Published online Jul 14, 2008. doi: 10.3748/wjg.14.4190
Revised: June 3, 2008
Accepted: June 10, 2008
Published online: July 14, 2008
AIM: To analyze pituitary hormone and melatonin circadian rhythms, and to correlate hormonal alterations with clinical performance, hepatic disease severity and diagnostic tests used for the detection of hepatic encephalopathy in cirrhosis.
METHODS: Twenty-six patients with cirrhosis were enrolled in the study. Thirteen patients hospitalized for systemic diseases not affecting the liver were included as controls. Liver disease severity was assessed by the Child-Pugh score. All patients underwent detailed neurological assessment, electroencephalogram (EEG), brain magnetic resonance imaging (MRI), assays of pituitary hormone, cortisol and melatonin, and complete blood chemistry evaluation.
RESULTS: Pituitary hormone and melatonin circadian patterns were altered in cirrhosis patients without clinical encephalopathy. Circadian hormone alterations were different in cirrhosis patients compared with controls. Although cortisol secretion was not altered in any patient with cirrhosis, the basal cortisol levels were low and correlated with EEG and brain MRI abnormalities. Melatonin was the only hormone associated with the severity of liver insufficiency.
CONCLUSION: Abnormal pituitary hormone and melatonin circadian patterns are present in cirrhosis before the development of hepatic encephalopathy. These abnormalities may be early indicators of impending hepatic encephalopathy. Factors affecting the human biologic clock at the early stages of liver insufficiency require further study.
- Citation: Velissaris D, Karanikolas M, Kalogeropoulos A, Solomou E, Polychronopoulos P, Thomopoulos K, Labropoulou-Karatza C. Pituitary hormone circadian rhythm alterations in cirrhosis patients with subclinical hepatic encephalopathy. World J Gastroenterol 2008; 14(26): 4190-4195
- URL: https://www.wjgnet.com/1007-9327/full/v14/i26/4190.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.4190
Hepatic encephalopathy, a major complication of cirrhosis, is a clinical syndrome characterized by mental status changes in patients with severe hepatic insufficiency. By contrast, the term “Minimal Hepatic Encephalopathy”, also known as subclinical hepatic encephalopathy (SHE) describes disturbances of several biological functions, including sleep and activities of daily living, in the absence of clinical neurologic symptoms[1–3]. Hormonal disorders and circadian rhythm abnormalities are often associated with liver disease[4], and the severity of these disorders is related to liver disease severity and duration. The role of melatonin is critical, as diurnal melatonin rhythm disruption may significantly contribute to circadian function alterations[5].
The main goal of the present study was to evaluate the circadian hormone secretion profile in cirrhosis patients without hepatic encephalopathy. Specifically, the study was designed to analyze the circadian rhythm of pituitary hormone, serum cortisol and melatonin, and correlate the hormone levels and 24-h hormone secretion abnormalities with brain magnetic resonance imaging (MRI) and electroencephalogram (EEG), which are used for the diagnosis of encephalopathy. In addition, the study also assessed the correlation between circadian hormone rhythm and the severity of hepatic disease as measured by the Child-Pugh score.
This was an observational study conducted at the University Hospital of Patras, Greece, in the years 2005-2006.
Twenty-six patients with cirrhosis were enrolled in the study. In addition, 13 patients hospitalized for various chronic diseases without liver or central nervous system (CNS) involvement were included as controls. We chose not to have a healthy control group, because hormone patterns in healthy people have been described in detail. Inclusion criteria were: age 35-75 years, abstinence from alcohol for at least 6 mo, cirrhosis confirmed by liver biopsy, and regular follow-up in our Liver Outpatient Clinic. Exclusion criteria were signs or symptoms of encephalopathy, any CNS or endocrine disease, use of medications with CNS effects, and illegal substance abuse.
Mean age was 64.6 ± 9.5 years in cirrhosis patients and 67.8 ± 10.8 years in controls. There were no significant differences between men and women. The etiology of disease in the two study groups is shown in Table 1.
| Cirrhosis patients | Controls | ||
| Cirrhosis etiology | Patient number | Admission diagnosis | Patient number |
| Alcohol | 13 | COPD | 5 |
| HBV infection | 9 | Cancer (no liver or CNS metastasis) | 4 |
| HCV infection | 1 | Ulcerative colitis | 3 |
| Alcohol + HBV | 1 | Crohn’s disease | 1 |
| Alcohol + HCV | 1 | ||
| Unknown | 1 | ||
The study was approved by the Institution Ethics Committee, and a written informed consent was obtained from all patients.
All study participants underwent comprehensive biochemical and clinical evaluation. The severity of cirrhosis was assessed by the Child-Pugh classification: 22 patients were Child-Pugh A (16 with score 5, and 6 with score 6), and 4 patients were Child-Pugh B (1 with score 7, 2 with score 8 and 1 with score 9). There was no patient with Child-Pugh class C.
Blood samples for cortisol, melatonin, prolactin and TSH levels were drawn at 09.00, 14.00 and 21.00 h in an attempt to make inferences about circadian hormone secretion patterns. Prolactin, TSH and cortisol levels were measured by the electrochemiluminescence technique (Elecsys 2010 ROCHE, Roche Diagnostics GmbH D-68298 Manheim), while melatonin levels were determined using a radio-immunoassay method (Biosourse, rue de1: Industrie-B-1400 Nivelles, Catalog Nr. KIPLO800).
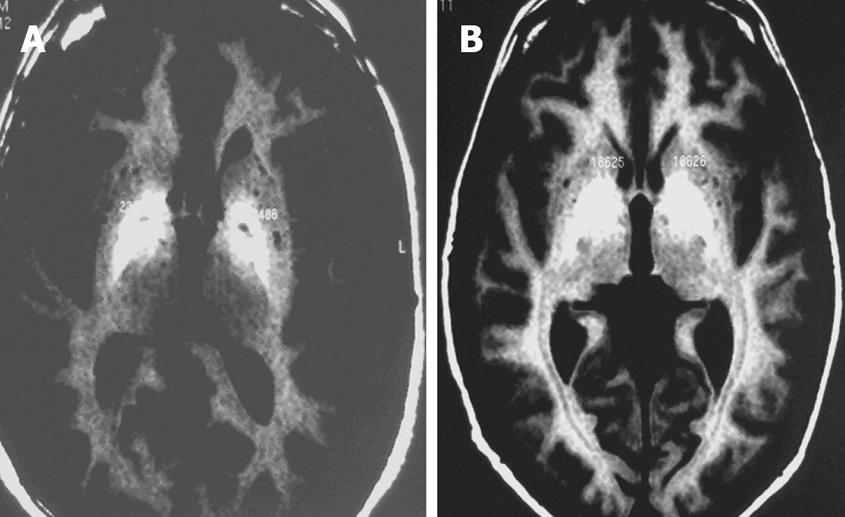
Brain MRI was performed without contrast with a 1 Tesla Gyroscan Intera MRI scanner using a head coil. Transverse and coronal sections were obtained with T1 sequences. Basal ganglia MRI signal was evaluated and compared with adjacent brain white matter MRI signal. The regions of interest (ROI) in the globus pallidus were defined bilaterally in axial and coronal images, whereby each ROI included a predetermined number of pixels. Quantitative image analysis was done by calculating the mean signal intensity for each ROI. MRI signal was classified as Grade 0 = no alterations, Grade 1= mild alterations (Figure 1A) or Grade 2 = severe alterations (Figure 1B).
All patients underwent comprehensive clinical neurologic examination with emphasis on cortical function assessment. An awake 16-channel digital EEG was obtained with a standard 10-20 scalp electrode system. Abnormal EEG findings were classified as specific (epileptiform or paroxysmal) or nonspecific (theta and delta waves in various combinations) disturbances. Nonspecific disturbances were further classified as mild, moderate or severe.
All statistical analysis was done with SPSS (Chicago, Illinois, USA) version 12 for Windows. Continuous data are presented as mean ± SD. The Student’s t-test was used to compare means and the Fischer’s exact test to compare proportions. Correlations between continuous variables were evaluated with Pearson’s correlation coefficient. Hormone assay results were analyzed with repeated measures ANOVA on the log of measured values. The Student-Neumann-Keuls test was used for post-hoc multiple comparisons. P < 0.05 was considered statistically significant for all tests.
All cirrhosis patients had normal muscle tone, normal tendon reflexes and no flapping tremors. Ascites was present in 2 patients, splenomegaly in 14 and endoscopically documented esophageal varices in 8 patients. Neurological evaluation did not reveal any abnormalities in the control subjects.
Baseline liver function tests showed minimal hepatic insufficiency without evidence of active liver disease (Table 2).
| Parameter | Cirrhosis | Control | P |
| Bilirubin (mg/dL) | 1.44 ± 0.95 | 0.89 ± 0.24 | < 0.01 |
| Albumin (q/dL) | 4.0 ± 0.5 | 3.7 ± 0.9 | |
| Globulin (q/dL) | 3.9 ± 0.7 | 3.8 ± 0.5 | |
| PT (s) | 14.4 ± 1.8 | 11.4 ± 0.8 | < 0.001 |
| SGOT (U/L) | 49 ± 31 | 42 ± 34 |
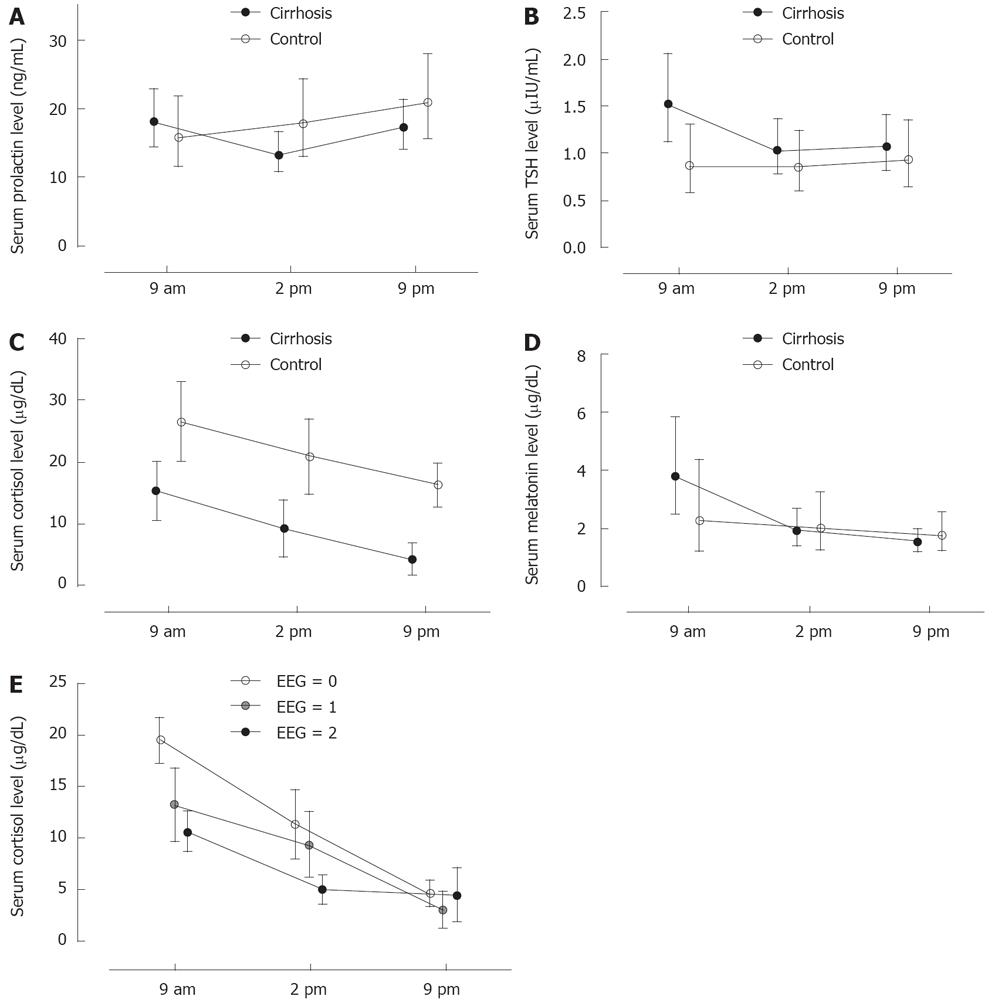
Hormone concentrations at 09.00 h, 14.00 h and 21.00 h are presented in Figure 2A-D.
Prolactin levels did not show significant variations during the day in the controls. By contrast, prolactin levels were significantly (P < 0.05) lower at 14.00 compared to 09.00 and 21.00 h in the cirrhosis group.
Morning TSH levels were significantly higher (P < 0.05) in cirrhosis patients compared to controls. Within the cirrhosis group, TSH levels were significantly higher (P < 0.001) at 9.00 h compared to 14.00 h and 21.00 h.
Cortisol levels were significantly lower (P < 0.001) at all times in cirrhosis patients compared to controls. However, the circadian cortisol secretion pattern was not altered compared to the pattern described in healthy individuals. Both the study groups (cirrhosis patients and controls) demonstrated a significant (P < 0.05) trend of decreasing cortisol levels from morning to night.
Melatonin levels were higher at 9.00 h compared to 14.00 h (P < 0.05) and 21.00 h (P < 0.01) in cirrhosis patients, whereas there was no such pattern in controls. The main difference between cirrhosis patients and controls was higher morning melatonin levels in patients with cirrhosis.
Brain MRI was abnormal in 18 of 26 cirrhosis patients, with high bilateral symmetrical signal intensity on T-1 images in the globus pallidus, the putamen, or both. The mean basal ganglia signal intensity was 1093.4 ± 171.8 units on T-1 images. We did not find any brain MRI abnormalities in controls. The MRI abnormalities and Child Scores are presented in Table 3.
| Child score | A5 (n = 16) | A6 (n = 6) | B7 (n = 1) | B8 (n = 2) | B9 (n = 1) | C (n = 0) | |
| Severity of brain MRI abnormalities | 0 | 8 (50) | - | - | - | - | - |
| 1 | 5 (31.20) | 5 (83) | 1 (100) | 1 (50) | - | - | |
| 2 | 3 (18.80) | 1 (17) | - | 1 (50) | 1 (100) | - |
EEG was performed in 22 of 26 cirrhosis patients and demonstrated nonspecific disturbances in 11 (50%) patients. Disturbances consisted of theta or delta waves, and were graded as mild (7 patients), moderate (3 patients), or severe (1 patient). We did not find epileptiform discharges in any patient. Cirrhosis patients with abnormal EEG had significantly (P < 0.05) higher mean basal ganglia MRI signal intensity (1151.1 ± 177.8 units) compared to those with normal EEG (1014 ± 135.3 units).
We did not find any correlation between melatonin, TSH and prolactin levels with MRI abnormalities. However, serum cortisol levels showed significant (P < 0.005) association with brain MRI abnormalities; the 9.00 (P < 0.04) and 14.00 h (P < 0.01) cortisol levels correlated with the severity of MRI disturbances.
We did not find any association between prolactin or TSH levels and cirrhosis severity as measured by the Child-Pugh score. However, there was an association between cortisol and melatonin levels in patients with Child-Pugh score of 5 (16 patients) compared with those with score > 5 (10 patients). Specifically, patients with Child score > 5 manifested impaired circadian cortisol variation (P < 0.05) and significantly lower morning cortisol levels (P < 0.01) compared to those with Child score of 5 (Figure 2E).
The evening melatonin levels were significantly (P < 0.04) lower in cirrhosis patients with Child-Pugh score > 5 (Group 2) compared to those with Child-Pugh score of 5 (Group 1).
We did not observe any association between prolactin, TSH, or melatonin levels and the severity of EEG disturbances (quantified as 0 = no EEG abnormalities, 1 = mild EEG abnormalities, 2 = severe EEG abnormalities). However, cortisol levels were higher in cirrhosis patients without EEG abnormalities compared to those with mild (P < 0.05) EEG disturbances, and were even higher compared to those with severe (P < 0.01) EEG abnormalities. The observed association between cortisol and EEG abnormalities was more pronounced in the morning (P < 0.001).
The presence of characteristic brain MRI and EEG disturbances, and hormone abnormalities in cirrhosis patients without hepatic encephalopathy is the main finding of the present study.
Hepatic encephalopathy is a syndrome characterized by abnormal mental status in patients with severe liver disease[6–8]. SHE is a milder condition associated with cirrhosis and/or porto-systemic shunts. The diagnosis of SHE is clinically relevant because it may precede the development of overt hepatic encephalopathy. Moreover, the psychomotor deficits in SHE may impair cognitive function and activities of daily living[23]. SHE diagnosis is based on psychometric tests, EEG and brain MRI. However, since there is no “gold standard” for diagnosing SHE[1–39], the prevalence of this condition in cirrhosis has been reported variously as 30% to 84%, possibly due to different diagnostic criteria used in the various studies.
Cirrhosis patients without clinical encephalopathy often demonstrate high basal ganglia MRI T-1 signal intensity, likely due to manganese deposition in the brain[10–12].
EEG is useful in the diagnosis of SHE, as slow (2-5 Hz) high-amplitude frontal lobe waves are characteristic of early hepatic encephalopathy. Although EEG abnormalities are not encephalopathy-specific, abnormal theta and delta wave activity correlates with disease severity [13].
Several biological rhythm abnormalities, including impaired arterial pressure diurnal variation, nocturnal portal pressure rise, melatonin secretion and sleep pattern alterations occur in cirrhosis. The various mechanisms proposed to explain these circadian abnormalities include the following: (a) effect of neurotoxins on the suprachiasmatic hypothalamic nucleus (SCN), which is the “human biologic clock”[1415], and (b) elevated morning melatonin levels due to impaired liver melatonin metabolism, causing a circadian clock phase-shift[1617].
Liver diseases are associated with several hormone disorders, including decreased serum levels of T3, cortisol, testosterone, FSH and insulin, and elevated prolactin concentrations[18–22]. In addition, a characteristic high daytime melatonin pattern[5] has been described; this may contribute to the sleep-wake cycle disturbances and hormone disorders, as SCN is located in the hypothalamus, which regulates pituitary hormones[523–26].
Melatonin may act as an internal circadian body rhythm “synchronizer”, and plasma melatonin profile may be a circadian pacemaker marker. Therefore, melatonin rhythm disruption observed in cirrhosis may reflect circadian alterations with significant clinical implications: high daytime melatonin levels can cause an endogenous clock phase-shift and may therefore partly explain the sleep disturbances observed in cirrhosis[527–29]. Additional factors, unrelated to melatonin but involved in liver failure, such as false neurotransmitters, cerebral amines and cerebral arteriovenous shunts may also contribute to hormonal circadian abnormalities in the early stages of hepatic encephalopathy. Our findings suggest that diurnal melatonin abnormalities correlate with the severity of liver disease in cirrhosis and may be identifiable early, before the development of clinical hepatic encephalopathy.
Prolactin secretion follows a pulsatile pattern, with a characteristic nocturnal rise, but cirrhosis is associated with elevated 24-h prolactin levels and loss of circadian prolactin rhythm[30–32]. However, our cirrhosis patients had significant prolactin circadian rhythm disturbances without baseline elevation. The differences between our findings and previous studies[3031] may be explained by patient selection, as we excluded patients with clinical encephalopathy.
A diurnal TSH secretion pattern, with the highest concentrations in late evening and the first hours of nocturnal sleep is well documented in normal subjects. Circadian TSH level variations may be modulated, in part, by a dopaminergic mechanism, which plays a major role in TSH rhythmicity in liver disease[33–35]. In our study TSH circadian abnormality was identified in the absence of clinical encephalopathy.
Although impaired cortisol inactivation is well documented in cirrhosis, basal circadian cortisol secretion remains stable[3637]. In the present study, patients with cirrhosis had low 24 h cortisol levels compared to controls.
Our data suggests that cirrhosis patients without encephalopathy have disrupted melatonin, TSH and prolactin circadian cycle, and suppressed 24-h cortisol levels, but the circadian cortisol rhythmicity is unaffected. More importantly, the melatonin abnormalities (lower night levels) are more pronounced in advanced liver failure (Child score > 5). As these findings were not seen in controls, the hormone abnormalities identified in cirrhosis could be specific for liver disease.
We did not find any correlation between melatonin, TSH or prolactin levels with the severity of brain MRI or EEG abnormalities, but the lack of association may be a type-II error due to the small number of patients. Cortisol levels correlated with brain MRI abnormalities, EEG abnormalities and severity of liver disease (Child score > 5). Melatonin and cortisol abnormalities correlated with severity of liver disease, while TSH and prolactin levels did not. As we could not find any similar findings in the literature, we believe these observations deserve further investigation.
Our cirrhotic patients with SHE had abnormal circadian pituitary hormone secretion and diurnal melatonin cycle. The abnormal hormonal pattern in SHE is different compared to patients with systemic diseases not affecting the liver and healthy individuals. The presence of these abnormalities in cirrhotics without clinical encephalopathy raises the possibility that these patterns may represent indicators of early hepatic encephalopathy.
We believe that originality is the main strength of our study, as there are no published reports on pituitary hormone abnormalities in relation to the severity of hepatic encephalopathy. The main limitations include the study design (observational, no randomization, no power analysis, small patient number), and the attempt to make inferences about circadian patterns from three measurements per day. An additional (fourth) measurement if obtained the following morning could have provided additional insight into the time course of the observed hormone changes. Since the number of statistical comparisons largely exceeded the number of cases and groups, positive findings should be interpreted with caution, because of the possibility of type I error. The inclusion of a healthy control group would have improved the study, and should perhaps be considered in future studies on this subject.
In conclusion, circadian hormone disturbances occur early in cirrhosis and are associated with disease severity. These observations raise the interesting hypothesis that alterations in circadian hormone secretion may be an early sign of impending clinical encephalopathy. This hypothesis has significant clinical implications and therefore, we believe, deserves further investigation.
Hepatic encephalopathy is a neuropsychiatric disorder associated with clinical manifestations ranging from slightly altered mental status to coma. The severity of symptom depends on the severity of liver disease, and the presence of metabolic or infectious complications. The term “Minimal Hepatic Encephalopathy” includes biological disturbances such as that of sleep and daily activities, in the absence of neurologic symptoms. Abnormalities of psychometric tests, electroencephalogram (EEG) and brain magnetic resonance imaging (MRI) may support the diagnosis of Minimal Hepatic Encephalopathy. Hormonal disorders and circadian rhythm abnormalities are often associated with liver disease, and the severity of these disorders is related to the severity and duration of the liver disease. The role of melatonin, a marker of intrinsic circadian pacemaker is critical, since diurnal melatonin rhythm disruption may reflect circadian function alterations leading to disturbances in the daily activities.
Circadian pituitary hormone alterations, brain MRI and EEG abnormalities have been described in cirrhosis. However, there is limited data on the relationship of these abnormalities with the severity of cirrhosis and hepatic encephalopathy. This field deserves further study.
This present study is the first attempt to evaluate circadian hormone abnormalities in relation to EEG, brain MRI, subclinical hepatic encephalopathy (SHE) and the severity of cirrhosis. The main finding is the presence of characteristic brain MRI, EEG and hormone secretion abnormalities in cirrhosis patients with SHE.
Our data suggests that melatonin and pituitary hormone circadian rhythm abnormalities are present early in the course of cirrhosis and are associated with the severity of liver disease in patients without clinical encephalopathy. These findings raise the interesting hypothesis that circadian hormone abnormalities may be an early sign of the development of hepatic encephalopathy.
Circadian rhythm means a predictable physiologic fluctuation in a 24 h period; SHE is a clinical entity consisting of mild neuropsychological abnormalities affecting the activities of daily living in cirrhosis patients without clinical encephalopathy.
This is a non-randomized observational study with novel and interesting findings, demonstrating that abnormal pituitary hormone and melatonin circadian patterns are present in cirrhosis patients without hepatic encephalopathy. The manuscript is well written.
| 2. | Groeneweg M, Quero JC, De Bruijn I, Hartmann IJ, Essink-bot ML, Hop WC, Schalm SW. Subclinical hepatic encephalopathy impairs daily functioning. Hepatology. 1998;28:45-49. |
| 3. | Groeneweg M, Moerland W, Quero JC, Hop WC, Krabbe PF, Schalm SW. Screening of subclinical hepatic encephalopathy. J Hepatol. 2000;32:748-753. |
| 4. | Blei AT, Zee P. Abnormalities of circadian rhythmicity in liver disease. J Hepatol. 1998;29:832-835. |
| 5. | Steindl PE, Finn B, Bendok B, Rothke S, Zee PC, Blei AT. Disruption of the diurnal rhythm of plasma melatonin in cirrhosis. Ann Intern Med. 1995;123:274-277. |
| 6. | Reichenbach A, Fuchs U, Kasper M, el-Hifnawi E, Eckstein AK. Hepatic retinopathy: morphological features of retinal glial (Muller) cells accompanying hepatic failure. Acta Neuropathol. 1995;90:273-821. |
| 7. | Haussinger D, Kircheis G, Fischer R, Schliess F, vom Dahl S. Hepatic encephalopathy in chronic liver disease: a clinical manifestation of astrocyte swelling and low-grade cerebral edema? J Hepatol. 2000;32:1035-1038. |
| 8. | Weissenborn K, Ennen JC, Schomerus H, Ruckert N, Hecker H. Neuropsychological characterization of hepatic encephalopathy. J Hepatol. 2001;34:768-773. |
| 9. | Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy--definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716-721. |
| 10. | Krieger D, Krieger S, Jansen O, Gass P, Theilmann L, Lichtnecker H. Manganese and chronic hepatic encephalopathy. Lancet. 1995;346:270-274. |
| 11. | Krieger S, Jauss M, Jansen O, Stiehl A, Sauer P, Geissler M, Theilmann L, Krieger D. MRI findings in chronic hepatic encephalopathy depend on portosystemic shunt: results of a controlled prospective clinical investigation. J Hepatol. 1997;27:121-126. |
| 12. | Skehan S, Norris S, Hegarty J, Owens A, MacErlaine D. Brain MRI changes in chronic liver disease. Eur Radiol. 1997;7:905-909. |
| 13. | Quero JC, Hartmann IJ, Meulstee J, Hop WC, Schalm SW. The diagnosis of subclinical hepatic encephalopathy in patients with cirrhosis using neuropsychological tests and automated electroencephalogram analysis. Hepatology. 1996;24:556-560. |
| 15. | Moore-Ede MC. The circadian timing system in mammals: two pacemakers preside over many secondary oscillators. Fed Proc. 1983;42:2802-2808. |
| 16. | Arendt J. Melatonin. Clin Endocrinol (Oxf). 1988;29:205-229. |
| 18. | Weitzman ED. Circadian rhythms and episodic hormone secretion in man. Annu Rev Med. 1976;27:225-243. |
| 19. | Weitzman ED. Biologic rhythms and hormone secretion patterns. Hosp Pract. 1976;11:79-86. |
| 20. | Barreca T, Franceschini R, Messina V, Bottaro P, Rolandi E. Changes in pituitary secretion after administration of branched-chain amino acids to patients with hepatic cirrhosis. Eur J Clin Pharmacol. 1983;25:763-766. |
| 21. | Kolster J, Castro de Kolster C, Bustillos R, de Pool I, Pepe M. [Pituitary-gonadal hormonal evaluation in male patients with alcoholic liver cirrhosis]. G E N. 1990;44:203-208. |
| 22. | De Besi L, Zucchetta P, Zotti S, Mastrogiacomo I. Sex hormones and sex hormone binding globulin in males with compensated and decompensated cirrhosis of the liver. Acta Endocrinol (Copenh). 1989;120:271-276. |
| 23. | Horne JA, Donlon J, Arendt J. Green light attenuates melatonin output and sleepiness during sleep deprivation. Sleep. 1991;14:233-240. |
| 24. | Skene DJ, Deacon S, Arendt J. Use of melatonin in circadian rhythm disorders and following phase shifts. Acta Neurobiol Exp (Wars). 1996;56:359-362. |
| 25. | Arendt J, Skene DJ, Middleton B, Lockley SW, Deacon S. Efficacy of melatonin treatment in jet lag, shift work, and blindness. J Biol Rhythms. 1997;12:604-617. |
| 26. | Skene DJ, Lockley SW, Arendt J. Use of melatonin in the treatment of phase shift and sleep disorders. Adv Exp Med Biol. 1999;467:79-84. |
| 27. | Arendt J. Importance and relevance of melatonin to human biological rhythms. J Neuroendocrinol. 2003;15:427-431. |
| 28. | Arendt J, Skene DJ. Melatonin as a chronobiotic. Sleep Med Rev. 2005;9:25-39. |
| 29. | Arendt J. Melatonin and human rhythms. Chronobiol Int. 2006;23:21-37. |
| 30. | Tarquini B, Gheri R, Anichini P, Neri B, Buricchi L. Circadian study of immunoreactive prolactin in patients with cirrhosis of the liver. Gastroenterology. 1977;73:116-119. |
| 31. | Nunziata V, Ceparano G, Mazzacca G, Budillon G. Prolactin secretion in nonalcoholic liver cirrhosis. Digestion. 1978;18:157-161. |
| 32. | Mukherjee S, Kar M, Dutta S. Observation on serum prolactin in hepatic cirrhosis. J Indian Med Assoc. 1991;89:307-308. |
| 33. | Besses GS, Burrow GN, Spaulding SW, Donabedian RK. Dopamine infusion acutely inhibits the TSH and prolactin response to TRH. J Clin Endocrinol Metab. 1975;41:985-988. |
| 34. | Van Thiel DH, McClain CJ, Elson MK, McMillin MJ. Hyperprolactinemia and thyrotropin-releasing factor (TRH) responses in men with alcoholic liver disease. Alcohol Clin Exp Res. 1978;2:344-348. |
| 35. | Van Thiel DH, Tarter R, Gavaler JS, Schade RR, Sanghvi A. Thyroid and pituitary hormone responses to TRH in advanced nonalcoholic liver disease. J Endocrinol Invest. 1986;9:479-486. |
| 36. | McCann VJ, Fulton TT. Cortisol metabolism in chronic liver disease. J Clin Endocrinol Metab. 1975;40:1038-1044. |
| 37. | Vogeser M, Fischer G, Jacob K. Quantification of cortisol inactivation in cirrhosis of the liver. Exp Clin Endocrinol Diabetes. 1998;106:410-414. |