Published online Jul 21, 2008. doi: 10.3748/wjg.14.4309
Revised: May 9, 2008
Accepted: May 16, 2008
Published online: July 21, 2008
AIM: To investigate the effects of the essential oil of Curcuma wenyujin (CWO) on growth inhibition and on the induction of apoptosis in human HepG2 cancer cells.
METHODS: The cytotoxic effect of drugs on HepG2 cells was measured by 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. DNA fragmentation was visualized by agarose gel electrophoresis. Cell cycle and mitochondrial transmembrane potential (ΔΨm) were determined by flow cytometry (FCM). Cytochrome C immunostaining was evaluated by fluorescence microscopy. Caspase-3 enzymatic activity was assayed by the cleavage of Ac-DEVD-R110. Cleaved PARP and active caspase-3 protein levels were measured by FCM using BD™ CBA Human Apoptosis Kit.
RESULTS: Treatment with CWO inhibited the growth of HepG2 cells in a dose-dependent manner, and the IC50 of CWO was approximately 70 &mgr;g/mL. CWO was found to inhibit the growth of HepG2 cells by inducing a cell cycle arrest at S/G2. DNA fragmentation was evidently observed at 70 &mgr;g/mL after 72 h of treatment. During the process, cytosolic HepG2 cytochrome C staining showed a markedly stronger green fluorescence than in control cells in a dose-dependent fashion, and CWO also caused mitochondrial transmembrane depolarization. Furthermore, the results clearly demonstrated that both, activity of caspase-3 enzyme and protein levels of cleaved PARP, significantly increased in a dose-dependent manner after treatment with CWO.
CONCLUSION: CWO exhibits an antiproliferative effect in HepG2 cells by inducing apoptosis. This growth inhibition is associated with cell cycle arrest, cytochrome C translocation, caspase 3 activation, Poly-ADP-ribose polymerase (PARP) degradation, and loss of mitochondrial membrane potential. This process involves a mitochondria-caspase dependent apoptosis pathway. As apoptosis is an important anti-cancer therapeutic target, these results suggest a potential of CWO as a chemotherapeutic agent.
-
Citation: Xiao Y, Yang FQ, Li SP, Hu G, Lee SMY, Wang YT. Essential oil of
Curcuma wenyujin induces apoptosis in human hepatoma cells. World J Gastroenterol 2008; 14(27): 4309-4318 - URL: https://www.wjgnet.com/1007-9327/full/v14/i27/4309.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.4309
Hepatocellular carcinoma (HCC) is the fifth most common cancer with more than 1 million fatalities occurring annually worldwide[1]. Most HCCs, unlike their normal counterparts, are quite resistant to death receptor-mediated apoptosis, because cell surface death receptors are cross-linked with either agonistic antibodies or soluble death ligand proteins[23]. HCCs also display high resistance to tumor necrosis factor-related apoptosis-inducing ligand-mediated cell death[45], which, together with other apoptosis resistance mechanisms, suggests that alternative approaches are needed to control HCC growth and metastasis.
Ezhu, as a Chinese traditional medicine has been used for a long time. It belongs to the family of Zingiberaceae. This genus is composed of about 70 species of rhizomatous herbs which are distributed all over the world, and about 20 species could be identified in China. Actually, Chinese Pharmacopoeia indicated that the rhizomes of three species including Curcuma phaeocaulis, C. kwangsiensis, and C. wenyujin are used as Ezhu, which has been used for removing blood stasis, alleviating pain, and liver disease protection[6]. In order to control the quality of Ezhu and develop Ezhu as an effective therapeutic agent, we have developed quality control methods and conducted studies comparing the quantities of several chemical components of different types of Ezhu[7]. Nowadays in China, the essential oil of Curcuma wenyujin (CWO) has been used as injection to cure paediatric diseases such as acute upper respiratory infections, viral myocarditis, or acute pneumonia[8]. Besides, the essential oil of Ezhu has been used as a preparation to treat vaginitis[9]. Also, in some other countries, as in France, Japan, or India, the essential oil of Ezhu has been reported to possess anti-microbial[10], anti-bacterial, vasorelaxant[11], and anti-inflammatory activities[12]. In China, the essential oil of Ezhu has shown promising effects in the treatment of liver[13], gastric, lung, and cervical cancers. For instance, inhibitory effects of CWO on the growth of SMMC-7721 cells, cervical cells, L615 cells, and K562 cells have been reported[14]. In addition, we recently identified furanodiene, one of Ezhu’s ingredients, to activate p38 and to inhibit of ERK mitogen-activated protein kinase (MAPK) signaling in HepG2 cells. The result suggests Ezhu as a potential candidate for the treatment of liver diseases[15]. Ezhu has a long history on treating liver disease and protecting liver functions; however, there is no report about inhibitory effects of the essential oil of Ezhu on human HepG2 cell growth and the underlying mechanism of action. This study aimed determining the cytotoxicity of CWO, one species of Ezhu, in human hepatoma HepG2 cells and the underlying molecular mechanism of action.
CWO was purchased from Zhejiang RuiAn Pharmaceutical Company (Lot No. 011001). CWO (0.1 mL) was diluted in 10 mL methanol. The solution thus obtained was filtered through a 0.45 &mgr;m Econofilter (Agilent Technologies, Palo Alto, CA, USA) and injected into Agilent Series 1100 (Agilent Technologies, USA) liquid chromatograph, equipped with a vacuum degasser, a quaternary pump, an autosampler, and a diode array detection (DAD) system, and analyzed under the conditions described in a previous report[7]. In brief, A Zorbax ODS column (250 mm × 4.6 mm I.D., 5 &mgr;m) with a Zorbax ODS C18 guard column (20 mm × 3.9 mm I.D., 5 &mgr;m) was used. Solvents that constituted the mobile phase were A (water) and B (acetonitrile). The elution conditions applied were: 0-15 min, linear gradient 30%-47% B; 15-30 min, isocratic 47% B; 30-40 min, linear gradient 47%-60% B; 40-50 min, linear gradient 60%-90% B; 50-60 min, linear gradient 90%-100% B; and finally, washing the column with 100% B for 10 min before reconditioning the column for 15 min with 30% B. The flow-rate was 1 mL/min and the injection volume was 10 &mgr;L. The column operated at 25°C. The analytes were monitored with DAD at 214 nm and 256 nm. In addition, methanol solutions, containing known concentrations of standards including curcumenone, curcumenol, neocurdione, curdione, isocurcumenol, furanodienone, curcumol, germacrone, curzerene, furanodiene, and β-elemene were prepared and subjected to the LC-DAD system for comparison. 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide (MTT), JC-1 dye, caspase-3 assay kit, and 488 cytochrome C apoptosis detection kit were purchased from Molecular Probes. Human apoptosis kits were purchased from BD Bioscience.
Cell culture and drug treatment: The human hepatoma cell line HepG2 was obtained from the American Type Culture Collection (ATCC, Rockville, MD). Cells were cultured in RPMI 1640 medium (GIBCO, Grand Island, NY) supplemented with 10% (v/v) heat-inactivated fetal bovine serum (FBS) (Life Technologies Inc., Gaithersburg, MD), 100 &mgr;g/mL streptomycin, and 100 U/mL penicillin in 75 cm2 tissue culture flasks in a humidified atmosphere at 37°C with 5% CO2. CWO was dissolved in 1 mL DMSO to make a 1 mmol/L stock solution and diluted to the concentrations as needed. The final volume of drug solution added to medium was 1%. Control samples contained 1% DMSO.
Growth inhibitory assay: Cells were seeded in 96-well microplates (1 × 105 cells/well in 100 &mgr;L medium). CWO was added to the cultures in serial concentrations and cultures were incubated for 48 h. Medium was discarded and 30 &mgr;L tetrazolium dye (MTT) solution (5 mg/mL in PBS) were added to each well. Plates were incubated for additional 4 h. DMSO (10 &mgr;L) was added to dissolve the formed formazan crystals. The plate was read in a microplate reader at 570 nm. MTT solution with DMSO (without cells and medium) acted as blank while the DMSO (1%)-treated cells served as control of 100% survival.
Agarose gel electrophoresis for analysis of DNA fragmentation: HepG2 cells were treated with the drug for 72 h while the DMSO (1%) containing medium treated cells served as control. Adherent cells (2 × 106 /mL) were harvested, washed once with 400 &mgr;L PBS, and taken up in 400 &mgr;L lysis buffer (containing 200 mmol/L Tris-HCl (pH 8.3), 100 mmol/L EDTA, and 1% SDS). Twenty &mgr;L of 10 mg/mL proteinase K were added for protein digestion, and the tubes were incubated in a 37°C water bath overnight. The samples were allowed to cool down to room temperature before 300 &mgr;L saturated NaCl solution were added. After centrifugation for 15 min at 9000 r/min, supernatants were collected. DNA fibers were obtained by adding 1 mL cold absolute ethanol (EtOH) and a centrifugation for 20 min at 4°C at 16 000 r/min. DNA fibers were washed once with 500 &mgr;L of -20°C 70% EtOH, and the DNA pellet was dried in a 70°C oven. Fifteen &mgr;L TE buffer (10 mmol/L Tris-HCl pH 8.0, 1 mmol/L EDTA) containing 0.2 mg/mL RNase A were added. After an incubation at 37°C for 90 min, 10 &mgr;L of each sample were loaded on a 1.5% TBE agarose gel to visualize DNA.
Cell cycle analysis: Cells were seeded in 6 well plates and treated with CWO at various concentrations for 48 and 72 h. DMSO (1%)-treated cells served as control. After treatment, media were discarded. The adherent cells were washed with PBS, and 300 &mgr;L trypsin were added for 5 min at room temperature to detach the cells. After centrifugation at 350 g at 4°C for 5 min, the cell pellet was resuspended with 1 mL cold 70% EtOH at 4°C for 12 h and centrifuged again for 5 min at 4°C at 350 g. Finally, 1 mL propidium iodide (PI) staining solution (20 &mgr;g/mL PI, 8 &mgr;g/mL DNase free RNase) was added to the samples. The samples were analyzed by flow cytometry (FCM) (BD FACS CantoTM). The results were analyzed by Mod Fit LT 3.0 software.
Measurement of mitochondrial transmembrane potential (ΔΨm):ΔΨm was assessed by JC-1 (5,5’,6,6’-tetrachloro-1,1’,3,3’-tetraethylbenzimidazolylcarbocyan-ine iodide), Mitochondrial Potential Sensors (Molecular Probes, Leiden, Netherlands). Red fluorescence J-aggregate form of JC-1 indicates intact mitochondria, whereas green fluorescence shows monomeric form of JC-1 due to breakdown of the mitochondrial membrane potential. Cells were seeded in 6-well plates and then incubated with the desired concentrations of CWO for 48 h. The medium of each well was discarded, cells were treated with 1 mL medium containing 5 mg/mL JC-1 for 15 min at 37°C and 5% CO2 in the dark, washed twice in PBS, resuspended in 1 mL medium and measured by FCM.
Immunostaining of cytochrome C: Cytochrome C release was assessed by SelectFX Alexa Fluor 488 cytochrome C apoptosis detection kit (Molecular Probes, Leiden, Netherlands). Cells were seeded in 24-well plates, and treated with various amounts of CWO in an humidified atmosphere (37°C in 5% CO2) for 24 h while the DMSO (1%)-treated cells served as control. Media were discarded and the cells were washed with warm PBS, fixed with freshly prepared 4% formaldehyde in PBS for 15 min at 37°C, and permeabilized with 0.2% Triton X-100 for 5 min at room temperature. The cells were washed, incubated in a blocking buffer (10% heat-inactivated normal goat serum (NGS) for 30 min at room temperature, and finally for 1 h with 1 &mgr;g/mL primary antibody (anti-cytochrome C, mouse IgG) at room temperature. Green fluorescence was observed by fluorescence microscopy.
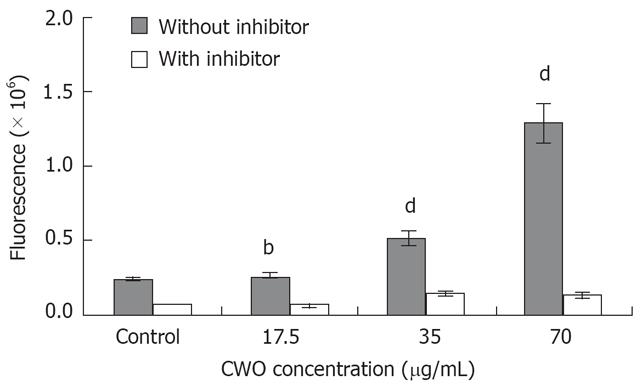
Caspase-3 enzymatic activity assay: Caspase-3 enzymatic activity was determined by measuring the cleavage of Ac-DEVD-R110 according to the protocol with the caspase-3 assay kit supplied by Molecular Probes. Cells were treated with various amounts of CWO in an humidified atmosphere (37°C in 5% CO2) for 24 h while DMSO (1%)-treated cells served as control. Cells were harvested at a concentration of a minimum of 1 × 106/mL, pellets were collected, appropriate cell lysis buffer was added, and the samples were incubated on ice for 30 min. The samples were then centrifuged and supernatants were collected and transferred to microplates. Cell lysis buffer was used as a no-enzyme control to determine the background fluorescence of the substrate. At the same time, 1 &mgr;L of 1 mmol/L Ac-DEVD-CHO inhibitor was added to selected samples. One &mgr;L of DMSO was added to no-inhibitor samples to serve as control and incubated for 10 min simultaneously. Finally, 0.05 mmol/L Z-DEVD-R110 substrate was added and samples were incubated for 30 min prior to the fluorescence measurement.
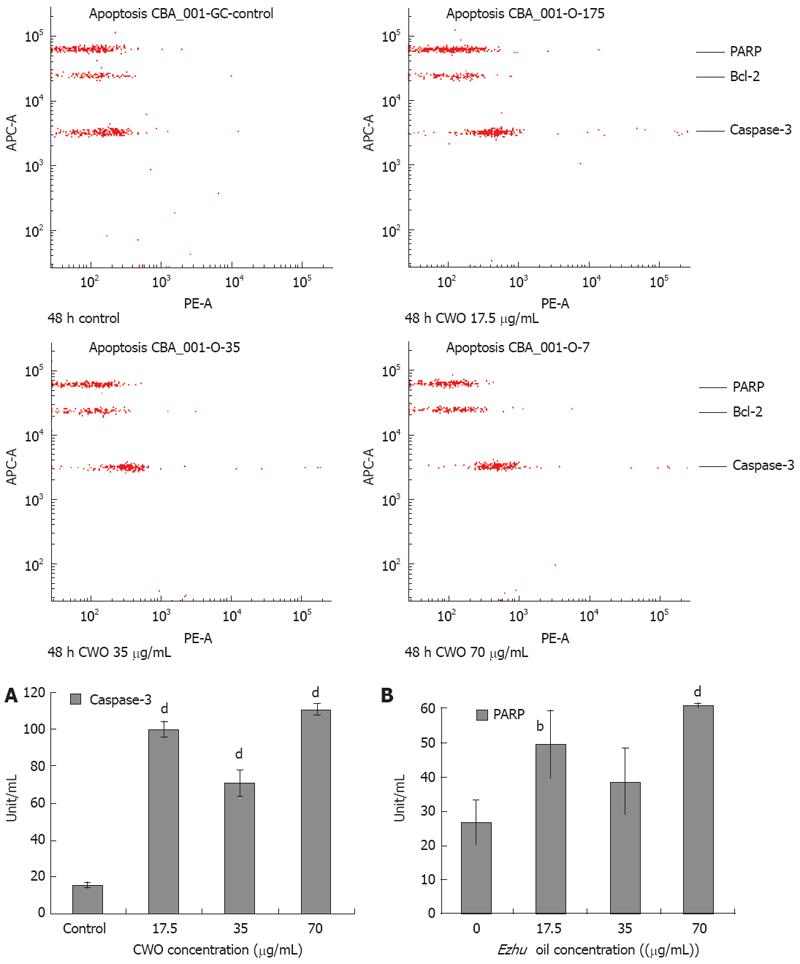
Measurement of cleaved PARP and active caspase-3 protein levels: The BD™ CBA human apoptosis kit (BD, Franklin Lakes, USA) was applied to quantify the active caspase-3 and Poly-ADP-ribose polymerase (PARP) protein levels; cytometric Bead Array (CBA) employs a particle with a discrete fluorescence intensity to detect a soluble analyte. This kit provided two types of bead populations with distinct fluorescence intensities that have been coated with capture antibodies specific for cleaved caspase-3 and PARP. Cells were seeded in 6-well plates and incubated with various concentrations of CWO in an humidified atmosphere (37°C with 5% CO2) for 48 h. 1.0 × 106 cells per sample were counted, harvested, and washed with PBS. Fifty &mgr;L of cell lysis buffer was added to each sample for 30 min on ice and samples were vortexed at 10-min intervals. Pellet cellular debris was removed by centrifugation at 12 500 r/min for 10 min. Protein concentrations were measured by 2-D Quant Kit (Amersham Biosciences, Piscataway, USA). Each sample was normalized in a final concentration of 0.2 &mgr;g/&mgr;L. Thirteen standard curves (standard ranging from 0 to 6000 U/mL) were obtained from one set of calibrators. For each sample and the standard mixture of lysate standard (caspase-3 and PARP beads), 50 mL of sample or standard of beads were added to the mixture of 50 mL of 2 mixed capture beads incubated for 1 h, and mixed 50 mL of PE detector bead for another 1 h. After that, samples were washed before data acquisition with a FCM. The results were analyzed by FCAP Array V1.0.
The data are expressed as mean ± SD from at least 3 independent experiments. Differences between groups were analyzed using a Student’s t-tests.
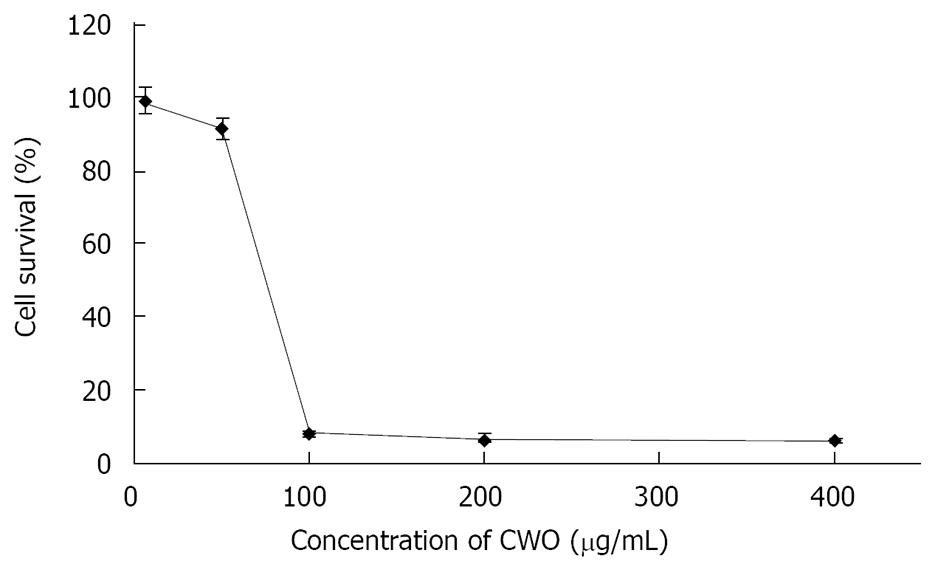
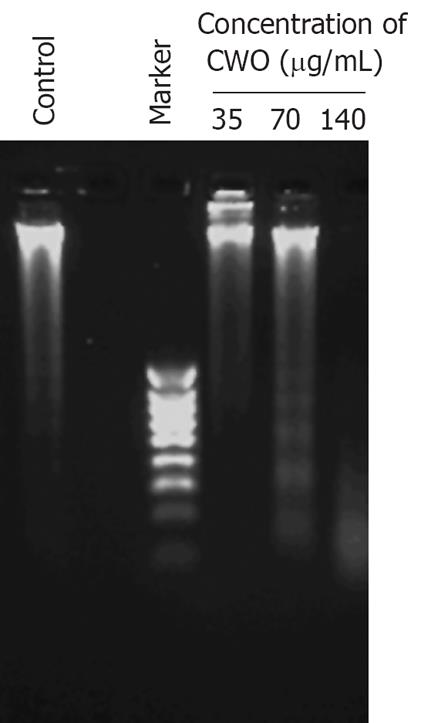
CWO treatment inhibited the growth of HepG2 cells in a dose-dependent manner, and the IC50 of CWO was approximately 70 &mgr;g/mL (Figure 1). We then examined whether CWO inhibited HepG cell growth through inducing cell death and apoptosis. HepG2 cell were treated with different concentrations of CWO for 72 h. Figure 2 shows that DNA fragmentation was evidently observed at a concentration of 70 &mgr;g/mL after 72 h of treatment.
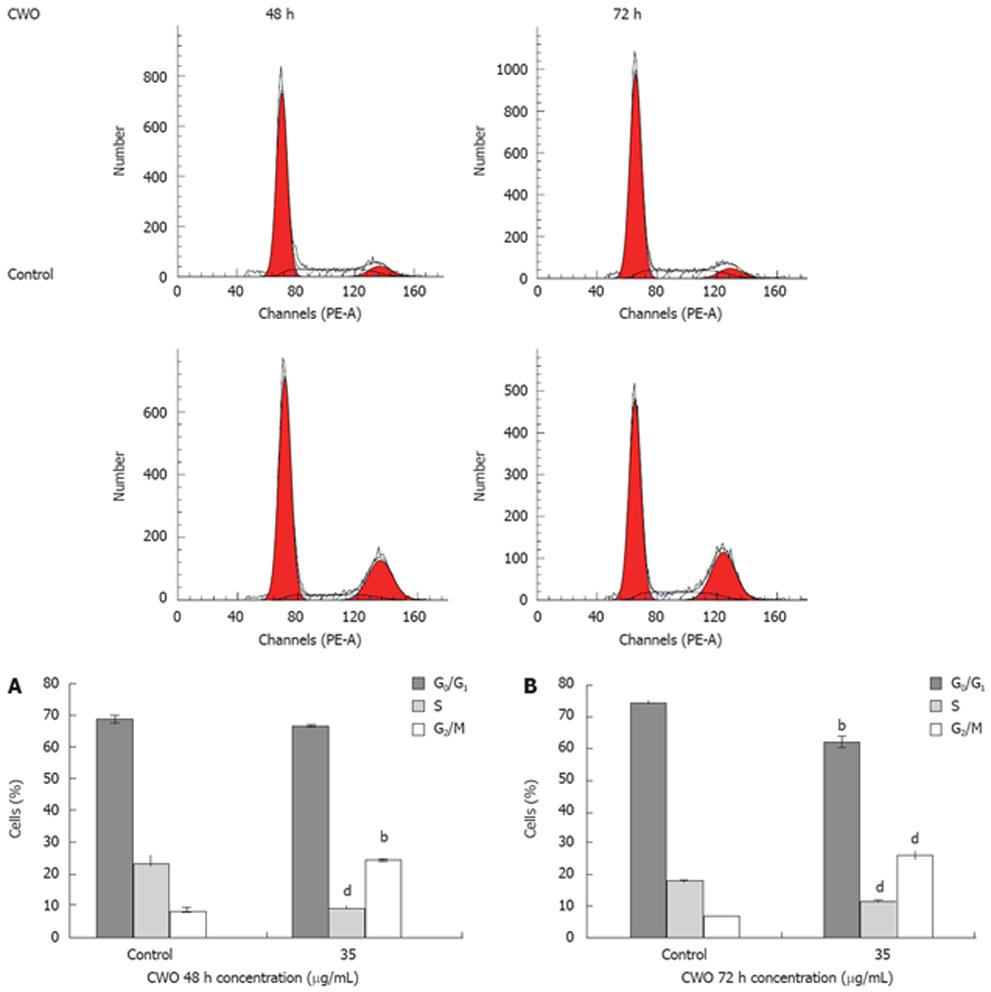
The effect of different concentrations of CWO on cell-cycle progression was studied after 48 and 72 h of drug exposure. CWO treatment resulted in the accumulation of cells in S/G2 phase with concomitant losses from G0/G1 phase (Figure 3). Since substantial proportions of cells were dead in groups treated with 50 &mgr;g/mL and 70 &mgr;g/mL CWO, only cultures treated with 35 &mgr;g/mL were used for the analysis as shown in Figure 4.
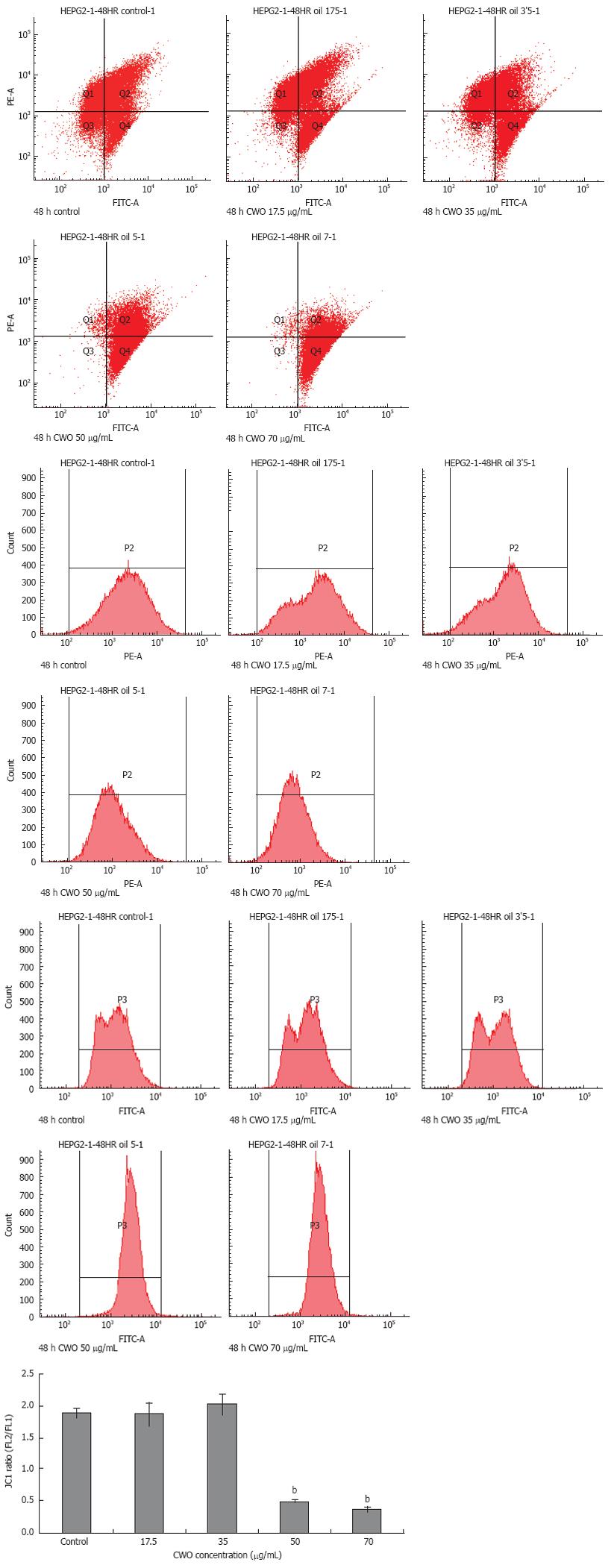
Some chemotherapeutic drugs induced apoptosis via mitochondrial pathways by altering ΔΨm. To monitor the ΔΨm, we used JC-1 probe to determine the ΔΨm in cells that were treated with CWO for 48 h at different concentrations. Mitochondria with normal ΔΨm concentrate JC-1 into aggregates (red/orange fluorescence), while in depolarized mitochondria, JC-1 forms monomers (green fluorescence). As compared to non-treated HepG2 cells, green fluorescence increased while red/orange fluorescence decreased after CWO exposure. Figure 4 indicates an only minor shift from red/orange to green fluorescence in groups treated with 17.5 &mgr;g/mL and 35 &mgr;g/mL CWO, while 50 &mgr;g/mL and 70 &mgr;g/mL CWO led to significant changes in ΔΨm.
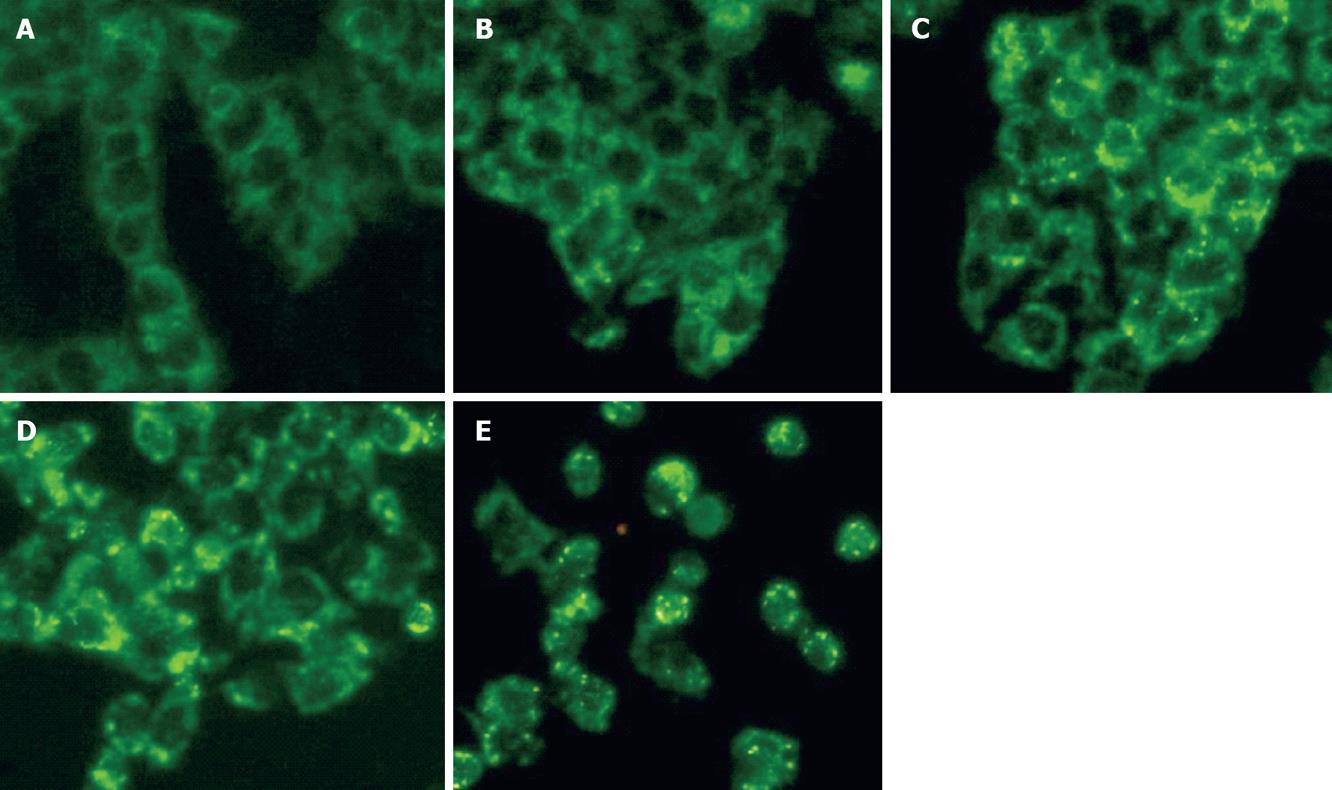
Cytochrome C release from mitochondria to the cytosol is implicated in mitochondria dependent apoptosis[16]. Cytochrome C staining in the cytosol of HepG2 cells showed markedly stronger green fluorescence than in control cells in a dose-dependent fashion (Figure 5). CWO treated cells showed obvious punctuate green fluorescence staining or appeared to have green fluorescence accumulated in large aggregates compared to the control.
Many studies previously have demonstrated that programmed cell death is associated with the activation of caspase as key elements involved in the sequence of events that lead to cell death[17]. Caspase-3 particularly, is essential for propagation of the apoptotic signal after exposure to many DNA-damaging agents and anticancer drugs. We examined caspase-3 activity after cells were treated with 17.5-70 &mgr;g/mL CWO for 24 h. The result clearly demonstrated that caspase-3 activity increased in a dose-dependent manner (Figure 6). Caspase-3 activities were 2 and 5.5 times higher in 35 &mgr;g/mL and 70 &mgr;g/mL CWO treated cultures, respectively, when compared to the control. When the cellular samples were incubated with specific Ac-DEVD-CHO inhibitors simultaneously, caspase-3 activity was blocked.
Drug-induced cell death via apoptosis pathway, signaling can generally be divided into receptor- and mitochondrial-mediated pathways. These pathways converge at several downstream points including the mitochondria, caspase activation, and substrate cleavage[18]. Figure 7 showed that there was significant increase in protein levels of cleaved PARP and active caspase-3 in a dose-dependent manner as determined by BD™ CBA Human Apoptosis Kit. HepG2 cells treated with 17.5, 35, and 70 &mgr;g/mL CWO were 6, 4, and 7 times higher in caspase-3 protein level than control group (Figure 7A). The level of cleavage of PARP had a 2-fold increase in HepG2 cells treated with 70 &mgr;g/mL CWO compared to the control (Figure 7B).
The essential oil of Ezhu and its ingredients have been widely used for treatment of malignant tumors in China[19] and have been identified to have hepatoprotective effects[2021]. Previously, we have already identified an active ingredient furanodiene, a sesquiterpene compound, which have been isolated from CWO, one of species of Ezhu. Furanodiene has been found to induce apoptosis in HepG2 cells through activation of mitochondrial and caspase dependent pathway which involved activation of P38, and inactivation of ERK1/2 MAPK signaling cascades[15]. In the present study, we have found that CWO inhibited HepG2 cells growth with IC50 at approximately 70 &mgr;g/mL and it has been identified to inhibit HepG2 cell growth via inducing apoptosis as evidenced by activation of depolarization of ΔΨm, mitochondrial cytochrome C release, caspase-3, PARP cleavage, S/G2 cell cycle arrest, and finally DNA fragmentation.
Active caspase-3 has been considered to be indicative of apoptosis[17] and another characteristic event of apoptosis is the proteolytic cleavage of PARP, a nuclear enzyme involved in DNA repair, DNA stability, and transcriptional regulation[22]. An experiment was performed to simultaneously and quantitatively evaluate CWO induced changes of active caspase-3 and cleaved PARP protein levels using CBA technology as demonstrated in Figure 7. As shown, active caspase-3 protein expressions were enhanced approximately 7-fold compared to the control. Meanwhile, we attempted to detect CWO-induced caspase-3 enzymatic activity increases using caspase-3 specific substrate Z-DEVE-R110, and have found that the enhancements disappeared when treated with Ac-DEVD-CHO inhibitor as shown in Figure 6. This showed the increases in caspase-3 enzymatic activities were specific to CWO treatment and suggested that CWO induces apoptosis via a caspase-3-dependent pathway.
CWO induced significant increase in caspase-3 enzymatic activity as well as the protein levels of active caspase-3 and cleaved PARP (Figure 7). These results suggested that CWO induced apoptosis via caspase pathway. Moreover, whether CWO induced apoptosis in HepG2 cell is mitochondria-dependent is unknown. To address this question, the change of ΔΨm and mitochondrial cytochrome C release were determined. Figures 4 and 5 suggested that CWO-mediated apoptosis was accompanied with the ΔΨm as well as the release of mitochondrial cytochrome C into cytosol. These results demonstrated that a mitochondrial pathway was also involved in CWO-induced apoptosis.
It is now widely believed that p38 and JNK mediate apoptotic signals, while ERK promotes growth, differentiation, and proliferation. Nowadays, many studies have shown that p38 MAPK activation is necessary for cancer cell death initiated by a variety of anti-cancer agents[23]. Furthermore, different MAPK signaling pathways can be coordinately manipulated to enhance the efficacy of anticancer drug. Co-treatment of anticancer drugs with ERK inhibitors has been found to enhance anticancer effects. Anti-cancer drug paclitaxel (Taxol) induces tumor cell apoptosis through activating endogenous JNK in tumor cells[24]. When paclitaxel and ERK inhibitor were combined in cancer treatment, ERK inhibitor significantly enhances the JNK activation-mediated cytotoxic effect of paclitaxel. ERK inhibitor also found to enhance docetaxel-induced apoptosis of androgen-independent human prostate cancer cells[25].
CWO, possibly act as chemopreventive agents with respect to inhibition of the growth of human HepG2 cells through the induction of apoptosis. As apoptosis has become a new therapeutic target in cancer research, these results confirm the potential of CWO as an agent of chemotherapeutic and cytostatic activity in human HepG2 cells. Besides, in our previous study, furanodiene, isolated from CWO, has been identified induce HepG2 cell apoptosis through alternating MAPK signaling. Furanodiene obviously elevated phosphorylated form of P38 and reduced phosphorylated form of ERK1/2 in a dose-dependent manner, but a slightly and statistically insignificant change in phosphorylated form of JNK. Therefore, furanodiene induced-apoptosis in HepG2, involve activation of P38 and inhibition of ERK MAPK signaling. Whether CWO acts on these signaling pathways should be an interesting area for further study.
In short, we conclude that CWO induces apoptosis in HepG2 cells through activation of mitochondrial and caspase-3 pathway.
Ezhu has a long history on treating liver diseases and protecting liver functions. Chinese Pharmacopoeia indicated that the rhizomes of three species including Curcuma phaeocaulis, C. kwangsiensis and C. wenyujin are used as Ezhu. The essential oil of Ezhu has been reported to possess various biological roles such as antimicrobial, anti-inflammatory, and anti-tumor activity. Some sesquiterpene compounds isolated from essential oil of Curcuma wenyujin (CWO) has been identified to have hepatoprotective effects. The total effects of the complex interactions of different compounds in extracts isolated from CWO are not well characterized.
Hepatocellular carcinoma (HCC) is the fifth most commonly diagnosed cancer with more than 1 million deaths reported annually worldwide. Many sesquiterpenes are identified to possess protective effects against carcinogenesis or tumor growth. For example, artemisinin, a sesquiterpene lactones, killed human oral cancer cells through apoptosis and may be useful as an alternative treatment for oral cancer. In order to develop effective means for prevention and treatment of HCC and related liver diseases, extensive research is being carried out to isolate and identify chemical extracts and pure compounds from Chinese medicine with hepatoprotective, anti-hepatoma, anti-multidrug resistant hepatoma, and anti-viral effects.
This is the first study to report the biological activity and mechanism of action of CWO on HCC cells.
CWO induce apoptosis in HepG2 cells through activation of mitochondrial and caspase-3-pathway and the result suggests the potential value of development of Ezhu on treatment of liver diseases and further study on anti-apoptotic effect of CWO may lead to identification of new lead compounds and novel drug targets for treatment of liver cancer and diseases.
This is an interesting study showing that CWO inhibits growth and induces apoptosis in human HepG2 hepatoma cells. These findings are of interest.
| 1. | Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10-30. |
| 2. | Kim HR, Park HJ, Park JH, Kim SJ, Kim K, Kim J. Characteristics of the killing mechanism of human natural killer cells against hepatocellular carcinoma cell lines HepG2 and Hep3B. Cancer Immunol Immunother. 2004;53:461-470. |
| 3. | Ochi M, Ohdan H, Mitsuta H, Onoe T, Tokita D, Hara H, Ishiyama K, Zhou W, Tanaka Y, Asahara T. Liver NK cells expressing TRAIL are toxic against self hepatocytes in mice. Hepatology. 2004;39:1321-1331. |
| 4. | Pei Z, Chu L, Zou W, Zhang Z, Qiu S, Qi R, Gu J, Qian C, Liu X. An oncolytic adenoviral vector of Smac increases antitumor activity of TRAIL against HCC in human cells and in mice. Hepatology. 2004;39:1371-1381. |
| 5. | Yamanaka T, Shiraki K, Sugimoto K, Ito T, Fujikawa K, Ito M, Takase K, Moriyama M, Nakano T, Suzuki A. Chemotherapeutic agents augment TRAIL-induced apoptosis in human hepatocellular carcinoma cell lines. Hepatology. 2000;32:482-490. |
| 6. | Pharmacopoeia of the People’s Republic of China (English edition); Pharmacopeia Commission of PRC, Eds.; Beijing: Chemical Industry Press. 2000;230. |
| 7. | Yang FQ, Wang YT, Li SP. Simultaneous determination of 11 characteristic components in three species of Curcuma rhizomes using pressurized liquid extraction and high-performance liquid chromatography. J Chromatogr A. 2006;1134:226-231. |
| 8. | He JS. Clinic application of oil of ezhu in paediatrics. Xiandai Zhongxiyi Jiehe Zazhi. 2006;15:501. |
| 9. | Yin H, Sun Y. Combination of Baofu Kang Shuan with nystatin on rosary fungus vaginitis. Xiandai Zhongxiyi Jiehe Zazhi. 2006;15:144. |
| 10. | Wilson B, Abraham G, Manju VS, Mathew M, Vimala B, Sundaresan S, Nambisan B. Antimicrobial activity of Curcuma zedoaria and Curcuma malabarica tubers. J Ethnopharmacol. 2005;99:147-151. |
| 11. | Sasaki Y, Goto H, Tohda C, Hatanaka F, Shibahara N, Shimada Y, Terasawa K, Komatsu K. Effects of curcuma drugs on vasomotion in isolated rat aorta. Biol Pharm Bull. 2003;26:1135-1143. |
| 12. | Uechi S, Ishimine Y, Hong F. Antibacterial activity of essential oil derived from Curcuma zedoaria against food borne pathogenic bacteria and their thermal stability. Rdngbm. 2000;47:129-136. |
| 13. | Wu WY, Xu Q, Shi LC, Zhang WB. Inhibitory effects of Curcuma aromatica oil on proliferation of hepatoma in mice. World J Gastroenterol. 2000;6:216-219. |
| 14. | Ding YL, Xu AX. Effects of oil of ezhu and its valid component against tumor. Zhongyaocai. 2005;28:152-156. |
| 15. | Xiao Y, Yang FQ, Li SP, Gao JL, Hu G, Lao SC, Conceicao EL, Fung KP, Wang YT, Lee SM. Furanodiene Induces G(2)/M Cell Cycle Arrest and Apoptosis Through MAPK Signaling and Mitochondria-Caspase Pathway in Human Hepatocellular Carcinoma Cells. Cancer Biol Ther. 2007;6:1044-1050. |
| 16. | Brenner C, Kroemer G. Apoptosis. Mitochondria--the death signal integrators. Science. 2000;289:1150-1151. |
| 18. | Ferri KF, Kroemer G. Organelle-specific initiation of cell death pathways. Nat Cell Biol. 2001;3:E255-E263. |
| 19. | Peng X, Zhao Y, Liang X, Wu L, Cui S, Guo A, Wang W. Assessing the quality of RCTs on the effect of beta-elemene, one ingredient of a Chinese herb, against malignant tumors. Contemp Clin Trials. 2006;27:70-82. |
| 20. | Matsuda H, Ninomiya K, Morikawa T, Yoshikawa M. Inhibitory effect and action mechanism of sesquiterpenes from Zedoariae Rhizoma on D-galactosamine/lipopolysaccharide-induced liver injury. Bioorg Med Chem Lett. 1998;8:339-434. |
| 21. | Morikawa T, Matsuda H, Ninomiya K, Yoshikawa M. Medicinal foodstuffs. XXIX. Potent protective effects of sesquiterpenes and curcumin from Zedoariae Rhizoma on liver injury induced by D-galactosamine/lipopolysaccharide or tumor necrosis factor-alpha. Biol Pharm Bull. 2002;25:627-631. |
| 22. | D’Amours D, Desnoyers S, D’Silva I, Poirier GG. Poly(ADP-ribosyl)ation reactions in the regulation of nuclear functions. Biochem J. 1999;342:249-268. |
| 23. | Olson JM, Hallahan AR. p38 MAP kinase: a convergence point in cancer therapy. Trends Mol Med. 2004;10:125-129. |
| 24. | MacKeigan JP, Collins TS, Ting JP. MEK inhibition enhances paclitaxel-induced tumor apoptosis. J Biol Chem. 2000;275:38953-38956. |
| 25. | Zelivianski S, Spellman M, Kellerman M, Kakitelashvilli V, Zhou XW, Lugo E, Lee MS, Taylor R, Davis TL, Hauke R. ERK inhibitor PD98059 enhances docetaxel-induced apoptosis of androgen-independent human prostate cancer cells. Int J Cancer. 2003;107:478-485. |