Published online Jul 28, 2008. doi: 10.3748/wjg.14.4540
Revised: July 19, 2008
Accepted: July 26, 2008
Published online: July 28, 2008
AIM: To explore the role of radio-frequency ablation (RFA) as a treatment for hilar cholangiocarcinoma.
METHODS: Eleven patients with obstructive cholestasis underwent Computed Tomography (CT) examination, occupying lesions were observed in the hepatic hilar region in each patient. All lesions were confirmed as cholangioadenocarcinoma by biopsy and were classified as type III or IV by percutaneous transhepatic cholangiography. Patients were treated with multiple electrodes RFA combined with other adjuvant therapy. The survival rate, change of CT attenuation coefficient of the tumor and tumor size were studied in these patients after RFA.
RESULTS: In a follow-up CT scan one month after RFA, a size reduction of about 30% was observed in six masses, and two masses were reduced by about 20% in size, three of the eleven masses remained unchanged. In a follow-up CT scan 6 mo after RFA, all the masses were reduced in size (overall 35%), in which the most significant size reduction was 60%. The survival follow-up among these eleven cases was 18 mo in average. Ongoing follow-up showed that the longest survival case was 30 mo and the shortest case was 10 mo.
CONCLUSION: RFA is a microinvasive and effective treatment for hilar cholangiocarcinoma.
- Citation: Fan WJ, Wu PH, Zhang L, Huang JH, Zhang FJ, Gu YK, Zhao M, Huang XL, Guo CY. Radiofrequency ablation as a treatment for hilar cholangiocarcinoma. World J Gastroenterol 2008; 14(28): 4540-4545
- URL: https://www.wjgnet.com/1007-9327/full/v14/i28/4540.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.4540
Hilar cholangiocarcinoma (also known as Klatskin Tumor) was first reported by Gerald Klatskin in 1965. Since then, many therapeutic methods have been established to treat this type of tumor. For those patients presenting with type I and type II tumors, surgical resection is good and has a high 5-year survival rate[1–5]. However, for those tumors classified as type III and IV tumors, the surgical prognosis is poor even when combined with local tumor resection and left or right hemi-lobectomy of the liver, which can itself lead to further complications[6–8]. Thus, finding a surgical approach for the treatment of type III and IV Klatskin tumors is problematic. Percutaneous image-guided radiofrequency ablation (RFA) has received increasing attention as a promising technique for the treatment of liver tumors. This technique permits the destruction of tumors without necessitating their removal, and in many cases, can be used in place of more invasive and expensive surgical treatment. Initial attempts at tissue ablation with radiofrequency have been limited to the 1.6-cm diameter coagulation necrosis obtained from a single conventional electrode[9]. In order to achieve larger thermal necrosis, internally cooled, single or clustered electrode technique, as well as expandable needle techniques has been introduced. Hence, from May, 2003 to December, 2005, we applied RFA therapy to a group of 11 patients with pathologically confirmed, type III and IV cholangiocarcinoma after percutaneous transhepatic cholangic drainage (PTCD) in order to determine its safety, efficacy, and outcome.
Eleven patients enrolled in this study were all male, ranging in age from 42 to 74 years, with a mean of 52 years. All patients presented with jaundice, and underwent CT examination, Occupying lesions were observed in hepatic hilar region in all cases. All lesions were confirmed as cholangioadenocarcinoma by biopsy and were classified as type III a (n = 4), type III b (n = 2), type IV (n = 5) tumors by percutaneous transhepatic cholangiography. The average dimensions of the tumor masses were 3.4-4.5 cm.
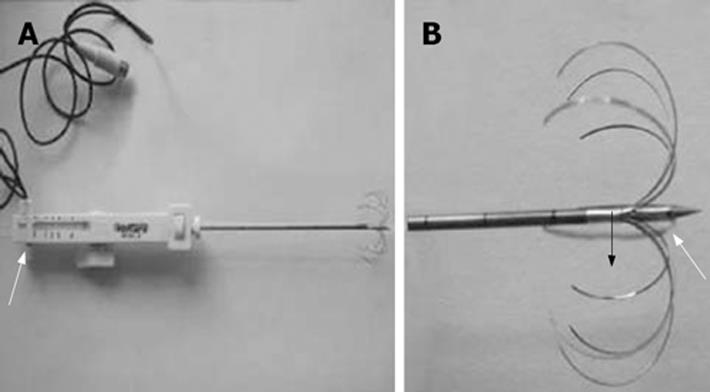
The Marconi CT-Twin flash, with the following parameters: volumetric scan with 5-10 mm thickness and pitch 1, WE7568 multiple electrode tumor RF ablator, 200W pulse output and 290 KHz pulse frequency, was applied (made by Beijing Welfare Electronic Ca.). A temperature sensor was installed in the electrode to monitor the temperature and the sensor deviation was ± 0.50°C. A WHK-4 multiple electrode tumor RFA electrode with side holes ablation needle was applied. These systems deploy an array of multiple curved stiff wires in the shape of an umbrella from a single 14-gauge or 16-gauge canula (Figure 1). This array can produce zones of coagulation necrosis of up to 4-5 cm compared to the bipolar electrode. Furthermore, there are tiny side holes in the distal end of the central electrode, which make it possible for saline infusion during RFA in order to avoid charring and improve the ablation effect.
All patients were hospitalized with proper blood analyses and preparations before synthetic interventional therapy. PTCD was performed for biliary drainage for 2 wk prior to RFA in order to preserve or improve hepatic function. The tumor size determined the number of sessions and duration of RFA.
By using a 22 gauge Chiba needle, PTC was performed from the right middle axillary line to the most dilated intrahepatic biliary duct according to a prior CT scan. When the needle punctured the biliary duct, we fixed the needle and injected contrast media to perform cholangiography. The contrast media allowed better depiction of the stenotic segment and determined whether the left and right hepatic ducts were involved, allowing the tumor to be staged further. A unilateral drainage catheter was applied for type III a and type III b. Bilateral kissing catheters were applied for type IV. The percutaneous drainage catheter was fixed onto the skin with stitches and connected with a drainage bag for daily observation of external bile drainage.
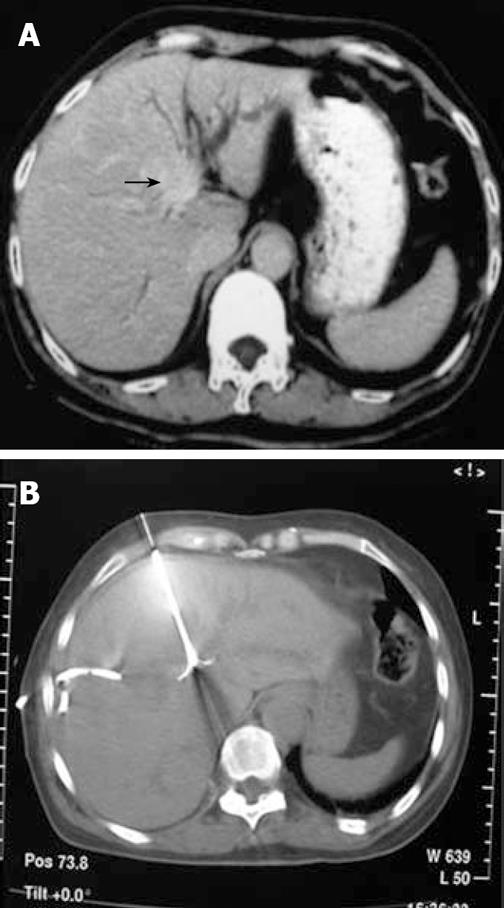
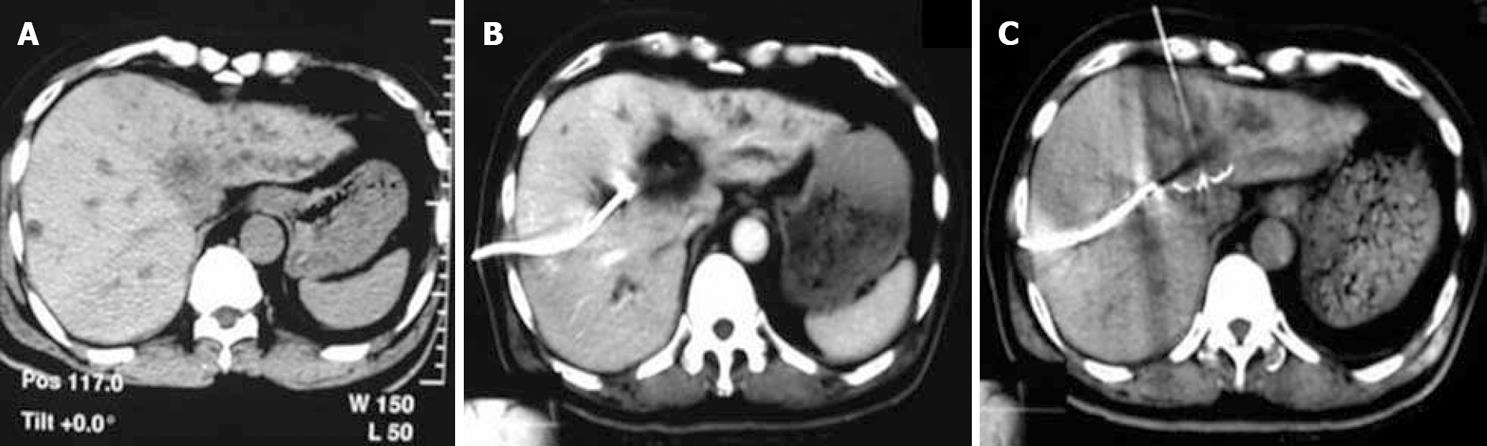
After 2 wk of biliary drainage, depending on the patient’s general condition and improvement of the hepatic function, RFA was applied by percutaneous puncture into the tumor mass with an ablation electrode needle under CT guiding (a density survey of the tumor mass was performed before the procedure). After optimal tumor puncture, we delivered the electrodes in appreciable diameter. The electrodes were distanced from the drainage catheter during the RFA procedure, thereby protecting the catheters from mechanical puncture and thermal damage. The duration of ablation was dependant on the size of the tumor mass, with 10 min for those ≤ 3 cm, and 10-15 min for those around 3.1-4.0 cm. We prolonged the procedure time during which the temperature slowly increased. Injection of 1 mL of 10% saline from the side aperture of the needle every minute enhanced the excitement of ion vibrations, increasing the capability of the device. A CT scan was performed after delivering the electrodes to ensure that the entire lesion was fully covered within the region of RFA. This would provide complete tumor necrosis and eliminate the possible residual tumor infiltration in the adjacent area (Figures 2 and 3).
After RFA, regular monthly abdominal CT scans including a plain scan, double-phase scan and delayed image were performed with biochemical blood analysis in the first 6 mo. For the next 6 mo, follow-up work was every 2 mo, and 1 year after RFA, follow-up was every 3 mo.
One month after the RFA synthetic therapy, CT scans were compared to the baseline data. The treatment was counted as effective, in case where there was significant density attenuation of the tumor mass with no contrast enhancement, regardless of whether the tumor size was reduced or remained unchanged. Conversely, cases were counted as non-effective, if the tumor mass was enlarged and there was no significant density attenuation and inhomogeneous enhancement by IV contrast.
The average density of the 11 tumor masses was 44.23 Hu before RFA therapy. One month after the therapy, it decreased to 21.6 Hu with the most marked case being a decrease of about 40 Hu.
After RFA treatment, six masses had diminished in size by approximately 30%, two masses had been reduced by approximately 20% and no significant change in size was noted in three masses. These results were confirmed 1 mo later by a second CT scan. After 6 mo, the average size reduction in all the masses was approximately 35%. The most significant size reduction was 60%.
One month after PTCD, the direct and indirect bilirubin levels returned to their normal range (direct bilirubin ≤ 8 &mgr;mol/L and indirect bilirubin ≤ 15 &mgr;mol/L) in nine cases. By 6 mo after RFA therapy, the bilirubin levels of all cases had returned to their normal range.
No severe complications occurred during or after each procedure with the exception of fever post RFA.
The 11 patients were all alive at the end of the study. The survival period among these 11 cases was 18 mo on average. Ongoing follow-up showed that the longest survival case was 30 mo and the shortest case was 10 mo.
Gerald Klatskin[10] first described the specific entities of adenocarcinoma as confined below, at or above the confluence of the common hepatic duct in 1965. Afterwards, Bismuth and Corlette[11] classified the tumor into four types: Type I: the lesion is confined to the common hepatic duct with no involvement of the left and right common ducts or confluence; Type II: confluence of the hepatic ducts is involved, but the lesion does not extend to the left and right intrahepatic biliary ducts; Type III a: the lesion spreads to the right hepatic duct; Type III b: the lesion spreads to the left hepatic duct; Type IV: involvement of the left and right hepatic ducts and confluence[12–18].
For Type I and II tumors, surgical resection is the most effective form of therapy. Furthermore, a surgical approach is still possible in cases of local tumor recurrence[1920]. However, for Type III tumors, radical resection is an aggressive procedure that might involve removal of the left or right hepatic lobe and caudate lobe of the liver. A mortality rate of up to 10% has been encountered, and survival after surgical resection on this type of tumor has been disappointing[121421–24]. Palliative resection of Type IV tumor is still controversial, but doctors tend to perform liver transplantation. Transartery chemoembolization (TACE) is not an ideal treatment for Type IV tumors. Since the tumor mass is hypo-vascular, only a faint tumor contrast dye will be observed by DSA (Digital Subtraction Angiography) resulting in inadequate lipiodol staining. Chemotherapy either general or intra-arterial does not improve survival[25–27]. Based on these reasons, we propose that RFA therapy should be used for the treatment of type III and IV cholangiocarcinoma.
Due to the massive and wide range of invasion of the tumor, the majority of patients with Type III and IV Klatskin’s tumor present with serious obstructive jaundice on admission. Therefore, with the aid of DSA, PTCD is the first step of the sequential interventional therapy since it anticipates jaundice and decompensates liver function by using an internal and external drainage. Two weeks following the drainage, until jaundice diminishes and hepatic function is amended, RFA is carried out and that is the most critical step of this sequential interventional therapy. RFA is becoming a widely used tool for treatment of liver metastatic tumors especially since Rossi et al introduced new needle electrodes capable of increasing the diameter of tissue necrosis to 4-5 cm[2829]. The tissue necrosis should include 5 mm of normal tissue around the lesion for oncological clearance.
The basic principle of RFA therapy is described below. Under CT or sonographic guidance, the electrode percutaneously punctures and is placed into the tumor tissue with single or multiple electrode probes. The cluster of electrodes at the end of the probe will emit median to high-frequency electromagnetic wave energy that may induce ionic vibrating friction of the target tissue cells resulting in the generation of heat. As the local temperature increases up to 80°C-90°C, it is sufficient to cause coagulation necrosis of the tumor tissue, and eventually, liquefaction or fibrosis. Coagulation of the peripheral vessel and tissue around the tumor will form a reactive zone. Thus, the tumor blood supplies are interrupted contributing to the prevention of metastasis.
The tumor size determines the number of sessions and duration of RFA. Furthermore, extending the range of RFA to 0.5 cm out of the normal tissue margin where possible will effectively eliminate potential minimal tumor infiltration in the normal liver tissue. This creates a free margin and may reduce the likelihood of tumor relapse.
RFA has a tendency to injure the biliary system when the lesions are near the porta hepatis. This injury may be a bile fistula or an obstruction of the biliary tract. In our study, none of these complications occurred, since PTCD was performed in all of our cases before RFA. The advantages of PTCD before RFA are that: (1) the probability of biliary tract injury decreases due to remission of the obstruction and dilation of the biliary tract after PTCD; and (2) the drain pipe which was detained in the biliary tract during PTCD can be used as a localization mark during RFA.
There are several large vessels near the porta hepatis. Thus, serious hemorrhage will occur if the puncture of the needle injures the large vessels. Hemorrhage will also occur if the dissepiment necrosis of the vessels happens during the RFA procedure due to the entry of the electrode. RFA treatment of all 11 cases in our study did not result in hemorrhagic complications. Our experience is that the radiologist who operates the RFA procedure must possess the imaging radiology and puncture technique, as well as being able to identify the large vessels in the porta hepatis and evade them. When the electrode enters the large vessels, the temperature remains low or the curve of the temperature offers crenate type and duty curve lasts high. At this time, the power source must be turned off immediately, and the location of the needle and electrode is adjusted. The cooling effect of the blood flow in the large vessels near the lesions results in lower efficacy in tumor necrosis. Our countermeasure is to inject 1 mL of 10% saline from the side aperture of the needle every minute to enhance ion vibration and excite the instrument to enhance the duty. It is also important to prolong the procedure time to ensure that the total time of effective temperature (higher than 70°C) lasts for 10-15 min.
Evaluation of the therapeutic effects of this treatment requires analysis of the changes in the density and size of the tumor mass by CT compared with base line data. One month follow-up after RFA showed that for all 11 cases, there were varying degrees of diminishing density and there was no enhancement in the contrast scans of the tumor mass. These effects were mainly due to the tumor coagulation and necrosis. In some cases, the lesion presented with an even lower density value because of the vacuum phenomenon resulting from long-term and multi-session therapy.
Six masses had diminished in size by approximately 30%, two masses had been reduced by approximately 20% and no significant change in size was noted in three masses.
Size reduction of the tumor mass was significantly noted in six out of 11 cases, two masses had been reduced by approximately 20%, and the other three lesions remained unchanged in other five patients in one month CT follow-up. However, 6 mo after synthetic sequential IR therapy, all 11 cases presented with varying degrees of size reduction. This was probably due to the slow progressive development of the coagulated necrosis, liquefaction and fibrosis of the lesion.
To date, all 11 patients are alive. A recent follow-up shows that the survival of a group of 29 cases of Klatskin tumor treated by surgical intervention. The mortality rate in the hospital (during and after surgery) was 17%, the average hospital stay was 71 d, and a survival rate of 1 year was observed in 50% of cases. Tsukada et al reported the survival after RFA therapy to be 18 mo on average, ranging between 10 mo and 30 mo so far. No complications among these 11 cases were observed during or after synthetic sequential interventional therapy. Parc et al[12] reported a group of 39 cases of Klatskin’s tumor treated by surgical intervention. Radical resection was performed in 18 cases, and the remaining 21 cases underwent palliative operation. Among the 18 cases treated with radical resection, one had type I, two had type II, eight had type III a, two had type III b and five had type IV. Four of the 18 cases developed post-operative complications and the survival rate ranged between 1 (67%) and 5 (47%) years. Of the 21 cases treated with palliative operation, the post-surgery mortality was 14% and the mean survival range was only 7 mo. Nimura[3031] performed a liver and bile duct resection combined with Whipple’s operation in five patients with hilar tumors. Two of the five patients died after surgery and the three survivors died 8, 10 and 27 mo later.
Compared with surgical intervention, the RFA treatment that we used for type III and IV Klatskin’s tumor is less invasive and involves less complications. Furthermore, a higher mean survival rate (up to 18 mo) has been shown after RFA than surgical intervention. Thus, we believe that RFA is a less invasive, safe, effective and promising therapy for type III and IV Klatskin tumors. The long-term curative effect requires further study.
Hilar cholangiocarcinoma (also known as Klatskin tumor) was first reported by Gerald Klatskin in 1965. Since then, many therapeutic methods have been established to treat this type of tumor. For those patients presenting with type I and type II tumors, surgical resection is good and has a high 5-year survival rate. However, for those tumors classified as type III and IV tumors, the surgical prognosis is poor even when combined with local tumor resection and left or right hemi-lobectomy of the liver, which can itself lead to further complications. Thus, finding a surgical approach for the treatment of type III and IV Klatskin tumors is problemtic.
Percutaneous image-guided radiofrequency ablation (RFA) has received increasing attention as a promising technique for the treatment of liver tumors. This technique permits the destruction of tumors without necessitating their removal, and in many cases, can be used in place of more invasive and expensive surgical treatment. Initial attempts at tissue ablation with radiofrequency have been limited to the 1.6-cm diameter coagulation necrosis obtained from a single conventional electrode. In order to achieve larger thermal necrosis, internally cooled, single or clustered electrode technique, as well as expandable needle techniques have been introduced.
To date, all 11 patients are alive. A recent follow-up shows that the survival range after RFA therapy is 18 mo on average, ranging between 10 mo and 30 mo so far. No complications among these 11 cases were observed during or after synthetic sequential interventional therapy. Compared with surgical intervention, the RFA treatment that we used for type III and IV Klatskin’s tumor is less invasive and involves less complications.
RFA is a less invasive, safe, effective and promising therapy for type III and IV Klatskin tumors. The long-term curative effect requires further study.
According to the study, we know that a new therapy for type III and IV Klatskin tumor, and RFA is less invasive, soft and have fewer complications than common surgical treatment. It is worth paying attention and we are looking forward to the better curative effect from RFA.
| 1. | Becker T, Lehner F, Bektas H, Meyer A, Luck R, Nashan B, Klempnauer J. [Surgical treatment for hilar cholangiocarcinoma (Klatskin's tumor)]. Zentralbl Chir. 2003;128:928-935. |
| 3. | Golfieri R, Giampalma E, Fusco F, Galuppi A, Faccioli L, Galaverni C, Frezza G. Unresectable hilar cholangiocarcinoma: multimodality treatment with percutaneous and intraluminal plus external radiotherapy. J Chemother. 2004;16 Suppl 5:55-57. |
| 4. | Lygidakis NJ, Singh G, Bardaxoglou E, Dedemadi G, Sgourakis G, Nestoriois J, Malliotakis A, Pedonomou M, Safioleas M, Solomou EK. Changing trends in the management of Klatskin tumor. Hepatogastroenterology. 2004;51:689-696. |
| 5. | Launois B, Terblanche J, Lakehal M, Catheline JM, Bardaxoglou E, Landen S, Campion JP, Sutherland F, Meunier B. Proximal bile duct cancer: high resectability rate and 5-year survival. Ann Surg. 1999;230:266-275. |
| 6. | Bismuth H, Nakache R, Diamond T. Management strategies in resection for hilar cholangiocarcinoma. Ann Surg. 1992;215:31-38. |
| 7. | Liu CL, Fan ST. Anterior approach for right hepatectomy for hilar cholangiocarcinoma. J Hepatobiliary Pancreat Surg. 2003;10:292-294. |
| 8. | Nagino M, Kamiya J, Uesaka K, Sano T, Yamamoto H, Hayakawa N, Kanai M, Nimura Y. Complications of hepatectomy for hilar cholangiocarcinoma. World J Surg. 2001;25:1277-1283. |
| 9. | Goldberg SN, Solbiati L, Hahn PF, Cosman E, Conrad JE, Fogle R, Gazelle GS. Large-volume tissue ablation with radio frequency by using a clustered, internally cooled electrode technique: laboratory and clinical experience in liver metastases. Radiology. 1998;209:371-379. |
| 10. | Klatskin G. Adenocarcinoma of the hepatic duct at its bifurcation within the porta hepatitis. An unusual tumour with distinctive clinical and pathological features. Am J Med. 1965;38:241-256. |
| 11. | Bismuth H, Castaing D, Traynor O. Resection or palliation: priority of surgery in the treatment of hilar cancer. World J Surg. 1988;12:39-47. |
| 12. | Parc Y, Frileux P, Balladur P, Delva E, Hannoun L, Parc R. Surgical strategy for the management of hilar bile duct cancer. Br J Surg. 1997;84:1675-1679. |
| 13. | Gerhards MF, van Gulik TM, Bosma A, ten Hoopen-Neumann H, Verbeek PC, Gonzalez Gonzalez D, de Wit LT, Gouma DJ. Long-term survival after resection of proximal bile duct carcinoma (Klatskin tumors). World J Surg. 1999;23:91-96. |
| 14. | Zovak M, Doko M, Glavan E, Hochstadter H, Roic G, Ljubicic N. Klatskin tumor--results of surgical therapy. Coll Antropol. 2004;28:317-323. |
| 15. | Ruiz E, Celis J, Sullon J. [Surgical treatment of Klatskin's tumor]. Rev Gastroenterol Peru. 1995;15:167-175. |
| 16. | Santoro E, Sacchi M, Carboni F, Santoro R, Scardamaglia F. Diagnostic and surgical features of Klatskin tumors. Chir Ital. 1999;51:1-7. |
| 17. | Tibble JA, Cairns SR. Role of endoscopic endoprostheses in proximal malignant biliary obstruction. J Hepatobiliary Pancreat Surg. 2001;8:118-123. |
| 18. | Lillemoe KD. Current status of surgery for Klatskin tumors. Curr Opin Gen Surg. 1994;8:161-167. |
| 19. | Acalovschi M. Cholangiocarcinoma: risk factors, diagnosis and management. Rom J Intern Med. 2004;42:41-58. |
| 20. | Yi B, Zhang BH, Zhang YJ, Jiang XQ, Zhang BH, Yu WL, Chen QB, Wu MC. Surgical procedure and prognosis of hilar cholangiocarcinoma. Hepatobiliary Pancreat Dis Int. 2004;3:453-457. |
| 21. | Iwatsuki S, Todo S, Marsh JW, Madariaga JR, Lee RG, Dvorchik I, Fung JJ, Starzl TE. Treatment of hilar cholangiocarcinoma (Klatskin tumors) with hepatic resection or transplantation. J Am Coll Surg. 1998;187:358-364. |
| 22. | Robles R, Parrilla P, Bueno FS, Ramirez P, Lujan JA, Rodriguez JM, Acosta F, Lopez J, Hernandez Q. Liver transplantation in the management of Klatskin's tumor. Transplant Proc. 1999;31:2494-2495. |
| 23. | Born P, Rosch T, Bruhl K, Sandschin W, Weigert N, Ott R, Frimberger E, Allescher HD, Hoffmann W, Neuhaus H. Long-term outcome in patients with advanced hilar bile duct tumors undergoing palliative endoscopic or percutaneous drainage. Z Gastroenterol. 2000;38:483-489. |
| 24. | Shimoda M, Farmer DG, Colquhoun SD, Rosove M, Ghobrial RM, Yersiz H, Chen P, Busuttil RW. Liver transplantation for cholangiocellular carcinoma: analysis of a single-center experience and review of the literature. Liver Transpl. 2001;7:1023-1033. |
| 25. | McDougall NI, Edmunds SE. An audit of metal stent palliation for malignant biliary obstruction. J Gastroenterol Hepatol. 2001;16:1051-1054. |
| 26. | Berr F, Tannapfel A, Lamesch P, Pahernik S, Wiedmann M, Halm U, Goetz AE, Mossner J, Hauss J. Neoadjuvant photodynamic therapy before curative resection of proximal bile duct carcinoma. J Hepatol. 2000;32:352-357. |
| 27. | Wiedmann M, Caca K, Berr F, Schiefke I, Tannapfel A, Wittekind C, Mossner J, Hauss J, Witzigmann H. Neoadjuvant photodynamic therapy as a new approach to treating hilar cholangiocarcinoma: a phase II pilot study. Cancer. 2003;97:2783-2790. |
| 28. | Gazelle GS, Goldberg SN, Solbiati L, Livraghi T. Tumor ablation with radio-frequency energy. Radiology. 2000;217:633-646. |
| 29. | Wiedmann M, Dietrich A, Mossner J, Witzigmann H, Caca K. Combined percutaneous transhepatic biliary drainage with port implantation for management of patients with malignant biliary obstruction. Gastrointest Endosc. 2004;60:117-120. |