INTRODUCTION
Patients with ulcerative colitis (UC) and Crohn’s disease (CD), the major forms of inflammatory bowel disease (IBD) in humans, are at increased risk of developing colorectal cancer (CRC)[12]. Chronic inflammation is believed to be the driving force for neoplastic transformation, and clinical factors that increase the risk include disease duration > 10 years, extensive disease, severity of colitis, a positive family history of sporadic CRC, and the concomitant presence of primary sclerosing cholangitis[3–5].
While in sporadic CRC, the dysplastic precursor is usually the adenomatous polyp, dysplasia in IBD patients can be both polypoid and flat, localized or diffuse. Detection of dysplasia during programmed screening and surveillance colonoscopy is the goal of the current strategy for CRC prevention in IBD patients. When dysplasia or CRC is identified, proctocolectomy is performed to remove the at-risk organ[6]. However, no evidence exists that screening for colonic dysplasia and cancer with surveillance colonoscopy prolongs survival in IBD patients. Indirect evidence also suggests that this is a cost-effective approach[78]. Therefore, gastroenterologists have recently shifted their attention towards alternative strategies of chemoprevention, and particular interest has been given to the possibility of reducing the risk of IBD-related CRC by using mesalazine or 5-aminosalicylic acid (5-ASA). Mesalazine is widely used in the maintenance of remission and in the treatment of mild flare-ups of IBD, and recent epidemiological studies have suggested that this drug is chemopreventive for CRC development in UC patients[9–11], even though some studies have documented no benefit[12]. The mechanisms behind the antineoplastic effect of mesalazine are incompletely understood, but it is likely that they are mostly dependent on the ability of the drug to attenuate ongoing mucosal inflammation. Indeed, it is well known that mesalazine can modulate various inflammatory pathways (e.g. production of inflammatory cytokines, activity of inducible nitric oxide synthase, activation of nuclear factor-κB) that are relevant to CRC initiation and progression[13–16]. There is also evidence that mesalazine inhibits the formation of reactive oxygen species (ROS) from polymorphonuclear leukocytes[17], which leads to a decrease or complete inhibition of DNA damage, a phenomenon that has been involved in colon carcinogenesis. More recently, it has also been shown that mesalazine can directly target CRC cells and interfere with biological pathways that control their growth and survival[18]. The object of this article is to summarize recent data that elucidate the basic mechanisms of the antineoplastic effect of mesalazine.
MESALAZINE HAS DIRECT INHIBITORY EFFECTS ON CRC CELL GROWTH AND SURVIVAL
Carcinogenesis is a complex and multistage process that involves interactions between genes and environmental insults which ultimately affect cell proliferation and apoptosis. Apoptosis progressively decreases and proliferation increases in the sequential stages from normal colonic mucosa to dysplastic and CRC tissue. Therefore, strategies that inhibit cell proliferation and/or restore cell susceptibility to apoptosis have been shown to be effective in interfering with CRC initiation and/or progression.
Accumulating evidence indicates that mesalazine can block growth and promote apoptosis of CRC cells. This was initially suggested by ex vivo studies in patients with colonic adenoma. In particular, Reinacker-Schick et al have analyzed the effect of orally administered mesalazine on apoptosis and proliferation of colorectal mucosa in 21 patients with sporadic polyps. An increase in the apoptotic rate and decrease in cell proliferation were seen 1 and 3 d, respectively, after the initiation of treatment with mesalazine[19]. Bus et al have demonstrated that 2 wk treatment with 4 g/d mesalazine enema in patient with sporadic CRC resulted in enhanced apoptosis of tumor cells, while no change was seen in the normal mucosa that surrounded the tumor lesion. Moreover, the cellular proliferation rate as assessed by means of Ki-67 expression was unchanged in both the tumor and normal tissue[20]. Studies in rodent models of CRC showed that mesalazine inhibits tumor growth and reduces the number of aberrant cryptic foci[2122]. Moreover, in a mouse model of colitis-associated CRC, Ikeda et al have shown that mesalazine, given in the remission stage of colitis, markedly suppresses the number and size of neoplasms. Notably, mesalazine treatment reduces the rate of proliferation of tumor cells, which leaves the proliferation of normal epithelial cells unaltered[23]. These observations have been reinforced by in vitro studies that show that mesalazine inhibits the growth and enhances apoptosis of several cultured CRC cell lines, in a time- and dose-dependent manner[1824]. Altogether, these later findings indicate that mesalazine has direct effects on CRC cells. This novel information has boosted new research aimed at dissecting the molecular mechanisms by which mesalazine interferes with CRC development/growth.
EFFECTS OF MESALAZINE ON REPLICATION FIDELITY
Many of the molecular alterations that are believed to play a major role in the development of sporadic CRC are also seen in IBD-associated CRC tissue. For instance, both these types of CRC are characterized by a very similar frequency of the two main types of genomic instability, namely chromosomal instability (CIN, 85%) and microsatellite instability (MSI, 15%)[25]. CIN results in abnormal segregation of chromosomes and abnormal DNA content (aneuploidy). As a result, loss of chromosomal material often occurs, which contributes to the loss of function of important tumor suppressor genes [e.g. adenomatous polyposis coli (APC) and p53]. The MSI pathway involves the primary loss of function of genes that usually repair DNA base-pair mismatches that occur during the normal process of DNA replication in dividing cells. During this process, frameshift mutations, called microsatellites, tend to accumulate. Since microsatellites are mainly located in intronic DNA sequences, microsatellite mutations generally result in no gene function alteration. However, if microsatellites are located in exonic gene regions, the mutations can lead to a shift in the codon reading frame and changes in the amino acid sequence during mRNA translation. The introduction of an early stop codon can eventually cause protein truncation, and this is generally associated with a loss of protein function[26]. Recently, Gasche and colleagues, using an assay based on a stable transfection of a plasmid carrying a microsatellite sequence into HCT-116 cells, have shown that mesalazine improves replication fidelity in cultured CRC cell lines by reducing frameshift mutations at microsatellites. Since the effect of mesalazine is seen in mismatch repair-deficient CRC cells, it is highly likely that mesalazine acts on replication fidelity independently of post-replicational mismatch repair. The molecular mechanism by which mesalazine inhibits the generation of frameshift mutations has not yet been characterized. However, studies by the same group have shown that mesalazine can interact with cellular machinery involved in cell-cycle progression[28]. In particular, it has been shown that mesalazine can slow down DNA replication and cell division, thus allowing cells to either repair DNA damage or undergo apoptosis. Analysis of cell-cycle phases has revealed that CRC cells are arrested in S-phase when treated with mesalazine for 24-48 h. Such results are, however, somewhat different from those published by Reinacher-Schick et al that have shown that CRC cells are arrested in G2-M phase when exposed to mesalazine[18]. The reasons for these discrepancies remain unclear, but it is likely that they are due to differences in the culture systems used by these investigators.
THE ANTI-MITOGENIC EFFECT OF MESALAZINE IS NOT STRICTLY DEPENDENT ON CYCLOOXYGENASE (COX)-2 INHIBITION
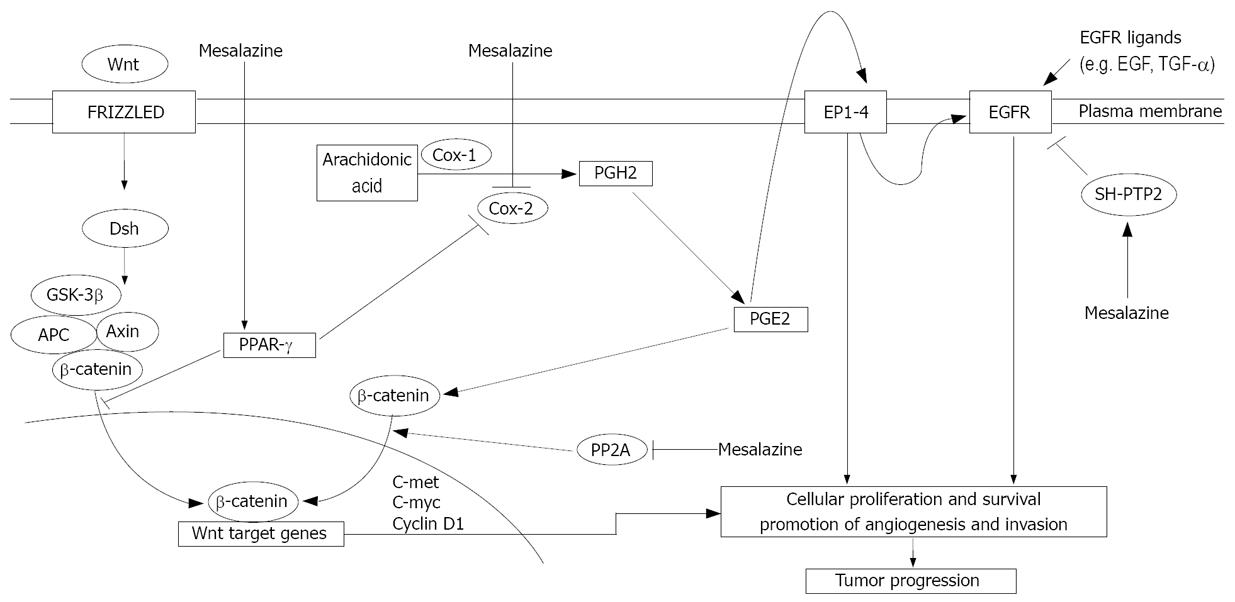
COX-2 is a major target of CRC chemopreventive programs, as it is highly expressed in both sporadic and familial CRC, and activation of COX-2 is known to trigger and/or amplify biological pathways that sustain CRC growth[29–32]. COX-2 is also over-expressed in IBD-related CRC tissue[33]. Since mesalazine inhibits COX-2 in inflammatory cells, it is hypothesized that the antineoplastic effect of this drug is strictly dependent on the inhibition of COX-2 in CRC cells. In this context, we have recently shown that mesalazine inhibits the growth of HT-115, a CRC cell line that expresses a functionally active COX-2, and that the anti-mitogenic effect of mesalazine is associated with a marked down-regulation of COX-2 at the protein and mRNA level[34]. Consistently, treatment of HT-115 cells with mesalazine causes a significant reduction in secretion of prostaglandin (PG) E2, a product of COX-2 activity that positively regulates CRC cell growth (Figure 1). However, exogenously added PGE2 does not abrogate the inhibitory effect of mesalazine on HT-115 cell growth. Moreover, mesalazine blocks the growth of DLD-1, a CRC cell line that does not express COX-2, which suggests that the effect of this drug on CRC cell growth is partially independent of inhibition of the COX-2/PGE2 axis.
Figure 1 Some putative molecular mechanisms that underlie the antineoplastic activity of mesalazine.
Mesalazine inhibits COX-2, thereby blocking synthesis of PGH2, an intermediate that is metabolized into various prostaglandins, including PGE2. PGE2 binds to its cell surface cognate receptors (EP1-4) and sustains various functions of tumor cells, including proliferation, survival, angiogenesis and invasion. These are mediated through the transactivation of EGFR or activation of other intracellular pathways. The antineoplastic effects of mesalazine rely also on its ability to inhibit Wnt/β-catenin, through inactivation of PP2A (and downstream down-regulation of β-catenin), and EGFR pathways. Moreover, mesalazine activates PPAR-γ, thus leading to inactivation of the Wnt/β-catenin pathway and down-regulation of COX-2.
MESALAZINE INHIBITS BOTH THE WNT/β-CATENIN AND EPIDERMAL GROWTH FACTOR RECEPTOR (EGFR) SIGNALING PATHWAYS IN CRC CELLS
An intriguing hypothesis that has emerged from the available experimental data is that mesalazine may act on one or more pathways that are both early and common in colorectal carcinogenesis. A potential candidate is the wingless and integration site growth factor (Wnt)/β-catenin pathway, since it is constitutively activated in the majority of CRC[35–37]. In this pathway, Wnt binds to the transmembrane Frizzled receptor, which leads to activation of the cytoplasmic disheveled (Dsh) protein. Dsh forms a complex with the β-catenin degradation complex, which consists of the APC gene product, glycogen synthase kinase-3β (GSK-3β), axin and β-catenin. The current model for the Wnt signaling pathway proposes that, in the absence of Wnt, GSK-3β phosphorylates β-catenin, thereby promoting ubiquitination and degradation of β-catenin[38]. In response to Wnt signals, β-catenin is no longer targeted for degradation and accumulates to high levels in the cytoplasm[39]. The accumulated β-catenin translocates to the nucleus, associates with the transcriptional enhancers of the lymphoid enhancer-binding factor/Tcf family, and stimulates the expression of genes, such as Myc, that play important roles in tumor progression[4041].
Elegant studies by Bos et al have shown that mesalazine inhibits the Wnt/β-catenin pathway in APC-mutated CRC cells with intact β-catenin (Figure 1)[42]. Consistent with this, mesalazine treatment reduces expression of nuclear β-catenin and Wnt/β-catenin target genes (e.g. cyclin D1, c-met and c-Myc), and increased β-catenin phosphorylation. Mesalazine fails to inhibit the expression of Wnt/β-catenin target genes in β-catenin mutant CRC cell lines, in which the Wnt/β-catenin pathway is not regulated by β-catenin phosphorylation. These observations suggest that inhibition of the Wnt/β-catenin pathway by mesalazine is dependent on increased phosphorylation of β-catenin. In line with this, pre-incubation of CRC cells with okadaic acid, a specific phospho-serine/phospho-threonine phosphatase inhibitor, prevents mesalazine-induced β-catenin phosphorylation. The precise mechanism by which mesalazine enhances β-catenin phosphorylation remains to be ascertained, even though Bos et al have demonstrated that mesalazine reduces the activity of protein phosphatase 2A (PP2A), a known regulator of the β-catenin phosphorylation status and Wnt/β-catenin pathway in CRC cells.
Another target of mesalazine is EGFR (Figure 1). This receptor is highly activated in CRC cells, in which it is supposed to trigger mitogenic and pro-survival signals[4344]. Consistent, EGFR inhibitors, such as monoclonal antibodies or small molecules that inhibit tyrosine kinase activity, have been shown to be effective in patients with advanced CRC[45]. A very high percentage of IBD-associated CRC displays immunohistochemical positivity for EGFR. Expression of EGFR is frequent in IBD-associated intestinal cancer[46]. Expression occurs at an early stage (i.e. premalignant lesions), as described in sporadic CRC[47], which suggests that blocking EGFR activity is useful for reducing the occurrence of IBD-related CRC[4849]. We have shown that exposure of CRC cell lines to mesalazine results in a marked suppression of EGFR phosphorylation/activation, and that mesalazine-induced EGFR dephosphorylation is dependent on neither CRC cell death induction nor shedding of the receptor[50]. Moreover, mesalazine suppresses EGFR activation induced by exogenous EGF or TGF-α, thus excluding the possibility that dephosphorylation of EGFR in mesalazine-treated cells is secondary to inhibition of EGFR ligand synthesis. EGFR phosphorylation is a tightly controlled phenomenon, which is the net result of the action of tyrosine kinase and phosphatases (PTPs). Therefore, we have examined whether mesalazine-mediated inhibition of EGFR activation reflects changes in the expression/activity of PTPs, which have been reported to control the extent of EGFR activation[51]. Notably, treatment of CRC cells with mesalazine causes a significant increase in the activity, but not expression, of phosphorylated-EGFR-targeting PTPs, and pre-incubation of cells with PTP inhibitors largely reduces the inhibitory effect of mesalazine on EGFR activation. Among these PTPs, both SH-PTP1 and SH-PTP2 interact with EGFR upon mesalazine treatment. However, targeted silencing of SH-PTP2, but not SH-PTP1 prevents mesalazine-induced EGFR dephosphorylation. Consistent with these data, mesalazine also attenuates EGFR phosphorylation in ex vivo organ cultures of human sporadic CRC explants.
MESALAZINE ACTIVATES PEROXISOME PROLIFERATOR ACTIVATED RECEPTOR-γ (PPAR-γ) IN CRC CELLS
PPAR-γ is a nuclear receptor that is highly expressed in the colon, and plays a key role in bacteria-induced inflammation. Many factors can modulate the activity of PPAR-γ, but the most important activating factor in colon epithelial cells appears to be the luminal flora[52]. Activation of PPAR-γ also has anti-tumorigenic effects that are manifested as both anti-proliferative and pro-apoptotic activities[5354], inhibition of the formation of aberrant cryptic foci[55], and inhibition of CRC development[56]. It has also been shown that PPAR-γ suppresses tumor formation by interfering with the Wnt/β-catenin signaling pathway[5758]. Recent in vitro and in vivo studies have shown that mesalazine can activate PPAR-γ (Figure 1). In particular, using HT-29 CRC cells, Rousseaux et al have shown that mesalazine enhances PPAR-γ expression, stimulates translocation of PPAR-γ to the nucleus, induces conformational changes in the PPAR-γ molecule, and increases the interaction between PPAR-γ and vitamin D3 receptor-interacting protein 205[59]. In competitive binding studies, mesalazine displaces rosiglitazone and the selective PPAR-γ ligand GW1929 from their binding sites on the PPAR-γ molecule[59]. In line with these in vitro findings, it has been shown that the antineoplastic effects of mesalazine are mediated by PPAR-γ in a model of CRC, in which SCID mice were engrafted with human CRC cells. In particular, in this model, locally administered mesalazine significantly reduced the growth of xenografts, and this effect was blocked by the selective PPAR-γ antagonist GW9662[60].
CONCLUSION
In recent years, there has been great interest in the possibility of chemoprevention of IBD-related CRC by mesalazine. Given the difficulty of performing double-blind, placebo-controlled, randomized clinical trials in patients, investigators have turned to experimental models of cancer, and indeed, the existing data suggest that mesalazine can reduce the risk of CRC by directly interfering with CRC cell biology, other than by simply controlling inflammation. However, definitive conclusions from experimental findings are not always completely correct, and therefore, future studies will be necessary to ascertain whether data generated from studies with cultured cells or animal models of CRC can be generalized to IBD-associated dysplasia or CRC.
Another important issue that needs further investigation regards the dosage/concentration of mesalazine required to interfere with CRC cell growth and survival. In vitro studies have indicated that the antineoplastic effect of mesalazine is seen with relative high drug dosages (e.g. 10-50 mmol/L), which are not always reached within the colonic tissue under standard oral treatment. In this context, it is also relevant to take into consideration mesalazine metabolism, for instance, mesalazine oxidation and acetylation, which could differ considerably between in vitro and in vivo circumstances, and limit the amount of biologically active compound.
Peer reviewer: Tamara Cacev, MSc, Division of Molecular
Medicine, Rudjer Boskovic Institute, Bijenicka c. 54, Zagreb
10000, Croatia