Published online Jul 21, 2008. doi: 10.3748/wjg.14.4352
Revised: June 10, 2008
Accepted: June 17, 2008
Published online: July 21, 2008
AIM: To study the influence of tumor removal on the serum level of IgG antibodies to tumor-associated Thomsen-Friedenreich (TF), Tn carbohydrate epitopes and xenogeneic αGal, and to elucidate on the change of the level during the follow-up as well as its association with the stage and morphology of the tumor and the values of blood parameters in gastrointestinal cancer.
METHODS: Sixty patients with gastric cancer and 34 patients with colorectal cancer in stages I-IV without distant metastases were subjected to follow-up. The level of antibodies in serum was determined by the enzyme-linked immunosorbent assay (ELISA) using synthetic polyacrylamide (PAA) glycoconjugates. Biochemical and haematological analyses were performed using automated equipment.
RESULTS: In gastrointestinal cancer, the TF antibody level was found to have elevated significantly after the removal of G3 tumors as compared with the preoperative level (u = 278.5, P < 0.05). After surgery, the TF and Tn antibody level was elevated in the majority of gastric cancer patients (sign test, 20 vs 8, P < 0.05, and 21 vs 8, P < 0.05, respectively). In gastrointestinal cancer, the elevated postoperative level of TF, Tn and αGal antibodies was noted in most patients with G3 tumors (sign test, 22 vs 5, P < 0.01; 19 vs 6, P < 0.05; 24 vs 8, P < 0.01, respectively), but the elevation was not significant in patients with G1 + G2 resected tumors. The postoperative follow-up showed that the percentage of patients with G3 resected tumors of the digestive tract, who had a mean level of anti-TF IgG above the cut-off value (1.53), was significantly higher than that of patients with G1 + G2 resected tumors (χ2 = 3.89, all patients; χ2 = 5.34, patients without regional lymph node metastases; P < 0.05). The percentage of patients with a tumor in stage I, whose mean anti-TF IgG level remained above the cut-off value (1.26), was significantly higher than that of patients with the cancer in stages III-IV (χ2 = 4.71, gastric cancer; χ2 = 4.11, gastrointestinal cancer; P < 0.05). The correlation was observed to exist between the level of anti-TF IgG and the count of lymphocytes (r = 0.517, P < 0.01), as well as between the level of anti-Tn IgG and that of serum CA 19-9 (r = 0.481, P < 0.05). No positive delayed-type hypersensitivity reaction in skin test challenges with TF-PAA in any of the fifteen patients, including those with a high level of anti-TF IgG, was observed.
CONCLUSION: The surgical operation raises the level of anti-carbohydrate IgG in most patients, especially in those with the G3 tumor of the gastrointestinal tract. The follow-up demonstrates that after surgery the low preoperative level of TF antibodies may be considerably increased in patients with the carcinoma in its early stage but remains low in its terminal stages. The stage- and morphology-dependent immunosuppression affects the TF-antibody response and may be one of the reasons for unresponsiveness to the immunization with TF-antigens.
- Citation: Smorodin EP, Kurtenkov OA, Sergeyev BL, Kodar KE, Chuzmarov VI, Afanasyev VP. Postoperative change of anti-Thomsen-Friedenreich and Tn IgG level: The follow-up study of gastrointestinal cancer patients. World J Gastroenterol 2008; 14(27): 4352-4358
- URL: https://www.wjgnet.com/1007-9327/full/v14/i27/4352.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.4352
Cancer immune surveillance is considered to be important in the anti-tumor protection of the host. However, immunity not only protects the host from cancer but also may promote tumor growth, creating an immuno-resistant cancer cell phenotype (“cancer immunoediting”). In the advanced stages of cancer, tumor escapes the immune control under the immunosuppressive conditions[1]. The removal of the tumor appears to disturb the immunoediting process and may reverse immunosuppression. The suppressed antigen-specific antibody responses in tumor-bearing mice may be reversed after the surgical removal of the primary tumor even in the existence of a disseminated metastatic disease[2].
Mucin-type tumor-associated carbohydrate antigens (TACA), the Thomsen-Friedenreich (TF) antigen and its precursor, Tn, are frequently expressed in malignant tumor cells. They deserve to be studied as targets for active specific immunotherapy[3]. Human blood serum contains natural TF- and Tn antibodies whose subpopulations may bind the corresponding antigens on human tumor cell lines[3–5]. It is not yet clear which role antibodies play in the natural anti-cancer defense system. The level of TF- and Tn antibodies was significantly decreased in the serum of primary (not operated) patients with cancer, including patients with the disease in its early stage[6–9]. Furthermore, the low level of anti-TF IgG was associated with a lower differentiated carcinoma and advanced gastric cancer that suggests an implication for antibodies in the progression and pathology of the tumor[10]. The high level of anti-TF IgG in the serum of primary patients with gastric cancer is closely associated with survival[11]. The dynamic changes of the level of TF- and Tn-antibodies in the serum of patients with cancer and its association with survival have been insufficiently studied. Investigations have mainly focused on clinical trials of antigen-specific immunotherapy[1213]. The authors have undertaken a long-term follow-up of cancer patients to determine changes in the postoperative level of TF- and Tn antibodies, as well as to elucidate the association of this level with the progression of cancer, and survival. For comparison, the level of xenoreactive antibodies to the αGal epitope was determined. In humans, the αGal epitope is absent because the α1-3 galactosyltransferase gene was inactivated in evolution, but approximately 1% of immunoglobulins have an anti-αGal specificity[14].
In this work, the influence of the surgical removal of the tumor on the level of antibodies and its change during the follow-up was investigated. The association of the level of antibodies with the stage and morphology of the tumor, as well as values of blood parameters was studied. Also, the delayed-type hypersensitivity reaction to TF-PAA conjugates in skin testing was examined.
The investigation was carried out in accordance with the ICH GCP Standards and approved by Tallinn Medical Research Ethics Committee. Informed consent was obtained from subjects under study. The follow-up study was undertaken of patients with a verified diagnosis of gastric (n = 60) and colorectal cancer (n = 34) of stages I-IV by using the pTNM system[15]. Patients with distant metastases of the cancer or those who received chemo- and X-ray therapy were not subjected to study. The median age of the patients was 60 years (the age ranging from 30 to 75 years). The venous blood samples were taken before and after surgery at intervals from three to sixteen months, with a further follow-up during two to twelve years. The extended D2 gastrectomy with lymphadenectomy or, additionally, with the splenectomy in gastric cancer, as well as the resection of local lesions of colorectal cancer was performed. In advanced cancer regional lymph node metastases were also removed. In some patients concomitant diseases were documented. Breast cancer was diagnosed in three, benign diseases, in five, anaemia and diabetes mellitus, in two cases. The other sporadic manifestations were Parkinson’s disease, carcinoma of the uterus and chronic hepatitis.
Synthetic polyacrylamide (PAA) glycoconjugates with a single reiterative epitope were used in comparative immunoassays[16]. The homogeneity of PAA-conjugates enables a precise detection of epitope-specific antibodies. The following PAA-conjugates were used: the TF disaccharide, Galβ1-3GalNAcα; Tββ, Galβ1-3GalNAcβ; Tn, GalNAcα; αGal or a B-blood group disaccharide, Galα1-3Galβ; SiaLea (CA 19-9 tetrasaccharide), Neu5Acα2-3Galβ1-3 (Fucα1-4) GlcNAcβ. Tris-PAA, tris (hydroxymethyl) aminomethane-PAA, was used as a negative control because of its low background and good reproducibility in immunoassay[8]. The TF-PAA as a substituted PAA containing 0.1 mol of TF per 1 mol of PAA was used because of its elevated binding to human IgG antibodies. The rest of the polyacrylethanolamide-conjugates had 0.2 mol of a saccharide per 1 mol of PAA. All PAA-conjugates were received from Lectinity, Russia.
The method has been described elsewhere[8]. The dilution of serum was 1:50-1:200. The antibody levels were calculated as a ratio Atest/Acontrol where Atest is the absorbance with PAA-glycoconjugate and Acontrol, with Tris-PAA. The variation coefficient was 3%. To diminish the variation of the test, the serum samples taken before and after surgery were analyzed using the same plate.
Biochemical and hematological analyses were performed at the Oncological Centre of the North-Estonian Regional Hospital. The following automatic equipment was used: a Hitachi 912 and Elecsys 2010, Roche Diagnostics; a Sysmex XE-2100, Sysmex Corporation.
Blood samples were taken before and after surgical operation during the planned visits to the physician for health control. The antibody levels were correlated with those of the C-reactive protein (CRP), tumor markers (CA19-9, CEA), the alanine aminotransferase, glucose, haemoglobin, circulating red blood cells (count), leukocytes (count), neutrophils (%, count), monocytes (%), lymphocytes (%, count), platelets (count) and eosinophils (%). The concentration of CRP was determined by a turbidimetric method and that of tumor markers, by an electrochemiluminescence immunoassay.
Antigens: TF-PAA, Mr 30 and 1000 ku; Tββ-PAA, Mr 1000 ku. The antigens (50-100 &mgr;g) were injected intradermally and the delayed-type hypersensitivity reaction was monitored twice: through 24 and 48 h. The reaction was considered positive if erythema > 5 mm was developed.
The Mann-Whitney (U-test), the sign test with a null hypothesis (median = 0) for a paired-sample comparison, the Chi-squared test and the regression analysis were used in the study. The differences were considered significant when P < 0.05. The graphs were plotted by means of a SigmaPlot 2000 program and Statgraphics Plus 5.1.
To investigate the effect of the surgical removal of the tumor on antibody levels, the serum samples taken before and after surgery were analyzed. The subtracted values of the postoperative minus preoperative level of antibodies varied and showed an abnormal distribution, however, were mostly positive. The median of differences was also positive (Tables 1 and 2). A sign test for a paired-sample comparison showed the postoperative level of TF- and Tn antibodies to have significantly increased in patients with gastric cancer. Patients with gastric cancer, whose postoperative level of TF- and Tn antibodies was higher than the preoperative one, predominated significantly over those having a lower postoperative level (Table 1, P < 0.05). In gastrointestinal cancer, the TF antibody level was found to have elevated significantly after the removal of G3 tumors as compared with preoperative level (median 1.42 and 1.23, respectively, u = 278.5, P < 0.05). The elevation of the level of anti-Tn and αGal IgG after surgery was not significant in U-test owing to the variation in levels. However, the paired-sample sign test demonstrated a significant (more than 75%) predomination of patients having a postoperative elevation of TF, Tn or αGal antibody level over those having a lower postoperative level after resection of G3 tumors. These differences were not significant in patients with G1 and G2 resected tumors (Table 2). The level of all three antibodies was increased after surgery in 12% (mainly patients having G3 tumors) and reduced in 4% of patients with gastrointestinal cancer. The change of antibody levels was not associated with the transfusion of erythrocytes after surgery.
| Grade1 | Detection of antibodies | Mean ± SD | Increase | Median of difference | Difference | P (sign test) | |
| + | - | ||||||
| G1 + G2 (n = 24) | TF, preoperative | 2.03 ± 2.04 | |||||
| postoperative | 2.43 ± 2.86 | 19.7% | 0.05 | 15 | 9 | ||
| G3 (n = 27) | TF, preoperative | 1.84 ± 1.71 | |||||
| postoperative | 3.03 ± 3.20 | 64.7% | 0.29 | 22 | 5 | < 0.01 | |
| G1 + G2 (n = 30) | Tn, preoperative | 2.46 ± 1.80 | |||||
| postoperative | 2.99 ± 2.45 | 21.5% | 0.12 | 18 | 12 | ||
| G3 (n = 25) | Tn, preoperative | 2.45 ± 2.18 | |||||
| postoperative | 3.15 ± 2.92 | 28.6% | 0.42 | 19 | 6 | < 0.05 | |
| G1 + G2 (n = 32) | αGal, preoperative | 4.10 ± 2.55 | |||||
| postoperative | 4.62 ± 3.20 | 12.7% | 0.17 | 20 | 12 | ||
| G3 (n = 32) | αGal, preoperative | 5.09 ± 3.34 | |||||
| postoperative | 6.61 ± 3.70 | 29.9% | 1.20 | 24 | 8 | < 0.01 | |
The clustered distribution of the combined pre- and postoperative level of antibodies was observed. The clusters were separated with the following cut-off values: TF 1.26, 1.53; Tn 1.88, 2.38; αGal 2.18; 2.80. Patients whose level of antibodies was higher than the first cut-off value were considered responders. The follow-up showed that the percentage of patients with G3 resected tumors of the digestive tract, whose individual mean level of anti-TF IgG remained above the cut-off value, exceeded significantly the percentage of patients with G1 + G2 resected tumors (Table 3). In the case of the other antibodies these differences were not observed.
| Cancer | G1 + G2 | G3 | χ2 | P | ||
| n | > cut-off | n | > cut-off | |||
| Gastric, all | 22 | 9.1% | 38 | 26.3% | 2.58 | 0.108 |
| Gastric, without metastases (N0) | 16 | 6.2% | 22 | 31.8% | 3.64 | 0.056 |
| Gastrointestinal, all | 47 | 8.5% | 47 | 23.4% | 3.89 | 0.049 |
| Gastrointestinal (N0) | 35 | 5.7% | 26 | 26.9% | 5.34 | 0.021 |
In gastric or gastrointestinal cancer, the percentage of anti-TF IgG responders was significantly higher in stage I than in stages III-IV (Table 4). In gastric cancer without metastases (N0 in stages I-II vs N1 + N2) the percentage of anti-TF IgG responders tended to increase. The proportion of Tn- or αGal-responders was not increased in stage I vs stages III-IV of the disease.
| Cancer | n | > cut-off | χ2 | P |
| Gastric, N0 | 38 | 39.5% | 2.92 | 0.088 |
| Gastric, N1 + N2 | 22 | 18.9% | ||
| Gastric, stage I | 24 | 41.7% | 4.71 | 0.03 |
| Gastric, stages III-IV | 18 | 11.1% | ||
| Gastrointestinal, stage I | 29 | 37.9% | 4.11 | 0.043 |
| Gastrointestinal, stages III-IV | 28 | 14.3% |
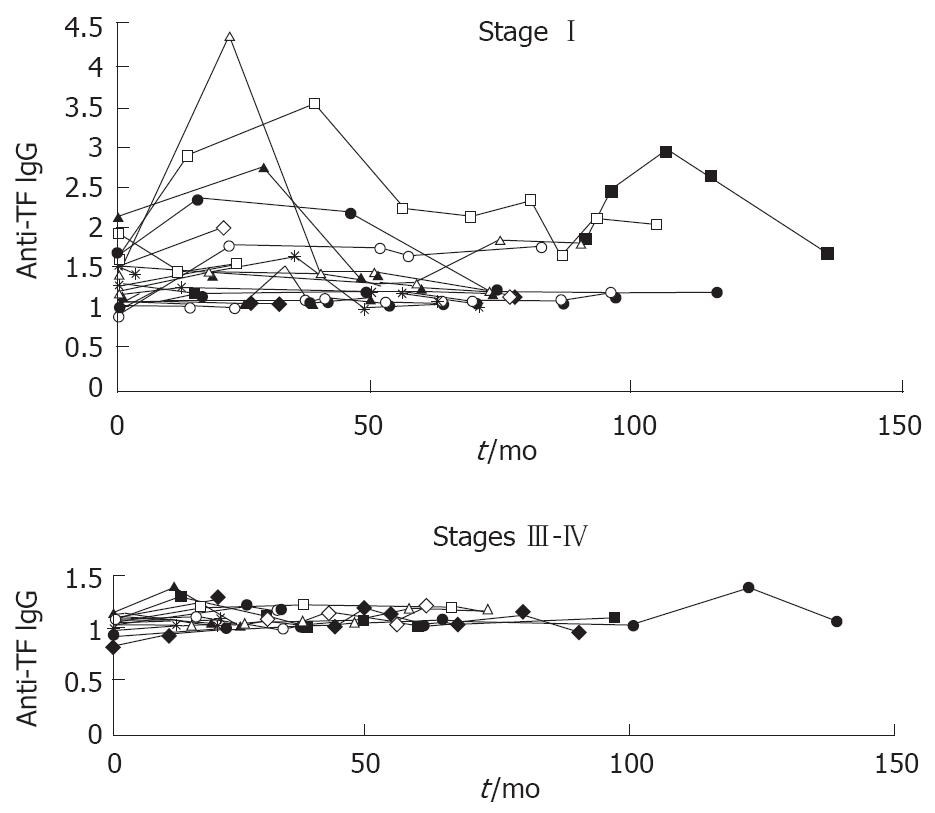
It was established that despite the elevation of anti-TF IgG level after surgery of patients with advanced cancer, its level during the follow-up varied only slightly, remaining mostly low (Figure 1).
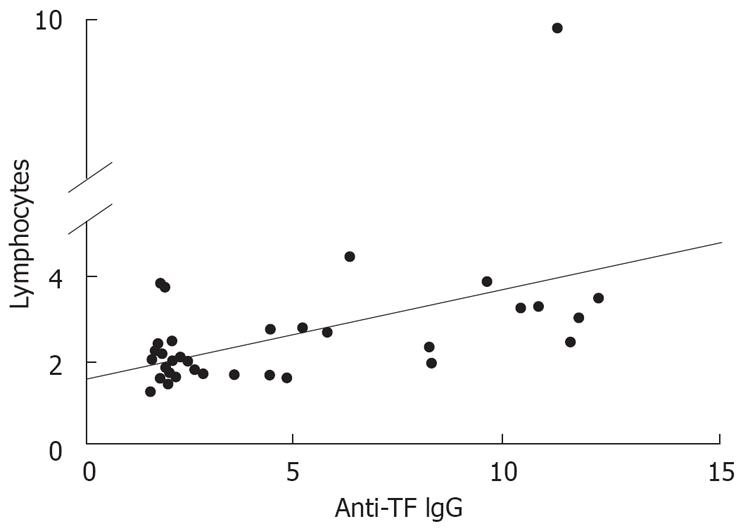
Linear regression analysis showed an existing correlation between the level of anti-TF IgG and the count of lymphocytes (Figure 2). As well, the correlation between the level of anti-Tn IgG and the serum level of tumor marker CA 19-9 was established: r = 0.481, P = 0.011, n = 27 (Tn-responders); r = 0.495, P = 0.002, n = 36 (all patients). No correlation between the level of antibodies and the values of other biochemical or hematological parameters was established.
No positive delayed-type hypersensitivity reaction in skin test challenges with TF-PAA in any of the fifteen patients, including those with a high level of anti-TF IgG, was observed. In some cases the weak erythema did not exceed 3 mm. Neither side effects nor complications were recorded.
The investigation of the level of serum IgG antibodies to tumor-associated carbohydrate epitopes (TF, Tn) and xenogeneic for human αGal epitope has been carried out earlier. The preoperative level of TF and Tn antibodies was significantly lower in patients with gastrointestinal cancer, including stage I, than in blood donors, whereas the difference in the level of αGal IgG between cancer patients and blood donors was not significant[8]. The preoperative level of anti-TF IgG antibodies in patients with gastric carcinoma having regional lymph node metastases was found to be significantly lower than that in patients without metastases[10].
In gastrointestinal cancer the level of TF antibodies in responders with regional metastases was increased after surgery but the follow-up showed the percentage of TF-responders to be significantly higher among patients with cancer in stage I (Table 4). This is due to a minor and short-term postoperative elevation of the antibody level in the serum of patients with lymph node metastases in stages III-IV of the disease. The level of anti-TF IgG in the serum of five patients with gastrointestinal cancer in stages II-IV, which was high prior to surgery rose even more after surgery and remained high for a long time (not shown). However, in most patients the TF IgG immune response in the terminal stages of the disease was suppressed whereas in stage I both the stimulation and suppression of the immune response took place (Figure 1). Since with cancer progression no significant differences were observed in the level of Tn and αGal antibodies, the immunosuppression associated with advanced cancer concerns TF antibodies mainly. In this study, the level of αGal antibodies was determined because their low level may be indicative of humoral immunodeficiency disorders[17]. However, the immunosuppression associated with advanced gastrointestinal cancer appeared not to affect the level of αGal antibodies. In gastric cancer the higher level of anti-αGal IgG was observed in patients with a larger tumor[10]. In patients with pancreatic cancer, who often suffer from severe immunosuppression[18], a high level of anti-αGal IgG was observed as well.
The positive correlation between the level of TF antibodies and the count of lymphocytes in TF-responders (n = 57, r = 0.400, P = 0.002) appeared to reflect the adaptive immune response and provided a further explanation for the involvement of anti-TF IgG in cancer-associated immunosuppression. In patients whose level of anti-TF IgG was higher than the second cut-off value (1.53, Figure 2) this correlation was more pronounced. However, during the follow-up of some patients no such correlation was observed. This is indicative of the existence of a complex relationship between both the parameters which depend on pathological conditions. A decreased preoperative count of lymphocytes and postoperative surgery-related lymphocytopenia occurred in patients with gastrointestinal cancer. Besides, the T helper deficiency was more frequently observed in patients with regional nodal metastases[18].
The above findings are supportive of the idea that patients with a disease of an early stage and its minimal residue after surgery are more responsive to active immunotherapy. The pre- and postoperative level of TF-antibodies, the pattern of TF-expression in tumor, and the individual profile of immune response to the immunization with TF-antigen should be taken into account when selecting the contingent for immunotherapy.
The postoperative elevation of the level of carbohydrate-specific antibodies, viz. Forssman antibodies, has been documented earlier[1920]. In our study, the postoperative elevation of antibody levels was found to be related to the low-differentiated carcinoma. Thus, the levels of all carbohydrate-specific antibodies, including those investigated earlier[21], were elevated in most patients after the surgical removal of G3 tumors, while in patients with G1 and G2 resected tumors, the differences in antibody levels before and after surgery were insignificant (Table 2).
In the preoperative examination of patients with gastrointestinal cancer, significant differences were established in the level of anti-TF IgG between them, namely, its lower level was associated with a lower-differentiated carcinoma in stages I-II[10]. These lower levels were increased significantly after surgery as shown in the present study. Moreover, the significant preponderance of patients with an elevated postoperative level of anti-TF IgG in the case of G3 resected tumors over those with G1 + G2 resected tumors was observed during the follow-up.
In general, the study shows that in most patients with the low-differentiated carcinoma the lower preoperative level of TF antibodies increases after surgery and has a tendency to remain elevated in the early stages of the cancer.
Taken together, the above results may be interpreted as follows: (1) A specific suppressive influence of the tumor on the production of TF antibodies is associated with the stage and grade of the tumor; the surgical removal of the primary tumor (especially G3 tumors) with lymphadenectomy may reverse the suppression of TF antibodies in the early stage of the disease; (2) The mainly low-differentiated carcinoma has an unspecific suppressive influence on the production of anti-carbohydrate antibodies. This influence may also be reversed by the removal of the tumor.
The specificity of human anticarbohydrate antibodies and their natural targets have been poorly studied. Natural anti-carbohydrate immunoglobulins are mostly antibodies of IgM-class. The high level of anti-TF and Tn IgG observed in some patients with cancer may be a sign of an acquired immune response which is indicative of the switching of antibody to the IgG-class. The anti-TF and anti-Tn IgG were affinity-purified from the serum of patients by using synthetic TF- and Tn-PAA sorbents[2223]. TF antibodies demonstrated a high activity of binding to mucins isolated from human malignant tumors, but only in 15% of tumor extracts, whereas the high activity of Tn antibodies was not observed. The analysis of the specificity of purified anti-TF IgG, the mutual and complete inhibition of serum antibodies by TFα and TFβ conjugates, and a good correlation between the levels of anti-TFα and anti-TFβ IgG in sera manifest that human anti-TF IgG is specific to both TFα and β anomers, with preference to the latter[23]. In this respect, antibodies resemble human monoclonal antibodies, which are able to bind different carcinoma cell lines and immunostained mucin-related tumor tissues[24].
The level of anti-Tn IgG is correlated with that of CA 19-9 in the serum. This seems to be unusual because the CA 19-9 antigen (SiaLea) differs structurally from Tn and contains no GalNAc residues. The correlation is not due to the cross-reactivity of antigens because the affinity-purified anti-Tn IgG did not react with SiaLea-PAA in the ELISA. This correlation may be explained by a similar relationship between both independent parameters and pathological conditions. Cancer progression or disorders of excretion (cholestasis and other disorders) may provoke the elevation of the level of CA 19-9[25] and Tn antibodies, respectively. Since the tumor marker CA 19-9 is a prognostic factor in colorectal cancer[26], the correlation between parameters may be indicative of the possible prognostic significance of antibodies as well.
Synthetic TF and Tn glycoconjugates deserve to be studied from a viewpoint of development of anti-cancer vaccines[1213]. Synthetic PAA-glycoconjugates may be promising preparations because they have been described well and can be modified by epitope density or supplemented with the amplifier of immune response and synthesized in necessary quantities. The results of the skin test performed by the authors show the TF-PAA to be a safe and non-toxic glycoconjugate. The lack of the delayed-type hypersensitivity reaction indicates that the TF antibody response took place through the T-cell independent mechanism, which is typical of carbohydrate antigens.
The high preoperative level of anti-TF as well as anti-MUC1 IgG was closely associated with the survival of patients with gastric cancer[11]. However, the possible protective mechanism of TF antibodies in cancer has yet remained unclear. The TF antigen seems to play a crucial role in the adhesion of cancer cells to the endothelium through the interaction of galectin-3[2728]. We suppose that even if TF antibodies are not cytotoxic for TF-expressed tumor cells, they may exhibit an anti-adhesive effect by blocking the TF- galectin-3 mediated metastatic spread. Whether anti-TF and anti-Tn IgG are prognostic factors and how their level in the follow-up is associated with survival will be shown by further investigations.
Tumor-associated carbohydrate antigens (TACA), the Thomsen-Friedenreich (TF) antigen and Tn are frequently expressed in malignant tumors. Human blood serum contains TF- and Tn antibodies but it is not yet clear which role antibodies play in the natural anti-cancer defense system. The TF antigen seems to play a crucial role in the metastatic mechanism due to adhesion of cancer cells to the endothelium. The high level of TF antibodies in the serum may be favourable if antibodies could block the TF- mediated metastatic spread. The level of TF- and Tn antibodies is significantly decreased in the serum of primary (not operated) patients with cancer. The high level of anti-TF IgG in the serum of primary patients with gastric cancer is closely associated with survival.
The relationship of the immune system with the tumor is far from a clear understanding. Cancer immune surveillance is considered to be important in the anti-tumor protection of the host. The growing tumor escapes the immune control under the immunosuppressive conditions. The surgical removal of the tumor may reverse the immunosuppression.
The dynamic changes of the level of TF- and Tn-antibodies in the serum of patients with cancer have been insufficiently studied. Investigations have mainly focused on short-term clinical trials of antigen-specific immunotherapy. Authors have undertaken a long-term follow-up of cancer patients to determine changes in the postoperative level of TF- and Tn antibodies, as well as to elucidate the association of this level with the progression of cancer, and survival. The association of the level of antibodies with the stage and morphology of the tumor, as well as values of blood parameters was studied. Also, the delayed-type hypersensitivity reaction to TF- polyacrylamide (PAA) conjugates in skin testing was examined.
The patients with a disease of an early stage may be more responsive to TF-specific active immunotherapy. Synthetic TF and Tn glycoconjugates deserve to be studied from a viewpoint of development of anti-cancer vaccines and in diagnostic aims.
The epitope is an antigenic determinant, viz. saccharide. The PAA glycoconjugate is a polymeric molecule with covalently bound saccharide. The anomer (α or β) is a spatial structure of saccharides.
This is interesting and provocative work. The authors present most of their results in terms of changes in antibody levels. It would be important for them to state what the basal level of these antibodies is, and to give us some idea of what percentage change is represented.
| 1. | Kim R, Emi M, Tanabe K. Cancer immunoediting from immune surveillance to immune escape. Immunology. 2007;121:1-14. |
| 2. | Danna EA, Sinha P, Gilbert M, Clements VK, Pulaski BA, Ostrand-Rosenberg S. Surgical removal of primary tumor reverses tumor-induced immunosuppression despite the presence of metastatic disease. Cancer Res. 2004;64:2205-2211. |
| 3. | Springer GF. Immunoreactive T and Tn epitopes in cancer diagnosis, prognosis, and immunotherapy. J Mol Med. 1997;75:594-602. |
| 4. | Avichezer D, Springer GF, Schechter B, Arnon R. Immunoreactivities of polyclonal and monoclonal anti-T and anti-Tn antibodies with human carcinoma cells, grown in vitro and in a xenograft model. Int J Cancer. 1997;72:119-127. |
| 5. | Wolf MF, Koerner U, Klumpp B, Schumacher K. Characterization of Thomsen-Friedenreich antibody subpopulations from normal human serum. Tumour Biol. 1987;8:264-272. |
| 6. | Smorodin EP, Jansson B, Miliukhina L, Paaski G, Bovin NV, Ovchinnikova TV, Kurtenkov O. [ELISA of IgM antibodies to Thomsen-Friedenreich (TF) hapten in cancer diagnostics: comparison of data obtained with four TF-glycoconjugates]. Bioorg Khim. 1997;23:795-799. |
| 7. | Smorodin JP, Kurtenkov OA, Miljukhina LM, Sergeyev BL, Hint EK, Bovin NV, Lipping AA, Chuzhmarov VJ. Thomsen-Friedenreich antigen-specific IgM antibodies: diagnostic significance for gastric and breast cancer. Exp Oncol. 1997;19:338-342. |
| 8. | Smorodin EP, Kurtenkov OA, Sergeyev BL, Lilleorg AL, Chuzmarov VI. Antibodies to tumor-associated carbohydrate epitopes in sera of cancer patients and blood donors. Exp Oncol. 2001;23:109i-113i. |
| 9. | Desai PR, Ujjainwala LH, Carlstedt SC, Springer GF. Anti-Thomsen-Friedenreich (T) antibody-based ELISA and its application to human breast carcinoma detection. J Immunol Methods. 1995;188:175-185. |
| 10. | Smorodin EP, Kurtenkov OA, Sergeyev BL, Lipping AA, Chuzmarov VI, Afanasyev VP. The relation of serum anti-TF, Tn and alpha-Gal IgG antibody levels to cancer progression and histopathological grading. Exp Oncol. 2002;24:270i-273i. |
| 11. | Kurtenkov O, Klaamas K, Mensdorff-Pouilly S, Miljukhina L, Shljapnikova L, Chuzmarov V. Humoral immune response to MUC1 and to the Thomsen-Friedenreich (TF) glycotope in patients with gastric cancer: relation to survival. Acta Oncol. 2007;46:316-323. |
| 12. | Slovin SF, Ragupathi G, Musselli C, Fernandez C, Diani M, Verbel D, Danishefsky S, Livingston P, Scher HI. Thomsen-Friedenreich (TF) antigen as a target for prostate cancer vaccine: clinical trial results with TF cluster (c)-KLH plus QS21 conjugate vaccine in patients with biochemically relapsed prostate cancer. Cancer Immunol Immunother. 2005;54:694-702. |
| 13. | Slovin SF, Ragupathi G, Musselli C, Olkiewicz K, Verbel D, Kuduk SD, Schwarz JB, Sames D, Danishefsky S, Livingston PO. Fully synthetic carbohydrate-based vaccines in biochemically relapsed prostate cancer: clinical trial results with alpha-N-acetylgalactosamine-O-serine/threonine conjugate vaccine. J Clin Oncol. 2003;21:4292-4298. |
| 14. | Galili U. The alpha-gal epitope and the anti-Gal antibody in xenotransplantation and in cancer immunotherapy. Immunol Cell Biol. 2005;83:674-686. |
| 15. | Sobin LH, Wittekind CH. TNM Classification of Malignant Tumours. 5th ed. Sobin LH, Wittekind CH editors. New York: Wiley. 1997;83-88. |
| 16. | Bovin NV. Polyacrylamide-based glycoconjugates as tools in glycobiology. Glycoconj J. 1998;15:431-446. |
| 17. | Galili U, Rachmilewitz EA, Peleg A, Flechner I. A unique natural human IgG antibody with anti-alpha-galactosyl specificity. J Exp Med. 1984;160:1519-1531. |
| 18. | Romano F, Uggeri F, Crippa S, Di Stefano G, Scotti M, Scaini A, Caprotti R, Uggeri F. Immunodeficiency in different histotypes of radically operable gastrointestinal cancers. J Exp Clin Cancer Res. 2004;23:195-200. |
| 19. | Mori T, Fujii G, Kawamura A Jr, Yasuda T, Naito Y, Tsumita T. Forssman antibody levels in sera of cancer patients. Immunol Commun. 1982;11:217-225. |
| 20. | Hirayama R, Hirokawa K, Takagi Y, Utsuyama M, Maejima S, Takemura K, Mishima Y, Makinodan T. Changes in serum levels of Forssman-like antibody in patients with gastric cancer. Cancer. 1989;63:1528-1533. |
| 21. | Smorodin EP, Kurtenkov OA, Sergeyev BL, Chuzmarov VI, Afanasyev VP. The relation of serum anti-(GalNAc beta) and -para-Forssman disaccharide IgG levels to the progression and histological grading of gastrointestinal cancer. Exp Oncol. 2007;29:61-66. |
| 22. | Smorodin EP, Kurtenkov OA, Sergeyev BL. The application of human natural polyclonal IgG-antibodies to Thomsen-Friedenreich epitope (TFE) for evaluation of TFE-expression in cancer-associated mucins. Exp Oncol. 2000;22:44-47. |
| 23. | Smorodin EP, Kurtenkov OA, Sergeyev BL, Pazynina GV, Bovin NV. Specificity of human anti-carbohydrate IgG antibodies as probed with polyacrylamide-based glycoconjugates. Glycoconj J. 2004;20:83-89. |
| 24. | Dahlenborg K, Hultman L, Carlsson R, Jansson B. Human monoclonal antibodies specific for the tumour associated Thomsen-Friedenreich antigen. Int J Cancer. 1997;70:63-71. |
| 25. | Fateh-Moghadam A, Stieber P. Sensible use of tumour markers. 2nd ed. Fateh-Moghadam A, editor. Jürgen Hartmann Verlag GmbH, Munich. New York: Wiley. 1993;20-38. |
| 26. | Reiter W, Stieber P, Reuter C, Nagel D, Lau-Werner U, Lamerz R. Multivariate analysis of the prognostic value of CEA and CA 19-9 serum levels in colorectal cancer. Anticancer Res. 2000;20:5195-5198. |
| 27. | Glinsky VV, Glinsky GV, Rittenhouse-Olson K, Huflejt ME, Glinskii OV, Deutscher SL, Quinn TP. The role of Thomsen-Friedenreich antigen in adhesion of human breast and prostate cancer cells to the endothelium. Cancer Res. 2001;61:4851-4857. |
| 28. | Yu LG, Andrews N, Zhao Q, McKean D, Williams JF, Connor LJ, Gerasimenko OV, Hilkens J, Hirabayashi J, Kasai K. Galectin-3 interaction with Thomsen-Friedenreich disaccharide on cancer-associated MUC1 causes increased cancer cell endothelial adhesion. J Biol Chem. 2007;282:773-781. |