Published online Jul 28, 2008. doi: 10.3748/wjg.14.4551
Revised: July 16, 2008
Accepted: July 23, 2008
Published online: July 28, 2008
AIM: To define the potential role of programmed death-1/programmed death-ligand (PD-1/PD-L) pathway in different hepatitis B virus (HBV) infection disease status; we examined the expression of PD-1 on antigen specific CD8+ T cells in peripheral blood of patients with chronic hepatitis B (CHB) and acute exacerbation of hepatitis B (AEHB) infection.
METHODS: The PD-1 level on CD8+ T lymphocytes and the number of HBV specific CD8+ T lymphocytes in patients and healthy controls (HCs) were analyzed by staining with pentameric peptide-human leukocyte antigen2 (HLA2) complexes combined with flow cytometry. Real-time quantitative polymerase chain reaction (PCR) was used to measure the serum HBV-DNA levels.
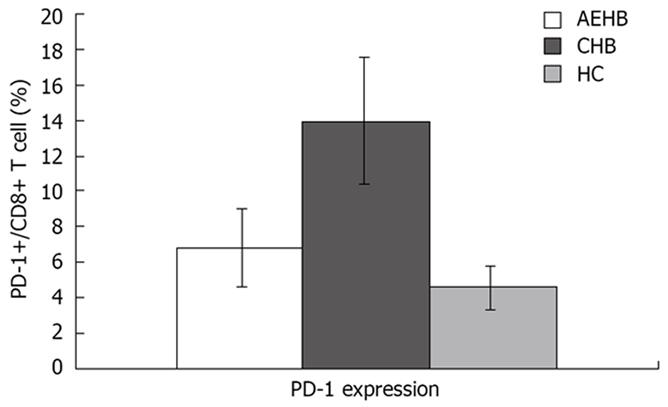
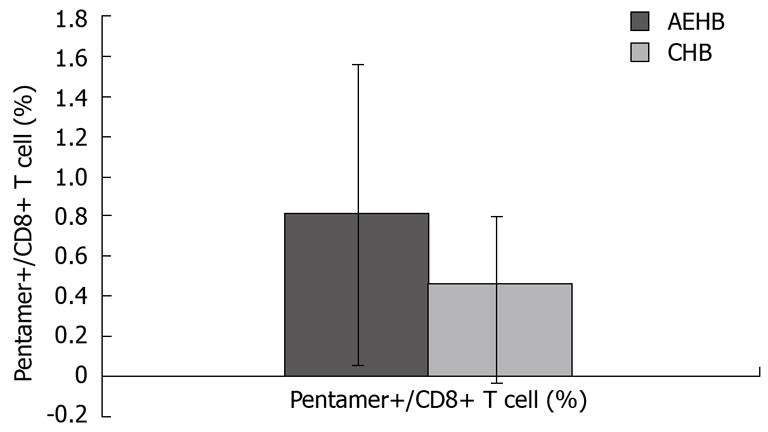
RESULTS: The level of PD-1 expression on total CD8+ T cells in CHB patients (13.86% ± 3.38%) was significantly higher than that in AEHB patients (6.80% ± 2.19%, P < 0.01) and healthy individuals (4.63% ± 1.23%, P < 0.01). Compared to AEHB patients (0.81% ± 0.73%), lower frequency of HBV-specific CD8+ T cells was detected in chronic hepatitis B patients (0.37% ± 0.43%, P < 0.05). There was an inverse correlation between the strength of HBV-specific CD8+ T-cell response and the level of PD-1 expression. Besides, there was a significant positive correlation between HBV viral load and the percentage of PD-1 expression on CD8+ T cells in CHB and AEHB subjects (R = 0.541, P < 0.01). However, PD-1 expression was not associated with disease flare-ups as indicated by alanine aminotransferase (ALT) levels (R = 0.066, P > 0.05).
CONCLUSION: Our results confirm previous reports that HBV specific CD8+ T-cell response in the peripheral blood is more intense in patients with AEHB than in chronic hepatitis B with persistent viral infection. Moreover, there is a negative correlation between the level of PD-1 and the intensity of virus specific CD8+ T cell response.
- Citation: Ye P, Weng ZH, Zhang SL, Zhang JA, Zhao L, Dong JH, Jie SH, Pang R, Wei RH. Programmed death-1 expression is associated with the disease status in hepatitis B virus infection. World J Gastroenterol 2008; 14(28): 4551-4557
- URL: https://www.wjgnet.com/1007-9327/full/v14/i28/4551.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.4551
Many individuals infected with hepatitis B virus (HBV) become chronic carriers and their liver disease may progress to chronic active hepatitis, cirrhosis, and hepatocellular carcinoma[1]. There is substantial evidence to suggest that adaptive immunity has a central role in determining whether HBV infection is followed by recovery or viral persistence[2]. In particular, the cellular immune response plays a critical role in the ability of HBV-infected individuals to control viral replication[3]. Patients who develop chronic HBV infection have progressive low frequency and functional impairment of T helper cells (Th cells), both in the peripheral blood and the liver[4]. The cytotoxic T lymphocyte (CTL) response is also impaired in chronic HBV infection with high viral load[5]. However, the molecular mechanisms underlying the exhaustion of memory T-cell subsets have not yet been elucidated. Understanding the immunopathogenesis of HBV infection is crucial for the development of effective strategies to control HBV.
Our present knowledge suggests that the interaction between positive and negative costimulatory molecules expressed on T cells and antigen presenting cells is essential for the development of T cell responses[67]. Among the many costimulatory molecules, programmed death-1 (PD-1) and its ligands programmed death 1-ligand 1 (PD-L1) and PD-L2 constitute important pathways that regulate and fine-tune immune responses[8–10]. Recent evidence indicates that the exhausted virus-specific CD8+ and CD4+ T cells in chronic viral infection hyperexpress PD-1 molecule[11–14]. However, the infected cells that remain productive resist early apoptosis by down regulation of PD-1[15]. PD-L1 expression on monocytes and dendritic cells is also increased in HIV infected individuals[16]. These findings indicates that the PD-1/PD-L1 pathway plays a crucial role in inhibiting the function of virus-specific CD8+ T cells in chronic viral infections, in mice and humans[17] [Human immunodeficiency virus (HIV)[18–20], hepatitis C virus (HCV)[2122] and HBV[23]]. These studies illustrated that the level of PD-1 expression correlates with the degree of HBV-specific T-cell impairment. Thus, induction of apoptosis may be a major mechanism employed by the PD-1/PD-L costimulatory pathway to affect the outcome of virus-specific T cell response. Such negative regulation of CD8+ T cell function by PD-1/PD-L system has also been observed in HBV infection[16].
It should be noted that the extent of HBV-specific T-cell exhaustion which influences the disease status of patients with HBV infection has thus far been analyzed only in small groups of patients, both those who were able to control an acute infection and those with established chronic infection[16]. However, little is known as to whether PD-1 expression on CD8+ T cells differs between patients with acute exacerbation of hepatitis B (AEHB) and chronic HBV infection.
To define the potential role of PD-1/PD-L pathway in acute exacerbation of HBV infection, we examined the expression of PD-1 on antigen specific CD8+ T cells in the peripheral blood in 32 patients with chronic untreated HBV infection and 11 patients with AEHB. The present study was performed by using pentamer technology combined with flow cytometry, allowing the direct visualization of HBV specific CD8+ T cells.
A total of 89 patients were enrolled in the study from either the outpatient clinic or the inpatient service of the Department of Infectious Diseases and Hepatology of the Union Hospital, Wuhan, China. The study group comprised of 66 chronic hepatitis B (CHB) patients [32 were human leukocyte antigen (HLA)-A2+], 23 AEHB (11 HLA-A2+) patients and 28 healthy donors. All subjects were anti-HBV treatment naive at the time of enrollment. Since APC-labeled HLA-A2 pentamer complexes specific for the HBV Core 18-27 (FLPSDFFPSU) can only identify HBV specific T cells in HLA-A2+ patients, all HBV-infected individuals subjected to fluorescent monoclonal antibodies and pentamer staining were first serologically identified as having the HLA-A2+ genotype. HLA typing was performed using flow cytometry by staining peripheral blood mononuclear cells (PBMCs) with a fluorescent anti-HLA-A2.01 antibody (Serotec). The criteria for the diagnosis of AEHB and CHB have been described previously in detail[24]. Based on the infection status, the subjects were divided into two groups: 11 patients had clinical, biochemical, and virologic evidence of AEHB infection [history of CHB, total bilirubin (TB) levels at least 10 times the upper limit of the normal, and plasma prothrombin activity (PTA) < 40%]. All patients were hepatitis B surface antigen (HBsAg) positive, and negative for antibodies against hepatitis C virus, hepatitis D virus (HDV), hepatitis E virus (HEV), HIV type-1 and HIV-2. Moreover, other causes of chronic liver disease were excluded. Patients with CHB patients had ALT levels ranging from 6 to 1485 U/liters and HBV DNA values from < 103 to 4.61 × 108 copies/mL. Patients with AEHB had TB levels ranging from 228 to 715.8 &mgr;mol/L, ALT from 36 to 174 U/liters, and HBV DNA from 4.3 × 104 to 8.9 × 107 copies/mL. A total of 28 HLA-A2+ uninfected age and sex matched healthy blood donors with normal liver functions were selected as healthy control (HC). Our study was approved by the local ethics committee, and all subjects gave a written informed consent.
The presence or absence of HBsAg, hepatitis B “e” antigen (HBeAg), antibody to hepatitis B surface antigen (anti-HBs), antibody to hepatitis B core antigen (anti-HBc), antibody to hepatitis B “e” antigen (anti-HBe), and antibodies to HCV, HDV, HIV-1, and HIV-2 were determined by using commercial enzyme immunoassay kits. Serum HBV viral load quantification was performed at the laboratory of Hepatology and Infectious Disease, Union Hospital by real-time polymerase chain reaction (PCR) (ABI PRISM 7300 Sequence Detector, PE Biosystems) based on the TaqMan technology using a commercial PCR diagnostic kit (Da An Gene Co. Ltd. of Zhong Shan University, Guangzhou, China). The viral DNA was extracted according to the manufacturer’s protocol. Sequences of forward primer (5′-ATCCTGCTGCTATGCCTCATCTT-3′), reverse primer (5′-ACAGTGGGGGAAAGCCCTACGAA-3′) and fluorogenic Taqman probes (5′-TGGCTAGTTTACTAG TGCCATTT-G3′) were designed against a highly conserved region of the HBV genome overlapping the genes encoding the X-protein and DNA polymerase. The cycling program was: 50°C for 2 min, 93°C for 5 min, 40 cycles of 93°C for 30 s, 53°C for 30 s, 72°C for 30 s, and 72°C for 7 min. A serum sample quantified by b-DNA method was used as the standard to estimate the number of virus and as the quality control for HBV quantitative PCR. The internal control was estimated by commercialized β-actin kit (PE Biosystems) with reaction condition at 50°C for 2 min first and 5 min at 93°C, followed by 40 cycles of denaturation at 93°C for 30 s and annealing-extension at 65°C for 1 min. The analysis was accomplished within 2 min automatically at the end of the run. The HBV DNA cut-off value was 1000 copies/mL.
The serum ALT levels were assayed by CL-7200 Fully-auto Chemistry Analyzer provided by Shimadzu Co. Ltd, at the Department of Clinical Laboratory, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, China.
Using centrifugation on Ficoll-Hypaque density gradient (HaoYang Biological Manufacture, Tianjin, China) and RBC lysis Solution (Roche), PBMC were isolated from fresh heparinized blood (5 mL) collected from each patient, washed twice in phosphate-buffered saline and analyzed immediately.
To measure the number of virus-specific CD8+ T cells and PD-1 expression on CD8+ T-cell subsets, cells were stained with fluorescein isothiocyanate (FITC)-, phycoerythrin (PE)-, and allophycocyanin (APC)-labeled monoclonal antibodies or pentamer, according to the manufacturers’ instructions. Briefly, a total of 1 × 106 to 5 × 106 freshly isolated PBMC were incubated for 30 min at room temperature with APC-labeled HLA-A2 pentamer complexes specific for the HBV Core 18-27 (FLPSDFFPSU) (Proimmune Oxford, United Kingdom) in RPMI 1640 and 10% fetal calf serum. After a wash with phosphate-buffered saline-0.1% fetal calf serum, cell surface staining was performed for 15 min in the dark by simultaneously using anti-CD8 (PE-conjugated), anti-PD-1 (FITC-conjugated) and the corresponding IgG2a isotype controls (eBioscience San Diego, CA).
Cells were washed twice with PBS and gated on CD8+ T cells; 1 × 106 events in the lymphogate were collected by a BD Biosciences multi-color flow cytometer (LSR2, Becton Dickinson Biosciences). The data were analyzed by using the FACS Diva software. Pentamer-positive responses were reported as a percentage of pentamer-positive T cells among the total CD8+ T cell population. The frequency of pentamer-positive cells exceeding 0.02% of CD8+ T cells indicated a positive response.
We used SPSS 12.0 software to perform statistical analyses. Frequency of pentamer positive CD8+ T cells and levels of PD-1 expression in HCs and patients with different viremia levels were compared using the Mann-Whitney test. Spearman correlation analysis was used to evaluate the correlation between viral load, frequency of HBV-specific T cells and PD-1 expression. All tests were two-tailed and P-values less than 0.05 were considered significant.
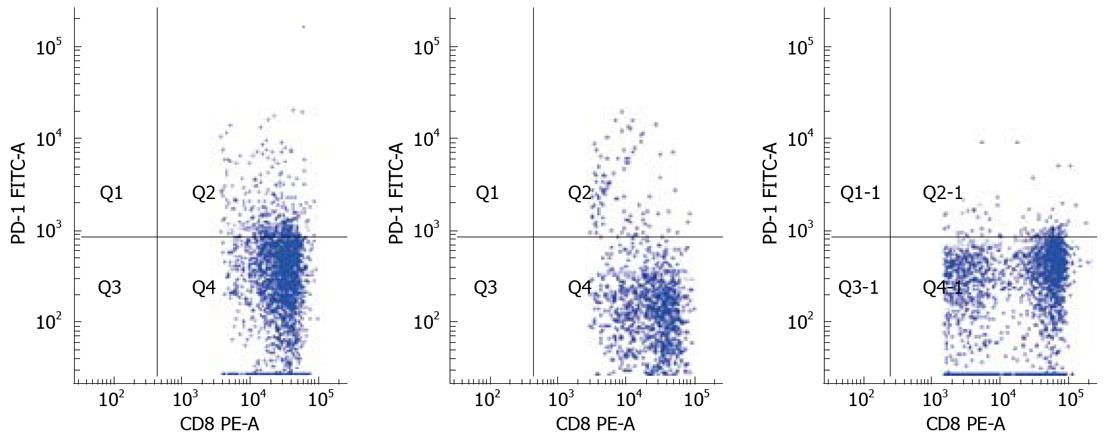
To determine PD-1 expression on CD8+ T cell population, we examined the frequency of PD-1-expression on CD8+ T-cell subsets using polychromatic flow cytometry in the three study groups: AEHB, CHB and HC. The PD-1 levels on the total CD8+ T cells in CHB patients (13.86% ± 3.38%) were significantly higher compared to AEHB patients (6.80% ± 2.19%, P < 0.01) and healthy individuals (4.63% ± 1.23%, P < 0.01) (Figure 1). Representative flow cytometry plots of PD-1 expression on CD8+ T cells from the peripheral blood of one health individual (5.8%) and two patients with CHB (12.71%) and AEHB (7.1%) are shown in Figure 2.
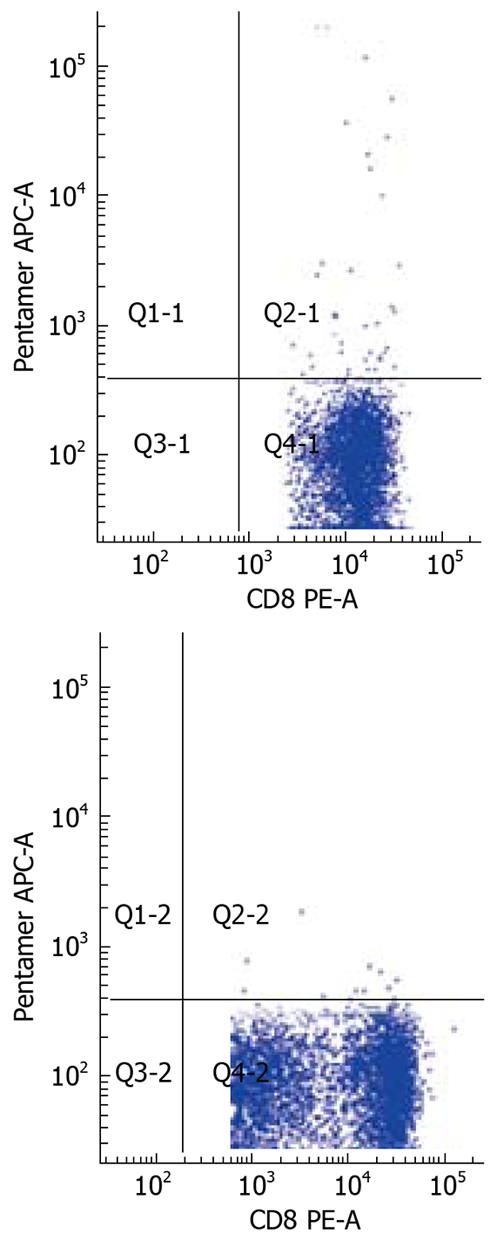
The frequency of HBV-specific CD8+ T cells in the three study groups was assessed. The detection limit was 0.02% for CD8+ T cells specific for the core peptide spanning amino acids 18 to 27. None of the 28 healthy individuals showed a positive response with pentamer. Compared to AEHB patients (0.81% ± 0.73%), there was a lower frequency of HBV-specific CD8+ T cells (0.37% ± 0.43%) in CHB patients (P < 0.05) (Figure 3). Representative plots from two patients with CHB (0.07%) and AEHB (0.3%) infection indicating the number of HBV-specific CD8+ T cells are shown in Figure 4.
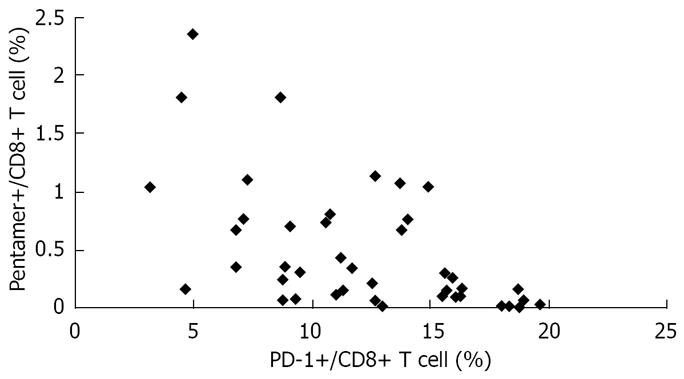
Using linear regression and Spearman’s correlation analyses, we found an inverse correlation between HBV-specific CD8+ T-cell response and level of PD-1 expression in AEHB and CHB patients. In other words, the higher the PD-1 expression level, the weaker (or totally absent) HBV-specific CD8+ T-cell response was observed (Figure 5).
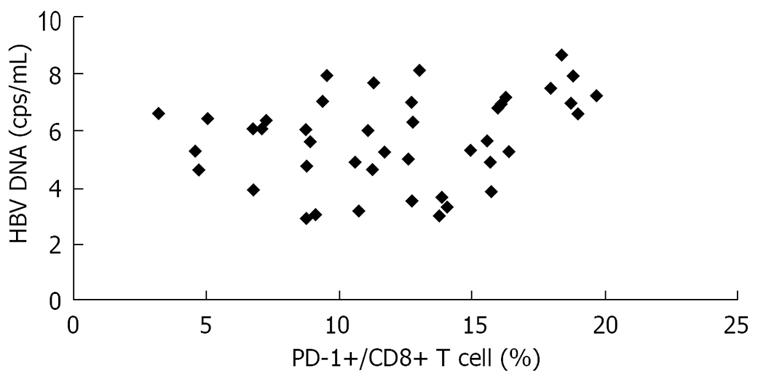
In the present study, there was a significant positive correlation between HBV viral load and PD-1 expression on CD8+ T cells in CHB and AEHB subjects. These findings indicate that high plasma viral load correlates with PD-1 upregulation on the total CD8+ T cells in viremic individuals (Figure 6).
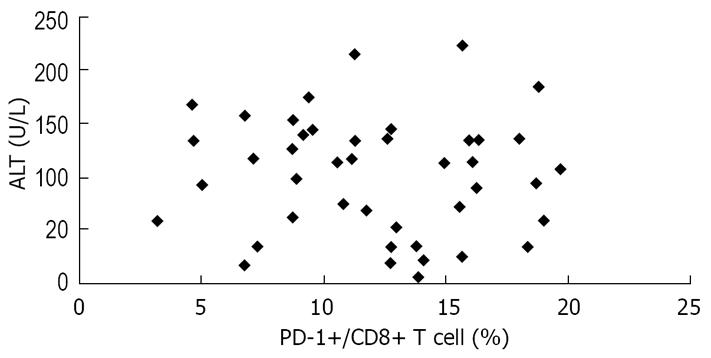
We also assessed the relationship between PD-1 expression and serum ALT levels, another predictor of HBV disease progression. There was no correlation between PD-1 expression and serum ALT levels in the study patients (Figure 7).
The development of chronic persistent HBV infection is usually associated with quantitative and qualitative exhaustion of functional T cells. Recent studies have shown that the negative costimulatory receptor PD-1 along with its ligand PD-L1 are upregulated on PBMC and virus specific T cells, to attenuate T cell responses which accounts for the impairment of their function[1516]. However, under acute hepatitis B (AHB) conditions, the levels of PD-1 are significantly down regulated on PBMC or on HBV specific CD8+ T cells compared to CHB patients. Several previous studies[102526] have suggested that operating co-stimulatory pathways may provide a new method to restore the impaired function of virus specific T cells and control chronic virus infection. Blocking the PD-1/PD-L1 pathway by anti-PD-L1 mAb or applying soluble PD-1 results in improvement of T cell functions such as survival, proliferation and cytokine production[27–29]. This would result in better control of the virus infection. Thus, an accurate balance between positive and negative co-stimulatory regulation such as PD-1/ PD-L pathway may contribute to the outcome of disease progression in HBV infection.
However, the mechanism by which PD-1 affects the function of CD8+ T cells in AEHB is unclear. To clarify the correlation between PD-1 expression and different presentations of HBV infection, we investigated the role of PD-1 in AEHB and chronic HBV infection, using a MHC class I pentamer specific for 18-27 epitopes, using the flow cytometry technology. Consistent with previous studies, we found that peripheral blood CD8+ T cells showed a higher PD-1 expression in CHB patients compared to AEHB patients and HCs. There was a negative correlation with impaired HBV-specific CD8+ T cell function and a positive correlation with the plasma viral load. However, we did not find an association between PD-1 expression and hepatic inflammatory indicator: serum ALT levels. Our data indicates that similar to AHB patients, CD8+ T cells in AEHB patients expressed a low level of PD-1 and an enhanced level of HBV specific CD8+ T cells compared to CHB patients. These results are in line with previous reports which indicate that virus specific T cell function is upregulated during acute exacerbation of hepatitis B infection compared to during persistent CHB. Furthermore, the proportion of CD8+ T cells expressing PD-1 correlated negatively with the intensity of virus specific CD8+ T cell response.
The low levels of PD-1expression were associated with low levels of HBV viremia. However, we did not find any correlation between PD-1 expression and disease flare-up indicator, ALT. Recent studies have shown that persistent antigenic stimulation has a suppressive effect on functional T cells. Briefly, T cells up-regulate their surface receptors such as PD-1 to restrict positive TCR signaling and, therefore, avoid the development of a strong immune response, including autoimmune diseases. However, some microorganisms may utilize this in vivo protection system to escape the host immune system and result in persistent viremia. However, why this phenomenon is broken in patients with AEHB remains to be investigated.
Although PD-1/PD-L pathway plays a prominent role in the regulation of T cell dysfunction, other costimulatory molecules cannot be excluded. In a recent study, it was observed that apart from PD-1, several other gene expressions were involved in promoting exhausted CD8+ T cells during chronic viral infection[13]. However, recent data indicates that PD-1 is a major factor of apoptosis sensitivity over and above other factors[9]. Future studies should be addressed to clarify the intracellular mechanisms used by the PD-1/PD-L1 pathway to influence disease status during viral infection. Importantly, the use of highly active anti-retroviral therapy (HAART), accompanied by the recovery of the host immune response, has been found to down-regulate PD-1 expression in ‘typical progressor’ (TP) patients infected with HIV[1130]. Therefore, it is important to determine whether direct suppression of PD-1/PD-L pathway could provide a potentially effective treatment for persistent viral infections.
Nearly, 2 billion people are infected with hepatitis B virus (HBV) worldwide. Persistent HBV infection of the liver is associated with end-stage liver diseases including cirrhosis and hepatocellular carcinoma. During chronic HBV infection, the effector functions of virus specific T cells often show an impaired activity indicated by low levels of cytokine production and cytotoxic activity. As a result, viruses can persist and establish long-term residency in vivo. However, the underlying mechanisms responsible for the induction of T-cell tolerance are not completely understood.
The aim of this study was to explore the potential role of programmed death-1/programmed death-ligand (PD-1/PD-L) pathway in antiviral immunity during HBV infection.
This is the first report on the differences in PD-1 expression on CD8+ T cells in acute exacerbation of hepatitis B (AEHB) and chronic HBV infection.
Blockade of PD-1/PD-L1 pathway may open a novel therapeutic strategy for restoring the function of the exhausted CD8+ T cells, and enhancing viral control during chronic viral infections. The perspective of future application: further studies to assess the intracellular mechanisms used by the PD-1/PD-L1 pathway to influence the disease status in viral infection.
This is a requisite investigation. The levels of PD-1 on CD8+ T cells has until now been analyzed only in small groups of patients with acute infection and established chronic infection. The manuscript provides new information about PD-1 expression on CD8+ T cells in different disease states of HBV infection including AEHB and chronic hepatitis B (CHB).
| 1. | Liu TT, Fang Y, Xiong H, Chen TY, Ni ZP, Luo JF, Zhao NQ, Shen XZ. A case-control study of the relationship between hepatitis B virus DNA level and risk of hepatocellular carcinoma in Qidong, China. World J Gastroenterol. 2008;14:3059-3063. |
| 2. | Zerbini A, Pilli M, Boni C, Fisicaro P, Penna A, Di Vincenzo P, Giuberti T, Orlandini A, Raffa G, Pollicino T. The characteristics of the cell-mediated immune response identify different profiles of occult hepatitis B virus infection. Gastroenterology. 2008;134:1470-1481. |
| 3. | Radziewicz H, Uebelhoer L, Bengsch B, Grakoui A. Memory CD8+ T cell differentiation in viral infection: a cell for all seasons. World J Gastroenterol. 2007;13:4848-4857. |
| 4. | Menne S, Tennant BC. Unraveling hepatitis B virus infection of mice and men (and woodchucks and ducks). Nat Med. 1999;5:1125-1126. |
| 5. | Rehermann B, Ferrari C, Pasquinelli C, Chisari FV. The hepatitis B virus persists for decades after patients' recovery from acute viral hepatitis despite active maintenance of a cytotoxic T-lymphocyte response. Nat Med. 1996;2:1104-1108. |
| 6. | Khoury SJ, Sayegh MH. The roles of the new negative T cell costimulatory pathways in regulating autoimmunity. Immunity. 2004;20:529-538. |
| 7. | Nurieva R, Thomas S, Nguyen T, Martin-Orozco N, Wang Y, Kaja MK, Yu XZ, Dong C. T-cell tolerance or function is determined by combinatorial costimulatory signals. EMBO J. 2006;25:2623-2633. |
| 8. | Muhlbauer M, Fleck M, Schutz C, Weiss T, Froh M, Blank C, Scholmerich J, Hellerbrand C. PD-L1 is induced in hepatocytes by viral infection and by interferon-alpha and -gamma and mediates T cell apoptosis. J Hepatol. 2006;45:520-528. |
| 9. | Bowen DG, Shoukry NH, Grakoui A, Fuller MJ, Cawthon AG, Dong C, Hasselschwert DL, Brasky KM, Freeman GJ, Seth NP. Variable patterns of programmed death-1 expression on fully functional memory T cells after spontaneous resolution of hepatitis C virus infection. J Virol. 2008;82:5109-5114. |
| 10. | Zhang Z, Zhang JY, Wherry EJ, Jin B, Xu B, Zou ZS, Zhang SY, Li BS, Wang HF, Wu H. Dynamic programmed death 1 expression by virus-specific CD8 T cells correlates with the outcome of acute hepatitis B. Gastroenterology. 2008;134:1938-1949, 1949. e1-e3. |
| 11. | Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350-354. |
| 12. | Latchman YE, Liang SC, Wu Y, Chernova T, Sobel RA, Klemm M, Kuchroo VK, Freeman GJ, Sharpe AH. PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells. Proc Natl Acad Sci USA. 2004;101:10691-10696. |
| 13. | Okazaki T, Honjo T. The PD-1-PD-L pathway in immunological tolerance. Trends Immunol. 2006;27:195-201. |
| 14. | Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC, Precopio ML, Schacker T, Roederer M, Douek DC. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med. 2006;203:2281-2292. |
| 15. | Venkatachari NJ, Buchanan WG, Ayyavoo V. Human immunodeficiency virus (HIV-1) infection selectively downregulates PD-1 expression in infected cells and protects the cells from early apoptosis in vitro and in vivo. Virology. 2008;376:140-153. |
| 16. | Meier A, Bagchi A, Sidhu HK, Alter G, Suscovich TJ, Kavanagh DG, Streeck H, Brockman MA, LeGall S, Hellman J. Upregulation of PD-L1 on monocytes and dendritic cells by HIV-1 derived TLR ligands. AIDS. 2008;22:655-658. |
| 17. | Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682-687. |
| 18. | Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350-354. |
| 19. | Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC, Precopio ML, Schacker T, Roederer M, Douek DC. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med. 2006;203:2281-2292. |
| 20. | Zhang JY, Zhang Z, Wang X, Fu JL, Yao J, Jiao Y, Chen L, Zhang H, Wei J, Jin L. PD-1 up-regulation is correlated with HIV-specific memory CD8+ T-cell exhaustion in typical progressors but not in long-term nonprogressors. Blood. 2007;109:4671-4678. |
| 21. | Golden-Mason L, Palmer B, Klarquist J, Mengshol JA, Castelblanco N, Rosen HR. Upregulation of PD-1 expression on circulating and intrahepatic hepatitis C virus-specific CD8+ T cells associated with reversible immune dysfunction. J Virol. 2007;81:9249-9258. |
| 22. | Urbani S, Amadei B, Tola D, Massari M, Schivazappa S, Missale G, Ferrari C. PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J Virol. 2006;80:11398-11403. |
| 23. | Boni C, Fisicaro P, Valdatta C, Amadei B, Di Vincenzo P, Giuberti T, Laccabue D, Zerbini A, Cavalli A, Missale G. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J Virol. 2007;81:4215-4225. |
| 24. | Chinese Society of Infectious Diseases and Parasitology and Chinese Society of Hepatology of Chinese Medical Association. The program of prevention and cure for viral hepatitis. Zhonghua Ganzangbing Zazhi. 2000;8:324-329. |
| 25. | Bukczynski J, Wen T, Wang C, Christie N, Routy JP, Boulassel MR, Kovacs CM, Macdonald KS, Ostrowski M, Sekaly RP. Enhancement of HIV-specific CD8 T cell responses by dual costimulation with CD80 and CD137L. J Immunol. 2005;175:6378-6389. |
| 26. | Yu Q, Yue FY, Gu XX, Schwartz H, Kovacs CM, Ostrowski MA. OX40 ligation of CD4+ T cells enhances virus-specific CD8+ T cell memory responses independently of IL-2 and CD4+ T regulatory cell inhibition. J Immunol. 2006;176:2486-2495. |
| 27. | Lukens JR, Cruise MW, Lassen MG, Hahn YS. Blockade of PD-1/B7-H1 interaction restores effector CD8+ T cell responses in a hepatitis C virus core murine model. J Immunol. 2008;180:4875-4884. |
| 28. | Ha SJ, Mueller SN, Wherry EJ, Barber DL, Aubert RD, Sharpe AH, Freeman GJ, Ahmed R. Enhancing therapeutic vaccination by blocking PD-1-mediated inhibitory signals during chronic infection. J Exp Med. 2008;205:543-555. |
| 29. | Onlamoon N, Rogers K, Mayne AE, Pattanapanyasat K, Mori K, Villinger F, Ansari AA. Soluble PD-1 rescues the proliferative response of simian immunodeficiency virus-specific CD4 and CD8 T cells during chronic infection. Immunology. 2008;124:277-293. |