Published online Jul 28, 2008. doi: 10.3748/wjg.14.4546
Revised: June 16, 2008
Accepted: June 23, 2008
Published online: July 28, 2008
AIM: To investigate the effect of delayed ethyl pyruvate (EP) delivery on distant organ injury, survival time and serum high mobility group box 1 (HMGB1) levels in rats with experimental severe acute pancreatitis (SAP).
METHODS: A SAP model was induced by retrograde injection of artificial bile into the pancreatic ducts of rats. Animals were divided randomly into three groups (n = 32 in each group): sham group, SAP group and delayed EP treatment group. The rats in the delayed EP treatment group received EP (30 mg/kg) at 12 h, 18 h and 30 h after induction of SAP. Animals were sacrificed, and samples were obtained at 24 h and 48 h after induction of SAP. Serum HMGB1, aspartate aminotransferase (AST), alanine aminotransferase (ALT), blood urea nitrogen (BUN), and creatinine (Cr) levels were measured. Lung wet-to-dry-weight (W/D) ratios and histological scores were calculated to evaluate lung injury. Additional experiments were performed between SAP and delayed EP treatment groups to study the influence of EP on survival times of SAP rats.
RESULTS: Delayed EP treatment significantly reduced serum HMGB1 levels, and protected against liver, renal and lung injury with reduced lung W/D ratios (8.22 ± 0.42 vs 9.76 ± 0.45, P < 0.01), pulmonary histological scores (7.1 ± 0.7 vs 8.4 ± 1.1, P < 0.01), serum AST (667 ± 103 vs 1 368 ± 271, P < 0.01), ALT (446 ± 91 vs 653 ± 98, P < 0.01) and Cr (1.2 ± 0.3 vs 1.8 ± 0.3, P < 0.01) levels. SAP rats had a median survival time of 44 h. Delayed EP treatment significantly prolonged median survival time to 72 h (P < 0.01).
CONCLUSION: Delayed EP therapy protects against distant organ injury and prolongs survival time via reduced serum HMGB1levels in rats with experimental SAP. EP may potentially serve as an effective new therapeutic option against the inflammatory response and multiple organ dysfunction syndrome (MODS) in SAP patients.
-
Citation: Yang ZY, Ling Y, Yin T, Tao J, Xiong JX, Wu HS, Wang CY. Delayed ethyl pyruvate therapy attenuates experimental severe acute pancreatitis
via reduced serum high mobility group box 1 levels in rats. World J Gastroenterol 2008; 14(28): 4546-4550 - URL: https://www.wjgnet.com/1007-9327/full/v14/i28/4546.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.4546
Excessive activation of the inflammatory mediator cascade during severe acute pancreatitis (SAP) is a major cause of multiple organ dysfunction syndrome (MODS), which leads to a high mortality rate[1]. Therapeutic strategies targeting these inflammatory mediators are thought to be an ideal way to reduce the severity of SAP. This is difficult, however, because the cytokines, such as TNF-α and IL-1β, are released early in the development of a systemic inflammatory response[2]. This leaves a narrow therapeutic window for the administration of therapeutics, and delayed delivery of anti-inflammatory therapeutics are not effective after the inflammatory mediator cascade has developed[2].
High mobility group box 1 (HMGB1) protein, which has been known as a ubiquitously expressed, intracellular DNA-binding protein for about 30 years, was recently identified as a late-acting mediator of endotoxin lethality[3]. It was reported that serum HMGB1 levels increased in patients with sepsis/endotoxemia[3–5], hemorrhagic shock[6], acute lung injury[78], rheumatoid arthritis[9] and disseminated intravascular coagulation[10]. HMGB1 is identified as a late mediator of endotoxin lethality because its systemic release during endotoxemia is delayed as compared with the rapid increase of the early proinflammatory cytokines, such as TNF-α and IL-1β. HMGB1 is released by endotoxin-stimulated macrophages only after a delay of 12-18 h. A similar delayed appearance of HMGB1 (8-32 h) was also observed in the serum of mice with endotoxemia after TNF-α had reached its peak and subsided[3]. Delayed anti-HMGB1 antibody dosing still confers significant protection against endotoxin lethality[3]. Strategies that target HMGB1 with specific antibodies or antagonists seem to have potential value for treating lethal systemic inflammatory diseases characterized by excessive HMGB1 release, and the delayed kinetics indicate that HMGB1 may provide a broader therapeutic window for treating those inflammatory disorders[11].
It has been recently demonstrated that the serum levels of HMGB1 were significantly elevated in patients with SAP on admission, and were correlated with the severity of the disease[12]. Early blockade of HMGB1 attenuates the development and associated organ dysfunction in experimental SAP[13]. These indicate that HMGB1 may be an effective therapeutic target of SAP. In a previous experimental study, we demonstrated that the serum levels of HMGB1 began to rise significantly at 12 h, and were maintained in high levels up to 48 h after induction of experimental SAP in rats[14]. Thus, we conceive that delayed therapeutic delivery targeting HMGB1 might attenuate SAP in rats.
Ethyl pyruvate (EP), a stable lipophilic pyruvate derivative, is an agent that can effectively protect animals from ischemia-reperfusion-induced tissue injury[15]. EP administration significantly improves the survival of lethal hemorrhagic shock in standard models[1617], and significantly inhibits the systemic release of both early (TNF-α) and late (HMGB1) cytokines that mediate the lethality of sepsis and systemic inflammation, even when administered 24 h after cecal puncture[18]. We hypothesize that delayed EP administration could reduce the severity of SAP in rats through inhibiting the systemic release of HMGB1.
Male Wistar rats weighing 200-250 g were obtained from the Experimental Animal Center of Tongji Medical College, Huazhong University of Science and Technology (Wuhan, China). Before the experiment, the animals were kept in rooms at 20 °C ± 2 °C in 12-h light-dark cycles for at least 1 wk to acclimate to the surroundings with free access to water and standard rat chow.
The animals were fasted with free access to water 12 h before surgery. The rats were then intra-abdominally anesthetized by 1% pentobarbital sodium (35 mg/kg body weight), and incised through a median incision of the abdomen. After the common bile duct was clamped in the hepatoduodenal ligament by a small bulldog clamp, the biliopancreatic duct was cannulated through mammary papilla from the anterior wall of the duodenum. 1 mL/kg body weight of 5% sodium taurocholate (Sigma, St. Louis, MO, USA) was injected by the cannula with an even speed of 0.1 mL/min, and the atraumatic vascular clamp was removed 10 min later. Finally, the abdominal incisions were closed in two layers. All procedures were performed using a sterile technique.
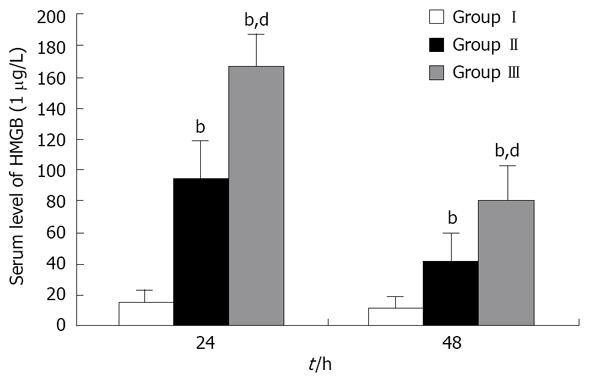
After the stabilization period, 96 male rats were randomly divided into three groups (each group n = 32), and each group was divided into two subgroups (each subgroup n = 16). Animals in a subgroup were sacrificed at either 24 h or 48 h after surgery. Rats in group I (sham group) underwent laparotomy with manipulation of the pancreas (sham procedure) and received 40 mL/kg normal saline subcutaneously (single dose). Groups II and III underwent laparotomy with induction of SAP, and subsequently received saline every 6 h after induction of SAP. Rats in group II (delayed EP treatment group) additionally received 30 mg/kg body weight EP intravenously at 12, 18 and 30 h after induction of SAP. EP (Sigma, St. Louis, MO, USA) was prepared in solution with sodium (130 mmol/L), potassium (4 mmol/L), calcium (2.7 mmol/L), chloride (139 mmol/L), and EP (28 mmol/L) (pH 7.0). Rats in group III (SAP group) intravenously received the same volume of vehicle at the same time as in group II. Twenty four hours after induction of SAP, the surviving rats were anesthetized with ether, and blood samples were taken from the inferior vena cava to measure serum HMGB1 levels and blood biochemical parameters. The animals were sacrificed, and the right lung was obtained to evaluate lung injury. 48 h after induction of SAP, only blood samples were taken in order to measure serum HMGB1 levels.
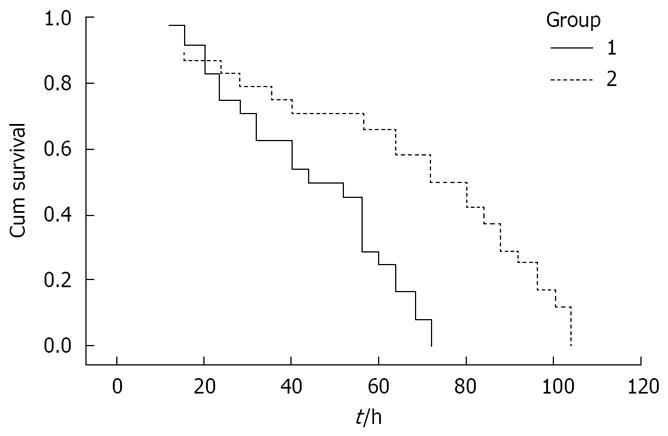
To investigate the effect of EP on the survival time of SAP rats, 48 male rats underwent laparotomy with induction of SAP, and were randomly divided into two groups (each group n = 24): SAP group and delayed EP treatment group. Rats in the EP treatment group received delayed EP delivery (30 mg/kg body weight) intravenously at 12, 18, 30 and 48 h after induction of SAP, and received the above-mentioned fluid resuscitation. Rats in the SAP group received fluid resuscitation and the same volume of vehicle intravenously as in the EP treatment group. The number of surviving rats was recorded every 4 h after induction of SAP.
HMGB1 was analyzed by Western blot as described by Wang et al[3]. Briefly, serum was first filtered with Centricon YM-100 (Millipore) to clear the samples from cell debris and macromolecular complexes formed during clotting. Samples were then concentrated 15-fold with Centricon YM-30 and separated on 12% SDS-polyacrylamide gels. Protein was transferred to nitrocellulose membranes (Pall) and HMGB1 was analyzed by using polyclonal anti-HMGB1 antibodies (Santa Cruz) and secondary anti-goat alkaline phosphatase (Beijing Zhongshan Biotechnology). The intensity of the 30-kDa band was analyzed by densitometry. Standard curves were constructed using r-HMGB1 (Sigma, St. Louis, MO, USA).
Serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), blood urea nitrogen (BUN), and creatinine (Cr) levels were measured using a standard clinical automated analyzer.
The right lung was wiped dry with filter paper and weighed. Then, it was dried to a constant weight at 60 °C for 72 h in an oven. W/D ratios could then be calculated. Routine paraffin sectioning was performed on the lung tissue. Pulmonary histological scores were graded using a Gloor score system (normal, 0; mild, 1; moderate, 2; severe, 3; overwhelming, 4) for intra-alveolar oedema, intra-alveolar haemorrhage, and neutrophil infiltration and these scores were then added to give a total score[19].
mean ± SD values for blood biochemical parameters, HMGB1 serum levels, lung W/D ratios and histological scores were determined. The differences between the two groups were further evaluated with the Mann-Whitney U test. Overall survival was calculated by the Kaplan-Meier Estimate. The log-rank test and the Breslow test were used to compare survival curves in the two groups. A P value < 0.05 was considered statistically significant.
At 24 h and 48 h after induction of SAP, serum HMGB1 levels of SAP rats were higher than those of the sham group. Delayed EP administration significantly reduced the serum HMGB1 levels of SAP rats (Figure 1).
Lung W/D ratios were observed to evaluate the severity of pulmonary edema. At 24 h after induction of SAP, W/D ratios were elevated in the SAP group in comparison with the sham group (9.76 ± 0.45 vs 5.43 ± 0.21, P < 0.01). The lung W/D ratio of rats that received delayed EP administration was significantly lower than that of the SAP group (P < 0.01), although it was significantly higher than that of the sham group (8.22 ± 0.42 vs 5.43 ± 0.21, P < 0.01).
The pulmonary histological scores, an all round evaluation for lung injury, were lower in the EP treatment group than that in the SAP group (7.1 ± 0.7 vs 8.4 ± 1.1, P < 0.01), although they were significantly elevated in comparison with the sham group (vs 0.5 ± 0.1, P < 0.01).
EP treatment protected against liver and renal injury. 24 hours after induction of SAP, serum AST, ALT, BUN and Cr levels were significantly elevated in groups II and III. Delayed EP administration significantly attenuated the elevated AST, ALT and Cr levels (Table 1).
SAP rats all died within 3 d without the delayed EP administration, and their median survival time was 44 h (95% confidence interval 29.6 h to 58.4 h). Delayed EP administration significantly prolonged the median survival time to 72 h (95% confidence interval 52.8 h to 91.2 h; Figure 2).
Extracellular HMGB1 was recently implicated as a late mediator of delayed endotoxin lethality. High serum HMGB1 levels in patients with sepsis are associated with increased mortality, and administration of HMGB1 produces acute inflammation in animal models of lung injury and endotoxemia. During lethal endotoxemia in mice, serum HMGB1 levels accumulate 8-32 h after LPS administration[3]. Passive immunization of mice with anti-HMGB1 antibodies attenuates LPS-induced lethality, even when antibody administration is delayed until after the onset of the early proinflammatory cytokine response (2 h after endotoxin administration)[3]. The delayed kinetics of HMGB1 appearance indicates that the therapeutic window may be significantly wider than for any previously described cytokine target.
In a previous experimental study, we demonstrated that the serum levels of HMGB1 began to increase significantly at 12 h after induction of SAP, after the TNF-α and IL-1β peak had already occurred. The delayed kinetics of HMGB1 release may provide a wider therapeutic window for SAP. It was shown that EP could inhibit HMGB1 release from macrophages and prevent the accumulation of serum HMGB1 levels in mice with lethal sepsis by inhibiting NF-κB and p38 MAPK signaling[18]. In this study, we administered SAP rats with EP intravenously. As a result, EP significantly reduced serum HMGB1 levels in rats with SAP, even though EP delivery began 12 h after induction of SAP.
In SAP, MODS, a consequence of the systemic inflammatory response syndrome, is a contributor to high mortality in the early phase[1]. It is conceivable that the release of mediators from the excess of activated macrophages/monocytes and neutrophils may lead to remote organ injury[1]. Serum HMGB1 levels are significantly elevated in patients with SAP on admission[12]. Purified rHMG-1 is lethal to both LPS-responsive (C3H/HeN, Balb/c) and LPS-resistant (C3H/HeJ) mice, indicating that HMG-1 mediates lethal toxicity in the absence of LPS signal transduction[3]. The cytokine activity of HMGB1 has been well-documented in many cell types. In cultured human primary macrophages/monocytes, HMGB1 stimulates the release of multiple proinflammatory cytokines, including TNF-α, IL-1α, IL-1β, IL-1RA, IL-6, IL-8, MIP-1α, and MIP-1β, but not IL-10 and IL-12[20]. In cultured human microvascular endothelial cells, addition of HMGB1 induces the expression of adhesion molecules, such as ICAM-1, VCAM-1, and RAGE, as well as the release of TNF-α and IL-8, MCP-1, PAI-1, and tPA[21]. HMGB1 also activates human neutrophils to produce proinflammatory mediators, such as TNF-α, IL-1β, and IL-8, suggesting an important role for HMGB1 in activation of neutrophils during inflammation[22]. HMGB1 also increases the permeability in cultured enterocytes via a nitric oxide (NO)-dependent pathway[2324]. These indicate that HMGB1 is potent in augmenting the inflammatory response. A previous study showed the early blockade of HMGB1 attenuated organ dysfunction in experimental SAP[13]. This study indicated that HMGB1 might be a good target to prevent organ injury in SAP. In this study, to evaluate the degree of injury in distant organs, such as lung, liver and kidney, serum AST, ALT, BUN and Cr levels were measured, and lung W/D ratios were calculated. Serum AST, ALT and Cr levels, and lung W/D ratios were lower in the EP treatment group compared to those in the SAP group. This indicates that delayed EP treatment protected against distant organ injury. An additional study was performed to evaluate the effect of EP on survival times of SAP rats. As a result, delayed EP administration significantly prolonged the survival times of SAP rats.
These results give direct evidence that the beneficial effects of EP are due to downregulation of HMGB1, and reveal that EP may be a useful new therapeutic option against the inflammatory response and MODS in SAP rats, and may also be effective in SAP patients, even when anti-inflammatory therapy is delayed in the early phase. In this study, EP and reduction in serum HMGB1 levels actually reduces the severity of acute pancreatitis, and prolongs survival time of SAP rats. All rats finally die, however. Further studies should be performed to elucidate the role of HMGB1 in SAP and to determine whether a higher dose or earlier delivery of EP could improve the survival rates of SAP rats. Subsequently, clinical investigations could be carried out to study whether EP can be used for SAP patients.
Excessive activation of inflammatory mediator cascade during severe acute pancreatitis (SAP) is a major cause of the high mortality. Cytokines such as TNF-α and IL-1β are released early in the development of systemic inflammatory response. This leaves a narrow therapeutic window for administration of therapeutics and delayed delivery of that anti-inflammatory therapeutics is not effective after the inflammatory mediator cascade has developed.
Extracellular high mobility group box 1 (HMGB1) was implicated as a late mediator of endotoxin lethality. The cytokine activity of HMGB1 has been well-documented in many cell types. It was reported that serum HMGB1 levels increased in patients with sepsis/endotoxemia, hemorrhagic shock, acute lung injury, rheumatoid arthritis and disseminated intravascular coagulation. It has been recently demonstrated that the serum levels of HMGB1 correlated with the severity of SAP.
In a previous experimental study, the authors demonstrated that the serum levels of HMGB1 began to rise significantly at 12 h, and maintained at high levels up to 48 h after induction of experimental SAP in rats. The delayed kinetics indicates that HMGB1 may provide a broader therapeutic window for treating this lethal systemic inflammatory disease.
Ethyl pyruvate (EP), a stable lipophilic pyruvate derivatives, is a nontoxic food additive. According to this study, it is potential to be used as an effective and low-cost therapeutic remedy for SAP patients.
This report is of interest and considerable potential importance, because it indicates that delayed treatment of rats with experimental SAP with EP is associated with a reduction of HMGB1 levels in blood; a decrease in lung, kidney, and liver injury; and prolonged survival. The "therapeutic window" for inhibiting this inflammatory mediator appears to be more favorable than for some other mediators which more rapidly reach a peak in the blood.
| 1. | Lankisch PG, Lerch MM. Pharmacological prevention and treatment of acute pancreatitis: where are we now? Dig Dis. 2006;24:148-159. |
| 2. | Tracey KJ, Beutler B, Lowry SF, Merryweather J, Wolpe S, Milsark IW, Hariri RJ, Fahey TJ 3rd, Zentella A, Albert JD. Shock and tissue injury induced by recombinant human cachectin. Science. 1986;234:470-474. |
| 3. | Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248-251. |
| 4. | Yang H, Ochani M, Li J, Qiang X, Tanovic M, Harris HE, Susarla SM, Ulloa L, Wang H, DiRaimo R. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc Natl Acad Sci USA. 2004;101:296-301. |
| 5. | Sunden-Cullberg J, Norrby-Teglund A, Rouhiainen A, Rauvala H, Herman G, Tracey KJ, Lee ML, Andersson J, Tokics L, Treutiger CJ. Persistent elevation of high mobility group box-1 protein (HMGB1) in patients with severe sepsis and septic shock. Crit Care Med. 2005;33:564-573. |
| 6. | Ombrellino M, Wang H, Ajemian MS, Talhouk A, Scher LA, Friedman SG, Tracey KJ. Increased serum concentrations of high-mobility-group protein 1 in haemorrhagic shock. Lancet. 1999;354:1446-1447. |
| 7. | Abraham E, Arcaroli J, Carmody A, Wang H, Tracey KJ. HMG-1 as a mediator of acute lung inflammation. J Immunol. 2000;165:2950-2954. |
| 8. | Ueno H, Matsuda T, Hashimoto S, Amaya F, Kitamura Y, Tanaka M, Kobayashi A, Maruyama I, Yamada S, Hasegawa N. Contributions of high mobility group box protein in experimental and clinical acute lung injury. Am J Respir Crit Care Med. 2004;170:1310-1316. |
| 9. | Taniguchi N, Kawahara K, Yone K, Hashiguchi T, Yamakuchi M, Goto M, Inoue K, Yamada S, Ijiri K, Matsunaga S. High mobility group box chromosomal protein 1 plays a role in the pathogenesis of rheumatoid arthritis as a novel cytokine. Arthritis Rheum. 2003;48:971-981. |
| 10. | Hatada T, Wada H, Nobori T, Okabayashi K, Maruyama K, Abe Y, Uemoto S, Yamada S, Maruyama I. Plasma concentrations and importance of High Mobility Group Box protein in the prognosis of organ failure in patients with disseminated intravascular coagulation. Thromb Haemost. 2005;94:975-979. |
| 11. | Yang H, Wang H, Czura CJ, Tracey KJ. HMGB1 as a cytokine and therapeutic target. J Endotoxin Res. 2002;8:469-472. |
| 12. | Yasuda T, Ueda T, Takeyama Y, Shinzeki M, Sawa H, Nakajima T, Ajiki T, Fujino Y, Suzuki Y, Kuroda Y. Significant increase of serum high-mobility group box chromosomal protein 1 levels in patients with severe acute pancreatitis. Pancreas. 2006;33:359-363. |
| 13. | Sawa H, Ueda T, Takeyama Y, Yasuda T, Shinzeki M, Nakajima T, Kuroda Y. Blockade of high mobility group box-1 protein attenuates experimental severe acute pancreatitis. World J Gastroenterol. 2006;12:7666-7670. |
| 14. | Yang ZY, Wang CY, Xiong JQ, Tao J, Xu YQ, Liu T. Serum levels and roles of high mobility group box 1 protein in severe acute pancreatitis rats. Huazhong Keji Daxue Xuebao (Yixueban). 2004;33:466-468. |
| 15. | Sims CA, Wattanasirichaigoon S, Menconi MJ, Ajami AM, Fink MP. Ringer's ethyl pyruvate solution ameliorates ischemia/reperfusion-induced intestinal mucosal injury in rats. Crit Care Med. 2001;29:1513-1518. |
| 16. | Tawadrous ZS, Delude RL, Fink MP. Resuscitation from hemorrhagic shock with Ringer's ethyl pyruvate solution improves survival and ameliorates intestinal mucosal hyperpermeability in rats. Shock. 2002;17:473-477. |
| 17. | Yang R, Gallo DJ, Baust JJ, Uchiyama T, Watkins SK, Delude RL, Fink MP. Ethyl pyruvate modulates inflammatory gene expression in mice subjected to hemorrhagic shock. Am J Physiol Gastrointest Liver Physiol. 2002;283:G212-G221. |
| 18. | Ulloa L, Ochani M, Yang H, Tanovic M, Halperin D, Yang R, Czura CJ, Fink MP, Tracey KJ. Ethyl pyruvate prevents lethality in mice with established lethal sepsis and systemic inflammation. Proc Natl Acad Sci USA. 2002;99:12351-12356. |
| 19. | Gloor B, Blinman TA, Rigberg DA, Todd KE, Lane JS, Hines OJ, Reber HA. Kupffer cell blockade reduces hepatic and systemic cytokine levels and lung injury in hemorrhagic pancreatitis in rats. Pancreas. 2000;21:414-420. |
| 20. | Andersson U, Wang H, Palmblad K, Aveberger AC, Bloom O, Erlandsson-Harris H, Janson A, Kokkola R, Zhang M, Yang H. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med. 2000;192:565-570. |
| 21. | Fiuza C, Bustin M, Talwar S, Tropea M, Gerstenberger E, Shelhamer JH, Suffredini AF. Inflammation-promoting activity of HMGB1 on human microvascular endothelial cells. Blood. 2003;101:2652-2660. |
| 22. | Park JS, Arcaroli J, Yum HK, Yang H, Wang H, Yang KY, Choe KH, Strassheim D, Pitts TM, Tracey KJ. Activation of gene expression in human neutrophils by high mobility group box 1 protein. Am J Physiol Cell Physiol. 2003;284:C870-C879. |
| 23. | Sappington PL, Yang R, Yang H, Tracey KJ, Delude RL, Fink MP. HMGB1 B box increases the permeability of Caco-2 enterocytic monolayers and impairs intestinal barrier function in mice. Gastroenterology. 2002;123:790-802. |