Published online Jul 21, 2008. doi: 10.3748/wjg.14.4324
Revised: June 2, 2008
Accepted: June 9, 2008
Published online: July 21, 2008
AIM: To evaluate the effects of ethanol on the insulin-like growth factor-I (IGF-I) system involved in c-Jun N-terminal kinase (JNK1/2) and alcoholdehydrogenase (ADH) activity in primary cultured rat hepatocytes.
METHODS: Hepatocytes isolated from male Sprague-Dawley rats were incubated with various concentrations of ethanol for different durations of time. The cells were pretreated with SP600125 (10 &mgr;mol/L) and 4-MP (200 &mgr;mol/L), and then treated with ethanol (200 mmol/L). We then measured IGF-Isecretion, IGF-I mRNA expression, cell viability and JNK1/2 activity by radioimmunoassay, RT-PCR, MTT assay and Western blot, respectively (n = 6).
RESULTS: Ethanol induced the activity of phospho (p)-JNK1/2, reaching a maximum at 60 min and then decreasing at 180 min. The effects of ethanol on the IGF-I system were increased at 60 min (secretion: 7.11 ± 0.59 ng/mg protein vs 4.91 ± 0.51 ng/mg, mRNA expression: 150.2% ± 10.2% vs 101.5% ± 11.3%, P = 0.045) and then decreased at 180 min (secretion: 3.89 ± 0.25 ng/mg vs 5.4 ± 0.54 ng/mg protein; mRNA expression: 41.5% ± 10.4% vs 84.7% ± 12.1%, P = 0.04), however cell viability was decreased in a dose- and time-dependent manner. SP600125 blocked the ethanol-induced changes (at 60 min). Additionally, 4-methylpyrazole prevented the ethanol-induced decreases in the IGF-I system, cell viability and p-JNK1/2 activity (at 180 min).
CONCLUSION: This study suggests that ethanol-induced p-JNK1/2 activation is associated with the IGF-I system and cell viability in hepatocytes. Furthermore, alcohol dehydrogenase is involved in the relationship between ethanol-induced inactivation of p-JNK1/2 and the changes of the IGF-I system and cell viability.
- Citation: Oh YI, Kim JH, Kang CW. Effects of ethanol on insulin-like growth factor-I system in primary cultured rat hepatocytes: Implications of JNK1/2 and alcoholdehydrogenase. World J Gastroenterol 2008; 14(27): 4324-4331
- URL: https://www.wjgnet.com/1007-9327/full/v14/i27/4324.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.4324
Ethanol abuse exerts deleterious effects on the internal organs of the body, particularly the liver and brain, and alcohol-induced liver damage is one of the major causes of morbidity and mortality in alcoholics[1]. Ethanol alters hepatic carbohydrate and lipid metabolism as well as the synthesis of protein and DNA, which leads to hepatic dysfunction and cirrhosis[2]. Although the spectrum of ethanol toxicity is well known, the underlying pathophysiology of the signal transduction pathways has not been elucidated.
Ethanol alters cell functions via multiple signaling pathways, particularly those involving mitogen-activated protein kinases (MAPKs), which are involved in a variety of cellular responses including proliferation, differentiation, and apoptosis[3]. Several MAPK cascades have been identified, including those involving p42/44 and p38 MAPKs, and c-Jun N-terminal kinase (JNK1/2, also known as stress-activated protein kinase)[4]. JNK1/2 activity has been linked to the proliferation and apoptosis of hepatocytes[5].
Ethanol also induces prolonged activation of tumor necrosis factor (TNF)-stimulated JNK1/2 after hepatocytes are stimulated with various agonists, and prolonged activation of JNK1/2 and activator protein 1 (AP-1) is associated with the apoptosis and necrosis of hepatocytes that occurs in response to oxidative stress[4] and ischemia/reperfusion injury[6].
Insulin-like growth factor (IGF)-I is a peptide that plays an important role in regulating cell metabolism, growth, and differentiation[7]. The dose-dependent effects of ethanol on the IGFs system have been previously described in male rats[8]. The cellular action of IGF-I is mediated via the insulin-like growth factor-I receptor (IGF-IR), which exhibits tyrosine kinase activity[7]. IGF-IR is a key regulator of normal cellular processes, and plays a critical role in the development and progression of many types of cancer[9]. It has been reported that the renin-angiotensin system regulates the IGF-I system in hepatocytes[10], and it is known that retinoic acid inhibits growth-hormone-stimulated IGF-I production via protein kinase C (PKC)-δ in breast cancer cells. We recently found that the inhibitory effects of the ethanol-induced IGF-I system are related to p42/44 activity[11]. Although the relationships between ethanol-induced cellular action and apoptosis via MAPK including JNK1/2 activity have been reported previously, the secretion control mechanisms of the IGF-I system (IGF-I secretion, IGF-I mRNA expression, and IGF-IR activity) remain to be elucidated in primary cultured hepatocytes.
In the present study, we investigated the effects of ethanol on the IGF-I system, with particular attention to the JNK1/2 activity and alcoholdehydrogenase (ADH) in primary cultured rat hepatocytes.
IGF-I antigen and IGF-I antibodies were purchased form GroPep (Adelaide, Australia), and the JNK1/2 inhibitor SP600125 was purchased from New England Biolabs (Beverly, MA, USA). An enhanced chemiluminescence (ECL) kit was purchased from Cell Signaling (Beverly, MA, USA). All routine culture media were obtained from Gibco-BRL (Grand Island, NY, USA). Aquasol, reflection X-ray film, and 125I isotope were purchased from Dupont-NEN (Boston, MA, USA). Polyvinylidene difluoride (PVDF) membranes were purchased from BioRad (Hercules, CA, USA). BSA (fraction V), glycine, SDS, acrylamide, glycerol, and Tween-20 were obtained from Sigma (St. Louis, MO, USA).
Recombinant human IGF-I was iodinated to a specific radioactivity of 150-300 Ci/g using the 125I isotope following a modified version of the chloramine-T (Kodak, Grand Island, NY, USA) method. The specific activity of the iodinated IGF-I was typically 60-110 Ci/g protein. The iodination mixture was purified on a Sephadex G-50 column (150 cm) and pre-equilibrated with phosphate-buffered saline (0.1 mol/L, pH 7.4). The samples was then separated, after which the immunoreactive IGF-I was determined as previously described[11] with some modifications. All IGF-I data were expressed as nanograms of pure human IGF-I per milliliter, while assuming that equal cross-reactivity occurred between the rat and human IGF-I in the radioimmunoassay. Fifty microliters of rat polyclonal IGF-I antibody diluted to 1:1500 was added to 100 &mgr;L of each sample/standard and then incubated for 1 h at room temperature. Next, [125I]-IGF-I was added at 20 000 cpm, and the samples and standards were then incubated for an additional 18 h at 4°C. Fifty microliters of horse serum (Sigma, St. Louis, MO, USA) was then added to the sample, which was then centrifuged at 3000 ×g for 30 min. After discarding this supernatants, the radioactivities of the precipitates containing the bound [125I]-IGF-I were counted with a gamma scintillation counter (Wallac, Finland). The intra- and interassay coefficients of variation for IGFs were 8% and 10%, respectively.
Hepatocytes were isolated from male Sprague-Dawley rats weighing 200-300 g by a two-step perfusion procedure using 0.05% collagenase as described previously[1213]. Cell viability, which was assessed by the exclusion of trypan blue, was 90% ± 5% (mean ± SD). Isolated hepatocytes were then plated onto collagen-coated plastic culture dishes (60 mm in diameter) at a density of 5 × 104 cells/cm2 in Williams’ medium E containing 10% FBS. The plates were then placed in a 5% CO2 incubator for 3 h at 37°C, after which the medium was changed to FBS-free Williams’ medium E. After an additional 30 min, ethanol, SP600125 and 4-methylpyrazole (4-MP) were added at various concentrations to the dishes, which were then immediately sealed with Parafilm. The cells were then incubated for 0-180 min at 37°C.
After incubation, cells were rinsed twice with ice-cold phosphate-buffered saline, followed by the addition of lysis buffer comprising 20 mmol/L HEPES (pH 8.8), 136 mmol/L NaCl, 1 mmol/L EDTA, 1 mmol/L EGTA, 1% Triton X-100, 10 mmol/L KCl, 2 mmol/L MgCl, 1 mmol/L phenylmethylsulfonyl fluoride, 1 mmol/L sodium orthovanadate, 1 mmol/L dithiothreitol, 1 mmol/L benzamidine, 10 mmol/L β-glycerophosphate, 10 &mgr;g/mL aprotinin, 10 &mgr;g/mL leupeptin, and 1 &mgr;g/mL pepstatin A. The cell lysates were then sonicated for 5 min using a Vibra Cell ultrasonic processor (Sonics and Materials, Danbury, USA). After centrifugation of the sonicated samples at 12 000 ×g for 10 min at 4°C, the supernatants were collected, and the protein concentrations were estimated using a bicinchoninic acid (BCA) protein assay kit (Pierce, Bonn, Germany).
Cell lysates containing equal amounts of protein (20-30 &mgr;g) were fractionated by 10% SDS-polyacry-lamide gel electrophoresis, after which the proteins were transferred to a PVDF membrane (Bio-Rad, Hercules, CA, USA) and then washed with 25 mmol/L Tris (pH 7.4) containing 137 mmol/L NaCl and 0.1% Tween-20. The membrane was then blocked with 25 mmol/L Tris (pH 7.4) containing 137 mmol/L NaCl and 0.1% Tween-20 containing 5% nonfat dry milk for 2 h at room temperature. The blots were then incubated with antibodies against p54/46 JNK1/2 and IGF-IR overnight at 4°C, after which they were incubated with antirabbit and antimouse horseradish peroxidase. After being washed, the blots were developed using an ECL kit and exposed to X-ray film to allow detection of the protein bands.
Standard MTT assay as described in literature was used with slight modification (2). MTT [3-(4,5-dimethylthiazole-2yl)-2,5-diphenyl-2H-tetrazolium bromide] (Sigma Co., MO. USA) was dissolved in isotonic phosphate buffer (IPB, pH 7.4) solution at 5 mg/mL and filtered to sterilize and remove insoluble residues. Hepatocytes were cultured in containing Williams’ medium E containing 10% FBS and incubated for 4 h at 37°C in serum free Williams’ medium E. Cell survival was assayed by measuring the conversion of yellow, water-soluble tetrazolium MTT to blue, water-insoluble formazan. The absorbance was measured at 570 nm.
The statistical significance of differences between groups was determined using a Student’s t test, with a probability value of P < 0.05 being considered to be indicative of statistical significance. All experiments were performed at least six times.
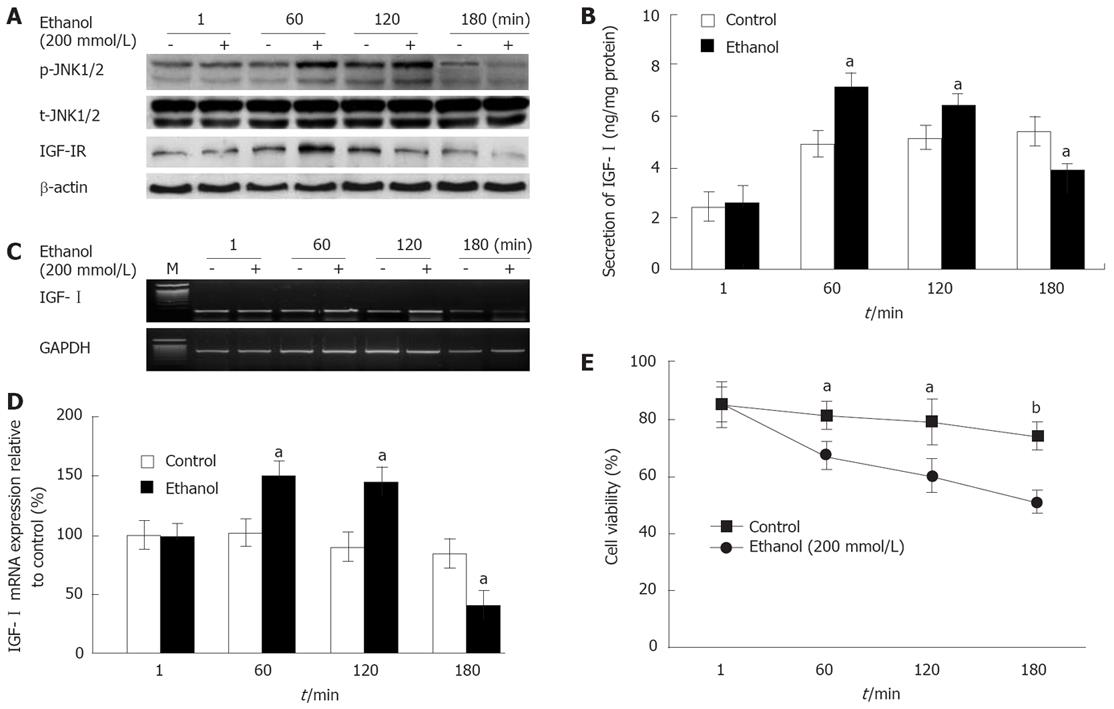
To evaluate the time course of the effects of exposure to ethanol on the IGF-I system, cell viability, and JNK1/2 activity, primary cultured rat hepatocytes were exposed to 200 mmol/L ethanol for different times (1, 60, 120 and 180 min). The activity of p-JNK1/2 was observed at 60 and 120 min, and was decreased relative to control at 180 min by ethanol exposure (Figure 1A). However, the total (t)-JNK1/2 activity was not affected. In addition, IGF-IR activity was also observed at 60 min, and it was decreased at 180 min (Figure 1A). The effects of ethanol on the secretion and mRNA expression of IGF-I were similar to changes in p-JNK1/2 activity, which increased at 60 (IGF-I secretion: 7.11 ± 0.59 ng/mg vs 4.91 ± 0.51 ng/mg protein; mRNA expression: 150.2% ± 10.2% vs 101.5% ± 11.3%, P = 0.045) and 120 min and then decreased at 180 min (IGF-I secretion: 3.89 ± 0.25 ng/mg vs 5.4 ± 0.54 ng/mg protein; mRNA expression: 41.5% ± 10.4% vs 84.7% ± 12.1%, P = 0.04; Figure 1B-D). The effects of ethanol on cell viability significantly decreased over time from 60 min onwards (at 60 min: 66.7% ± 5.12% vs 80.45% ± 5.21%, P = 0.035; Figure 1E).
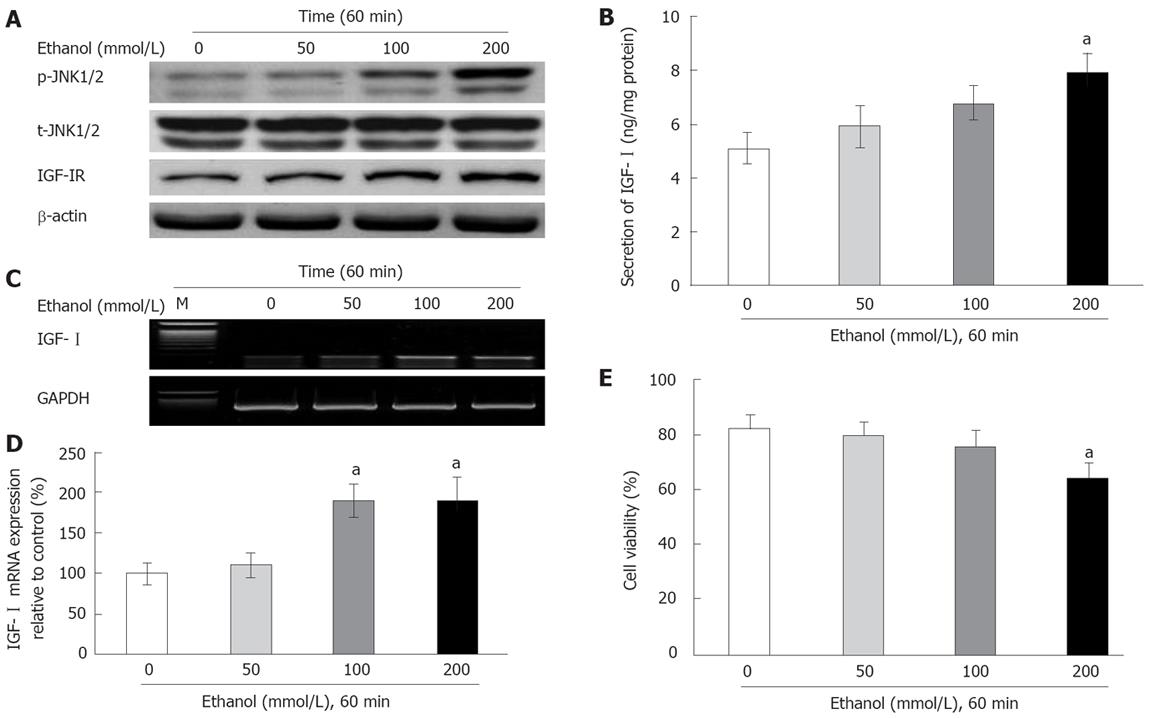
To investigate the dose-response effects of ethanol on the IGF-I system and JNK1/2 activity, the cells were exposed to ethanol at different concentrations (0, 50, 100 and 200 mmol/L) for 60 min. The effects of ethanol on the p-JNK1/2 activity increased in a dose-dependent manner (Figure 2A). In addition, the activity of p-JNK1/2 relative to control was maximal in response to exposure to 200 mmol/L ethanol, whereas the t-JNK1/2 activity was not affected (Figure 2A). Furthermore, the changes in IGF-I secretion, mRNA expression, and IGF-IR activity were increased by ethanol in a dose-dependent manner (Figure 2A-C). The treatment with 200 mmol/L ethanol showed significantly decrease of IGF-I secretion, mRNA expression and IGF-IR activity when compared with the control (IGF-I secretion: 7.89 ± 0.71 ng/mg vs 5.09 ± 0.56 ng/mg protein, mRNA expression: 191.5% ± 27.4% vs 100% ± 13.1%, P = 0.032; Figure 2A-D). These results were similar to those of the ethanol-induced p-JNK1/2 activity. However, cell viability was significantly decreased by exposure to 200 mmol/L ethanol (65.2% ± 4.9% vs 83.2% ± 4.2%, P = 0.024; Figure 2E).
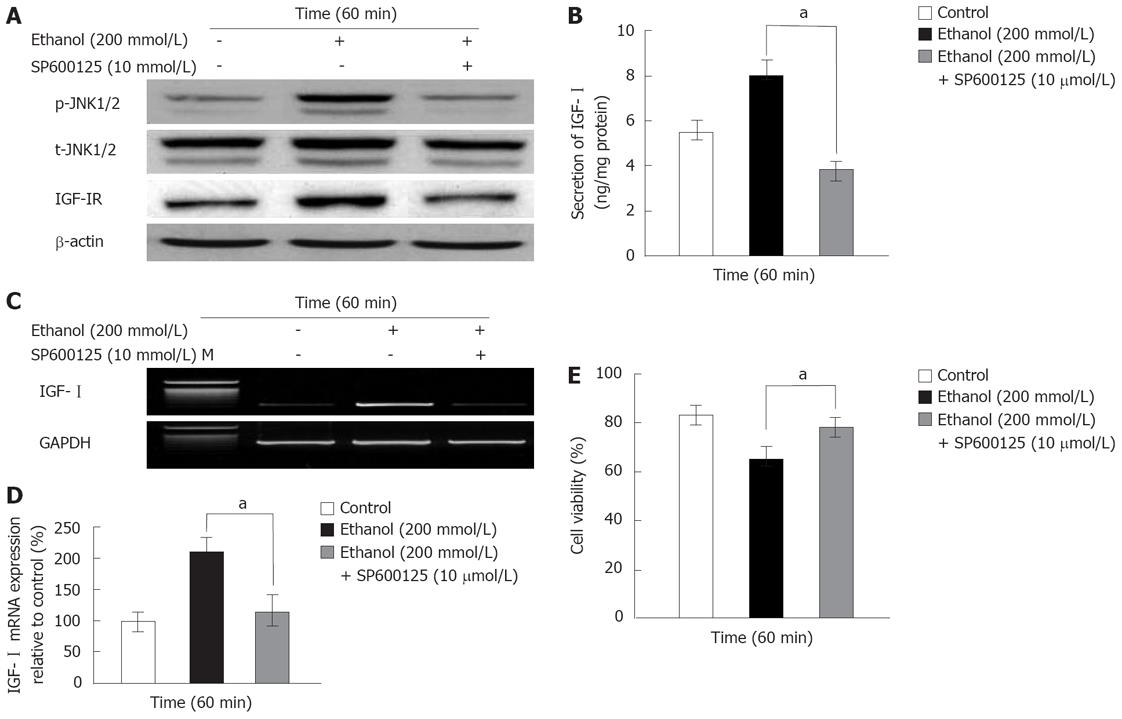
The JNK1/2 inhibitor SP600125 was used to determine if ethanol-induced activation of p-JNK1/2 (at 60 min) was related to the IGF-I system and cell viability. The ethanol-induced activations of p-JNK1/2 and IGF-IR were blocked by treatment with 10-5 mol/L SP600125 (Figure 3A). In addition, the temporary increases in secretion (8.02 ± 0.67 ng/mg protein) and mRNA expression (208.8% ± 23.4%) of IGF-I induced by ethanol were also blocked by SP600125 (IGF-I secretion: 3.78 ± 0.42 ng/mg protein, mRNA expression: 113.87% ± 27.5%, P = 0.024; Figure 3B-D), whereas t-JNK1/2 activity was not affected (Figure 3A). The ethanol-induced decrease in cell viability (64.2% ± 5.5%) was recovered by SP600125 (77.6% ± 4.1%, P = 0.045; Figure 3E). These results together demonstrate that the transient changes in the ethanol-induced IGF-I system and the decreased cell viability were related to p-JNK1/2 activity.
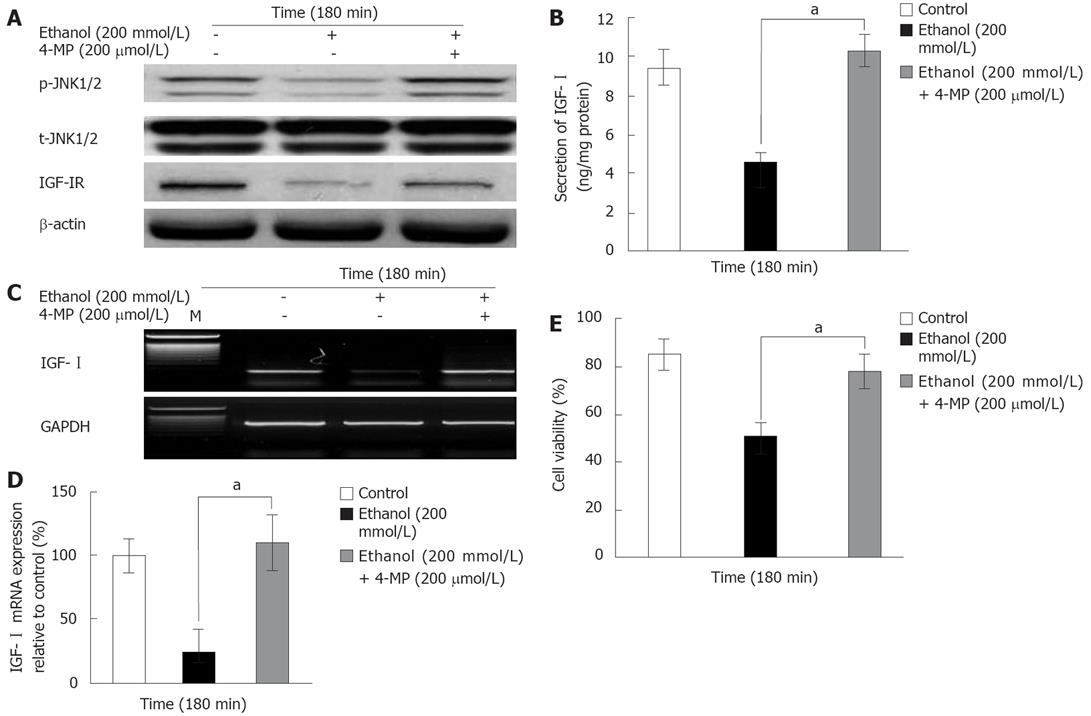
To determine the effects of ADH (alcohol dehydrogenase) on the ethanol-induced inactivation of p-JNK1/2 in the IGF-I system and decreased cell viability at 180 min, cells were exposed to 200 mmol/L ethanol after being pretreated with the ADH inhibitor 4-MP (200 &mgr;mol/L). The ethanol-induced inactivation of p-JNK1/2 and IGF-IR was recovered by 10-5 mol/L 4-MP, whereas the t-JNK1/2 activity was not affected (Figure 4A). The ethanol-induced decreases in the secretion (4.54 ± 0.52 ng/mg protein) and mRNA expression (24.5% ± 17.1%) of IGF-I and cell viability (50.3% ± 5.5%) were also recovered by pretreatment with 4-MP (IGF-I secretion: 10.3 ± 0.79, mRNA expression: 109.4% ± 21.8%, cell viability: 77.2 ± 7.2, P = 0.035; Figure 4B-E). These results together indicate that the decreases in ethanol-induced p-JNK1/2 activity, IGF-I system and cell viability were related to the ADH.
Ethanol exerts toxic effects on almost all organs, particularly liver and brain. The liver is a major metabolic organ in which most IGF-I is produced and secreted, and its mediators are responsible for alcohol-induced liver injury[14].
In the present study, we showed that ethanol transiently increased p-JNK1/2 activity at 60 min and then decreased it at 180 min. It has been reported that ethanol activates MAPKs[12], and acute exposure of primary cultured rat hepatocytes to ethanol for 60 min increases the activities of p42/44 MAPK and p-JNK1/2, with both activities gradually decreasing thereafter[15]. Exposure to ethanol has been shown to cause prolonged activation of p-JNK1/2; however, this response was attenuated in hepatocytes obtained from rats chronically exposed to ethanol for 6 wk[16]. Our result is similar with those of previous studies mentioned above, in which exposure time to ethanol causes activation and inactivation of p-JNK1/2 activation in rat hepatocytes.
The activation of p-JNK1/2 normally occurs in response to growth stimuli and is involved in cell proliferation[17]. This is also related to apoptosis and antiapoptosis, and is activated by cytokines or stress stimuli such as osmotic shock, UV light, and heat[918]. IGF-I system has been reported to protect against a variety of chemical cellular injuries that induce apoptosis[19]. We previously showed that ethanol decreased the synthesis and secretion of IGF-I and the activity of IGF-IR, an effect that is related to cell proliferation and differentiation[11]. In the present study, ethanol-induced transient activation of p-JNK1/2 increased in the IGF-I system, but this decreased when p-JNK1/2 was inactivated. Furthermore, IGF-IR activity also regulates ethanol-induced secretion and synthesis of IGF-I. These results are consistent with our previous study that ethanol-induced p42/44 activity was related to the secretion and synthesis of IGF-I in primary cultured rat hepatocytes[11]. In our previous study, although there were different doses (5%-20%), chronic ethanol treatment caused dose-dependent decreases in the secretion and synthesis of IGF-I in liver and blood in vivo[8].
We also used a JNK1/2 inhibitor to verify whether the ethanol-induced transient activation of p-JNK1/2 may alter the IGF-I system. The results reported here confirmed that the effects of ethanol on p-JNK1/2 activation, the IGF-I system, and cell viability were recovered by an inhibitor of the JNK1/2 activity. We suggest that acute exposure to ethanol affects not only p42/44 MAPK but also p-JNK1/2 activities, which in turn alter the IGF-I system. It has been reported that ethanol-induced transient activation of p-JNK1/2 indicates pro-apoptosis, while a prolonged activation induces anti-apoptosis in hepatocytes[20]. Hepatocytes express two JNK genes (JNK1 and JNK2) and bile acids cause activation of both JNK1 and JNK2, but JNK1 activation causes apoptosis whereas JNK2 activation protects against apoptosis[21]. It has been reported that ethanol causes more pronounced activation of JNK 1 compared to JNK 2, suggesting a role for this preferential activation of JNK 1 in ethanol-induced apoptosis of hepatocytes[15].
Interestingly, we found that cell viability is always decreased by ethanol. However, there was a transient activation of p-JNK1/2 and the subsequent inactivation of p-JNK1/2 in parallel with changes of the IGF-I system. These results suggest that transient activation of p-JNK1/2 with increment of the IGF-I system lead to pro-apoptotic events and transient resistance of hepatocytes. Also, the ethanol-induced inactivation of p-JNK upon decrease of the IGF-I system indicate that the cells have already passed the threshold for proliferation or survival against the ethanol-induced toxicity.
The ADH is an enzyme involved in ethanol metabolism that appears to provide the link between the effects of ethanol-induced p-JNK1/2 activity on the IGF-I system and cell viability at 180 min but not at 60 min (data not shown). It has been reported that the level of 4-MP decreased by approximately 90% in rat hepatocytes following exposure to ADH and ethanol[22]. Ethanol rapidly activates p-JNK1/2, which is associated with the response of the endoplasmic reticulum to stress, which in turn causes inhibition of ADH[23]. Acute exposure to 200 mmol/L ethanol may also activate p-JNK1/2 via acetaldehyde-dependent[15] and acetaldehyde-independent[24] pathways in rat hepatocytes. It was reported that acetaldehyde produced by ethanol oxidation activates p42/44 MAPK and p-JNK1/2 in rat hepatocytes[1215]. We previously reported that ethanol-induced changes of the IGF-I system are related to ADH activity[11]. These results suggest that the decrease in p-JNK1/2 activity induced by exposure to ethanol for 180 min regulates the decrease of IGF-I system and cell viability, with these effects being related to ADH. However, the effects of the ethanol-induced transient activation of p-JNK1/2 on the increment of IGF-I system were not due to ADH.
In conclusion, this study suggest that ethanol-induced p-JNK1/2 activation is related to changes in the IGF-I system and cell viability in hepatocytes. Furthermore, ethanol-induced inactivation of p-JNK1/2 is involved in the IGF-I system and cell viability via ADH. These findings might be helpful to understand the pathogenesis of liver damage induced by ethanol, and may lead to a rational therapeutic intervention against ethanol toxicity.
Ethanol-induced liver damage is unavoidable upon exposure to alcohol. Moreover, enhanced c-Jun N-terminal kinase (JNK1/2) and alcohol dehydrogenase (ADH) activity have been linked to the ethanol induced hepatotoxicity. Insulin-like growth factor-I (IGF-I) system has been reported to protect against a variety of chemical cellular injuries that induce apoptosis; however it has not been well defined whether the IGF-I system is associated with the ethanol-induced JNK and ADH activity.
It was reported that acetaldehyde produced by ethanol oxidation activates p42/44 MAPK and p-JNK1/2 in rat hepatocytes. We previously reported that ethanol-induced changes of the IGF-I system are related to p42/44 activity. To investigate the importance of IGF-I system via JNK and ADH, we performed this study using specific inhibitors.
We found that there was increase and then decrease in the IGF-I secretion and mRNA expression during ethanol treatment. The activity of JNK was also temporary increased and then decreased by ethanol. However, cell viability was monotonically decreased. Both JNK and ADH inhibitors blocked ethanol-induced changes of IGF-I system and cell viability.
The present study evaluated the changes of the IGF-I system indicating that the potential value of IGF-I system for patients with ethanol-induced liver damage. Moreover, this study demonstrates that ethanol-induced IGF-I system is involved in the activities of JNK1/2 and ADH.
IGF-I system has been reported to protect against a variety of chemical cellular injuries and promote proliferation of hepatocytes. The liver is a major metabolic organ in which most IGF-I is produced and secreted, and its mediators are responsible for alcohol-induced liver injury.
This manuscript describes the activation of JNK1/2 activity by ethanol treatment of rat hepatocytes and subsequent changes in IGF expression and secretion as well as changes in proliferation. These effects could be inhibited by the specific inhibitors of JNK1/2 as well as an inhibitor of ADH. This manuscript is well-written, clear and concise with a thorough results section.
| 1. | Reddy MA, Shukla SD. Potentiation of mitogen-activated protein kinase by ethanol in embryonic liver cells. Biochem Pharmacol. 1996;51:661-668. |
| 2. | Lieber CS. Mechanism of ethanol induced hepatic injury. Pharmacol Ther. 1990;46:1-41. |
| 3. | Tombes RM, Auer KL, Mikkelsen R, Valerie K, Wymann MP, Marshall CJ, McMahon M, Dent P. The mitogen-activated protein (MAP) kinase cascade can either stimulate or inhibit DNA synthesis in primary cultures of rat hepatocytes depending upon whether its activation is acute/phasic or chronic. Biochem J. 1998;330:1451-1460. |
| 4. | Czaja MJ. The future of GI and liver research: editorial perspectives. III. JNK/AP-1 regulation of hepatocyte death. Am J Physiol Gastrointest Liver Physiol. 2003;284:G875-G879. |
| 5. | Schwabe RF, Bradham CA, Uehara T, Hatano E, Bennett BL, Schoonhoven R, Brenner DA. c-Jun-N-terminal kinase drives cyclin D1 expression and proliferation during liver regeneration. Hepatology. 2003;37:824-832. |
| 6. | Bradham CA, Stachlewitz RF, Gao W, Qian T, Jayadev S, Jenkins G, Hannun Y, Lemasters JJ, Thurman RG, Brenner DA. Reperfusion after liver transplantation in rats differentially activates the mitogen-activated protein kinases. Hepatology. 1997;25:1128-1135. |
| 7. | Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16:3-34. |
| 8. | Park SH, Heo JS, Kang CW. Dose-dependent effect of alcohol on insulin-like growth factor systems in male rats. Clin Exp Pharmacol Physiol. 2004;31:22-28. |
| 10. | Isenovic ER, Meng Y, Divald A, Milivojevic N, Sowers JR. Role of phosphatidylinositol 3-kinase/Akt pathway in angiotensin II and insulin-like growth factor-1 modulation of nitric oxide synthase in vascular smooth muscle cells. Endocrine. 2002;19:287-292. |
| 11. | Lee SM, Alam R, Ho CJ, Kim JH, Kang CW, Park JH, Lee MS. Involvement of p42/44 MAPK in the effects of ethanol on secretion of insulin-like growth factor (IGF)-I and insulin-like growth factor binding protein (IGFBP)-1 in primary cultured rat hepatocytes. Int J Neurosci. 2007;117:187-201. |
| 12. | Seglen PO, Reith A. Ammonia inhibition of protein degradation in isolated rat hepatocytes. Quantitative ultrastructural alterations in the lysosomal system. Exp Cell Res. 1976;100:276-280. |
| 13. | Weng Y, Shukla SD. Ethanol alters angiotensin II stimulated mitogen activated protein kinase in hepatocytes: agonist selectivity and ethanol metabolic independence. Eur J Pharmacol. 2000;398:323-331. |
| 14. | Resnicoff M, Cui S, Coppola D, Hoek JB, Rubin R. Ethanol-induced inhibition of cell proliferation is modulated by insulin-like growth factor-I receptor levels. Alcohol Clin Exp Res. 1996;20:961-966. |
| 15. | Lee YJ, Aroor AR, Shukla SD. Temporal activation of p42/44 mitogen-activated protein kinase and c-Jun N-terminal kinase by acetaldehyde in rat hepatocytes and its loss after chronic ethanol exposure. J Pharmacol Exp Ther. 2002;301:908-914. |
| 16. | Bailey SM, Cunningham CC. Acute and chronic ethanol increases reactive oxygen species generation and decreases viability in fresh, isolated rat hepatocytes. Hepatology. 1998;28:1318-1326. |
| 17. | Aroor AR, Shukla SD. MAP kinase signaling in diverse effects of ethanol. Life Sci. 2004;74:2339-2364. |
| 18. | Saklatvala J, Dean J, Finch A. Protein kinase cascades in intracellular signalling by interleukin-I and tumour necrosis factor. Biochem Soc Symp. 1999;64:63-77. |
| 19. | Matthews CC, Feldman EL. Insulin-like growth factor I rescues SH-SY5Y human neuroblastoma cells from hyperosmotic induced programmed cell death. J Cell Physiol. 1996;166:323-331. |
| 20. | Lee YJ, Shukla SD. Pro- and anti-apoptotic roles of c-Jun N-terminal kinase (JNK) in ethanol and acetaldehyde exposed rat hepatocytes. Eur J Pharmacol. 2005;508:31-45. |
| 21. | Qiao L, Han SI, Fang Y, Park JS, Gupta S, Gilfor D, Amorino G, Valerie K, Sealy L, Engelhardt JF. Bile acid regulation of C/EBPbeta, CREB, and c-Jun function, via the extracellular signal-regulated kinase and c-Jun NH2-terminal kinase pathways, modulates the apoptotic response of hepatocytes. Mol Cell Biol. 2003;23:3052-3066. |
| 22. | Carter EA, Wands JR. Ethanol-induced inhibition of liver cell function: I. Effect of ethanol on hormone stimulated hepatocyte DNA synthesis and the role of ethanol metabolism. Alcohol Clin Exp Res. 1988;12:555-562. |
| 23. | Nishitani Y, Matsumoto H. Ethanol rapidly causes activation of JNK associated with ER stress under inhibition of ADH. FEBS Lett. 2006;580:9-14. |
| 24. | Cabrales-Romero Mdel P, Marquez-Rosado L, Fattel-Fazenda S, Trejo-Solis C, Arce-Popoca E, Aleman-Lazarini L, Villa-Trevino S. S-adenosyl-methionine decreases ethanol-induced apoptosis in primary hepatocyte cultures by a c-Jun N-terminal kinase activity-independent mechanism. World J Gastroenterol. 2006;12:1895-1904. |