koyama S, Fujiyama Y, Bamba T. Haemorrhagic cerebral sinus thrombosis associated with ulcerative colitis: a case report of successful treatment by anticoagulant therapy. J Gastroenterol Hepatol. 2000;15:688-692. |
| 13. | Umit H, Asil T, Celik Y, Tezel A, Dokmeci G, Tuncbilek N, Utku U, Soylu AR. Cerebral sinus thrombosis in patients with inflammatory bowel disease: a case report. World J Gastroenterol. 2005;11:5404-5407. |
| 14. | Twig G, Zandman-Goddard G, Szyper-Kravitz M, Shoenfeld Y. Systemic thromboembolism in inflammatory bowel disease: mechanisms and clinical applications. Ann N Y Acad Sci. 2005;1051:166-173. |
| 15. | Kao A, Dlugos D, Hunter JV, Mamula P, Thorarensen O. Anticoagulation therapy in cerebral sinovenous thrombosis and ulcerative colitis in children. J Child Neurol. 2002;17:479-482. |
| 16. | Danese S, Papa A, Saibeni S, Repici A, Malesci A, Vecchi M. Inflammation and coagulation in inflammatory bowel disease: The clot thickens. Am J Gastroenterol. 2007;102:174-186. |
| 17. | Nakano E, Taylor CJ, Chada L, McGaw J, Powers HJ. Hyperhomocystinemia in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2003;37:586-590. |
| 18. | Romagnuolo J, Fedorak RN, Dias VC, Bamforth F, Teltscher M. Hyperhomocysteinemia and inflammatory bowel disease: prevalence and predictors in a cross-sectional study. Am J Gastroenterol. 2001;96:2143-2149. |
| 19. | Oldenburg B, Fijnheer R, van der Griend R, vanBerge-Henegouwen GP, Koningsberger JC. Homocysteine in inflammatory bowel disease: a risk factor for thromboembolic complications? Am J Gastroenterol. 2000;95:2825-2830. |
| 20. | Renowden S. Cerebral venous sinus thrombosis. Eur Radiol. 2004;14:215-226. |
| 21. | Risch L, Huber AR, Schmugge M. Diagnosis and treatment of heparin-induced thrombocytopenia in neonates and children. Thromb Res. 2006;118:123-135. |
| 22. | Poncz M, Rauova L, Cines DB. The role of surface PF4: glycosaminoglycan complexes in the pathogenesis of heparin-induced thrombocytopenia (HIT). Pathophysiol Haemost Thromb. 2006;35:46-49. |
| 23. | Kuo KH, Kovacs MJ. Fondaparinux: a potential new therapy for HIT. Hematology. 2005;10:271-275. |
| 24. | Savi P, Chong BH, Greinacher A, Gruel Y, Kelton JG, Warkentin TE, Eichler P, Meuleman D, Petitou M, Herault JP. Effect of fondaparinux on platelet activation in the presence of heparin-dependent antibodies: a blinded comparative multicenter study with unfractionated heparin. Blood. 2005;105:139-144. |
| 25. | Havrda DE, Rathbun S, Scheid D. A case report of warfarin resistance due to azathioprine and review of the literature. Pharmacotherapy. 2001;21:355-357. |
| 26. | Begelman SM, Hursting MJ, Aghababian RV, McCollum D. Heparin-induced thrombocytopenia from venous thromboembolism treatment. J Intern Med. 2005;258:563-572. |