Published online Jul 14, 2008. doi: 10.3748/wjg.14.4179
Revised: June 17, 2008
Accepted: June 24, 2008
Published online: July 14, 2008
AIM: To investigate the distribution of the placental form of glutathione-S-transferase (GST) in colon polyps in order to evaluate the role of GST-pi in these tissues.
METHODS: Sixteen polyp tissues removed at colonoscopy were examined. Tissues were investigated histologically and ultrastructurally. GST-pi expression was also analysed immunohistochemically, using peroxidase anti-peroxidase (PAP) method and immunogold labelling method, for light and electron microscope respectively.
RESULTS: All polyp tissues examined were adenoma of low, mild and high- grade dysplasia as shown in the histopathological reports. Nevertheless, the examination of the above specimens with electron microscope revealed that 3 of 9 adenoma of mild dysplasia had ultrastuctural features similar to high-grade dysplasia adenoma. GST-pi was variably expressed in adenoma, with the lowest relative levels occurring in low-grade adenoma and the highest levels found in high-grade adenoma. GST-pi was located mainly in undifferentiated epithelial cells. GST-pi positive particles were found in the cytoplasm and especially in the nucleus adjacent to the nuclear membrane of these cells.
CONCLUSION: The overexpression of GST-pi in mild-grade adenomas with significant subcellular changes and in the majority of high-grade dysplasia adenoma suggests that this might be related to the carcinogenetic proceeding. Immunohistochemical localization of GST-pi in combination with ultrastructural changes indicate that GST-pi might be a sensitive agent for the detection of preneoplastic transformations in adenoma.
- Citation: Gaitanarou E, Seretis E, Xinopoulos D, Paraskevas E, Arnoyiannaki N, Voloudakis-Baltatzis I. Immunohistochemical localization of glutathione S-transferase-pi in human colorectal polyps. World J Gastroenterol 2008; 14(26): 4179-4184
- URL: https://www.wjgnet.com/1007-9327/full/v14/i26/4179.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.4179
Glutathione S-transferases (GST) are a family of enzymes that play an important role in the prevention of cancer by detoxifying numerous potentially carcinogenic compounds[12]. In this respect high tissue levels of GST’s are protective against cancer. In preneoplastic cells as in neoplastic cells, specific molecular forms of GST are known to be expressed and have been known to participate in their resistance to drugs. GSTs are present in most epithelial tissues of the human gastrointestinal tract, as it is an important site of contact with compounds from food, drugs or medication[34]. The cytoplasmic GSTs have been grouped into four main classes, each with a different tissue-specific expression[5]. Significant amounts of the class pi GST were expressed in the majority of human tumors and human tumor cell lines[6]. Recent studies have shown increased levels of human placental glutathione S- transferase (GST-pi) in different tumors of gastrointestinal tract as well as in precancerous lesions[578]. GST-pi was significantly increased in proliferative hepatic nodules induced by chemical carcinogen and in well-differentiated carcinoma[9]. Studies have shown that GST-pi is expressed highly in neoplasms and could be regarded as a tumor marker[10–12].
There are immunohistochemical studies about colorectal carcinoma, suggesting that GST-pi is located in cancer cells[1314].
As the description of the immunohistochemical and the immunoelectron microscopic localization of GST-pi in human polyps is not available, we performed a combined study to examine the distribution of GST-pi in these tissues.
In this study, 16 polyp tissues were removed at colonoscopy from the Department of Gastroenterology of the Regional Anticancer-Oncologic Hospital of Athens “Agios Savvas”. Paraffin-sections were obtained from all tissues and examined by pathologists. When histological grading was performed, all polyp tissues were adenoma of different stage of dysplasia (3 of low-grade, 9 of mild-grade and 4 of high-grade).
Specimen preparation for light microscopy: Samples for light immunohistochemical study were immediately placed in ice-cold saline in the endoscopic room. In the laboratory, the tissue was washed free of blood with ice-cold saline, frozen in liquid nitrogen and kept at -80°C until further use. The specimens were cut in frozen sections (6 &mgr;m).
Electron microscopy: Shortly after colonoscopy, tissues for electron microscopy were fixed in 2.5% glutaraldehyde in 0.1 mol/L sodium phosphate buffer (pH 7.3) for 1 h at room temperature, and postfixed with 1% osmium tetroxide for 1 h at room temperature. All samples were then dehydrated via graded alcohol (25%, 30%, 50%, 70%, 90%, and 100%) and propylene oxide and embedded in Araldite resin. Semi-thin sections (1 &mgr;m) were cut, stained with toluidine blue, and examined under light microscope. Silver sections (500 A) were collected on uncoated copper grids for ultrastructural observation and on uncoated nickel grids for immunolabeling. These sections were further stained with uranyl acetate and lead citrate and examined with a C-100 Phillips Electron Microscope.
Peroxidase anti-peroxidase (PAP) method for light microscopy: Sections on gelatin coated slides were treated in 0.3% H2O2. Following incubation for 30 min in a blocker solution containing 1/30 normal goat serum in phosphate buffered saline (PBS), sections were immunostained with antiserum to glutathione-S-transferase-pi (Dako Company). Then PAP was applied and diluted in bovine serum albumin (BSA) and PBS 1/400 for 30 min. Finally, the sections were incubated for 5 min in a solution containing diaminobenzidine (DAB), H2O2 and PBS.
Following that, the slides were rinsed twice with tap water and counterstained in hematoxylene for 2 min. They then were visualized using an optical microscope and were photographed using a Nikon Coolpix 3100.
Immunogold labeling for electron microscopy: All steps except for incubation with antibodies were carried out at room temperature. Ultra-thin sections in silver uncoated grids were treated in 8% H2O2 for 8 min and etched with saturated aqueous sodium metaperiodate for 30 min. After incubating for 30 min. in a blocker solution containing 5% normal goat serum in PBS, sections were immunostained with antiserum to glutathione S-transferase-pi. The rabbit monoclonal anti S-transferase pi antiserum (Dako Company) was used at a dilution of 1:20 to 1:40. The incubations were carried out at 4°C, overnight. Following several washings in BSA, immunoreactivity was visualized by incubating the sections in 20 nm colloidal gold-labeled goat anti-rabbit IgG (British BioCell International, Cardiff, UK). The specificity of the immunolabeling was assessed by incubation of the sections with non-immune serum. When we performed the appropriate negative controls, we followed the same procedure described above except for incubation in the absence of primary antibody after the treatment with non-immunoreactive goat serum. After counterstaining with uranyl acetate and lead citrate, the sections were viewed under the Philips C100 Electron Microscope.
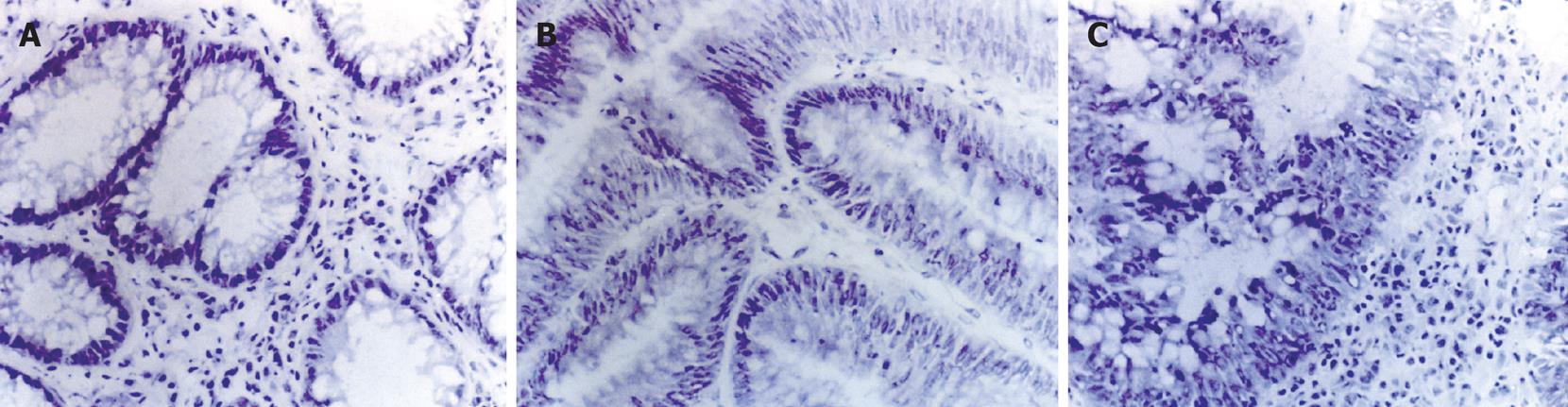
Histological examination of the tissues demonstrated that all were colorectal adenomas of low-grade dysplasia to high-grade dysplasia. Morphologically, adenomas showed abnormalities in epithelial cells that agree with the histopathological reports. Low-grade dysplasia adenoma is characterized by tall epithelial cells with elongated and hyperchromatic nuclei (Figure 1A). In mild-grade dysplasia adenoma, crypt architecture tents to be distorted. Nuclei are crowded, hyperchromatic and may be stratified near the base of the crypts without reaching the lumen (Figure 1B). High-grade dysplasia adenoma is characterized by a true nuclear stratification and a back-to-back pattern, and nuclei extend all the way to the lumen (Figure 1C).
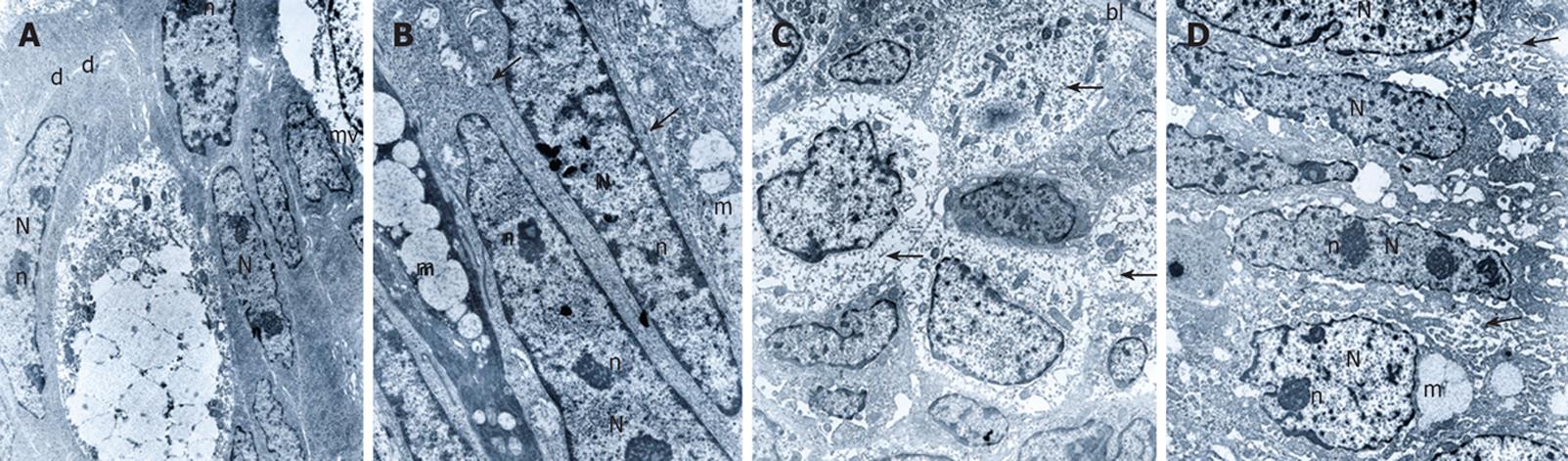
All cases were examined under the electron microscope. At least four specimens from each case were examined and the most representative block was used for electron microscopic examination. The relative number of each cell type in the low, mild and high-grade dysplasia varied. Low-grade dysplasia tissues closely mimicked those of normal colorectal mucosa but goblet cells were fewer and nuclei were elongated. The luminal surface of epithelial cells exhibited microvilli with core rootlets and glycocalyceal bodies (Figure 2A). Mild-grade dysplasia tissues contained not only fewer but also incompletely differentiated goblet cells. In mild-grade dysplasia, we observed an increased nuclear cytoplasm ratio. The nuclei were large, elongated with peripheral (around the nuclear membranes) aggregation of heterochromatin, and nucleoli were increased in number. There were no significant changes in intercellular relationships. The junctions, especially desmosomes, were well developed (Figure 2B). In 3 out of 9 tissues histologically characterized as mild-grade dysplasia, we observed more significant subcellular changes. Almost every cell was undifferentiated with an abundance of free polyribosomes. Intercellular junctions appeared poorly differentiated. Nevertheless, the basal lamina was reduplicated (Figure 2C). In high-grade dysplasia tissues, intercellular junctions were slightly opened and in many cases we could not confirm cell borders. Epithelial cells and especially goblet cells were undifferentiated. The most significant ultrastructural change occurred in the nucleus. Nuclei were elongated or round with prominent nucleoli. Nucleoli were also increased in number and were marginated near the nucleus periphery (Figure 2D).
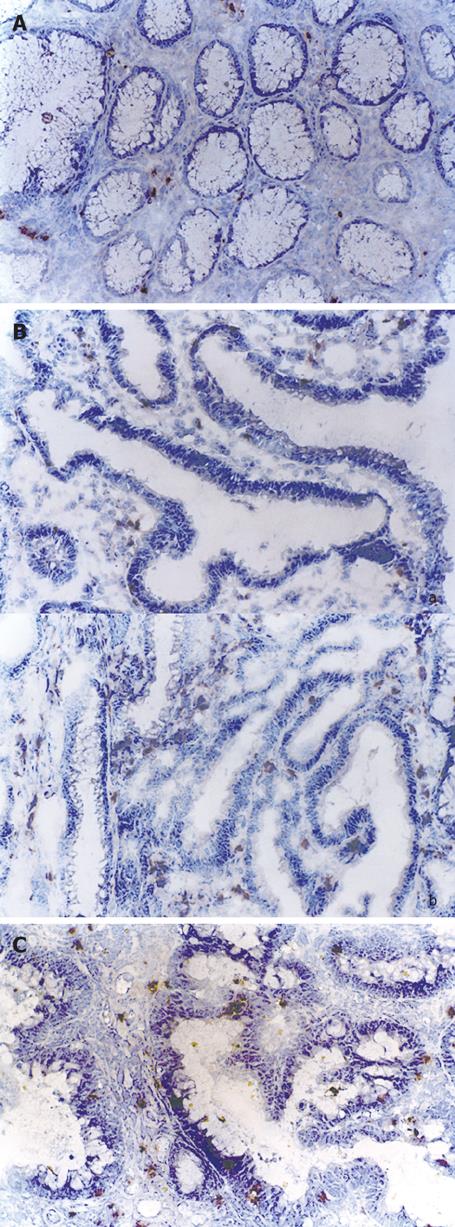
The examined colorectal adenoma tissues exhibited positive immunoreaction to glutathione S-transferase-pi with different density, which is expressed as weak, moderate and strong (Table 1). Low-grade dysplasia adenoma showed mainly a weak immunoreactivity to GST-pi (Figure 3A). Five out of nine adenoma of mild-grade dysplasia immunoreacted moderately and three out of nine showed a strong immunoreaction (Figure 3B), respectively. Three out of four adenoma of high-grade dysplasia showed a strong immunoreactivity to GST-pi (Figure 3C). In all the above specimens, GST-pi was positive in epithelial cells and lamina propria cells.
| Adenoma | Dysplasia | Intensity of staining for GST-pi |
| 1 | Low-grade | Moderate |
| 2 | Low-grade | Weak |
| 3 | Low-grade | Weak |
| 4 | Mild-grade | Moderate |
| 5 | Mild-grade | Weak |
| 6 | Mild-grade | Moderate |
| 7 | Mild-grade1 | Strong |
| 8 | Mild-grade1 | Strong |
| 9 | Mild-grade1 | Strong |
| 10 | Mild-grade | Moderate |
| 11 | Mild-grade | Moderate |
| 12 | Mild-grade | Moderate |
| 13 | High-grade | Strong |
| 14 | High-grade | Strong |
| 15 | High-grade | Strong |
| 16 | High-grade | Moderate |
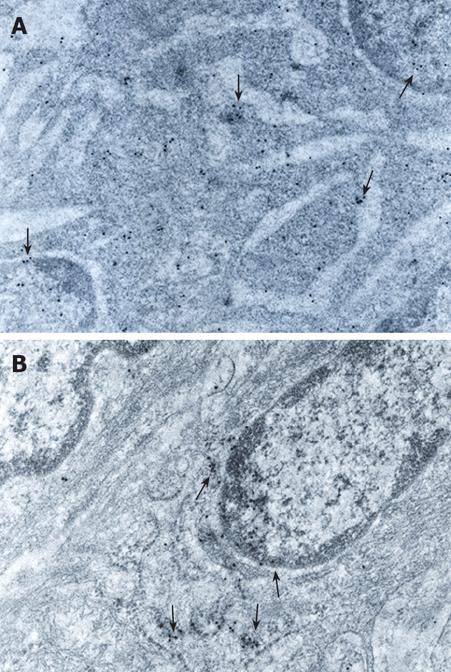
In low and mild-grade dysplasia tissues, GST-pi was located in the cytoplasm, mainly ribosomes, and nucleus adjacent to nuclear membrane, and the intensity of the immunostaining was moderate (Figure 4A). In histologically and ultrastructurally high-grade dysplasia cases, GST-pi was located mainly in undifferentiated epithelial cells. GST-pi positive particles were found in membranes of the cytoplasm and especially the nucleus adjacent to the nuclear membrane of these cells. The positive particles accumulated in lumps and the intensity of the immunostaining was strong (Figure 4B).
Investigations revealed that GST-pi was widely distributed in the human gastrointestinal tract[15–17]. GST-pi may be over expressed in an early phase of malignant transformation in premalignant and malignant cells[1819]. Nevertheless, the description of the immunohistochemical and immunoelectron microscopical localization of GST-pi in human polyps is not available. In this respect, we performed a combined study to explore the distribution of GST-pi in these tissues.
We combined the power of morphologic evaluation that is obtained through the use of light and electron microscopy with the detection of GST-pi in tissue and in subcellular structures of colorectal polyp tissues. We believe it was necessary for the correct assessment of changes associated with premalignant transformation in these tissues.
Our study revealed that the immunoreaction of GST-pi was in specific cell-types in adenoma tissues. The location of GST-pi was mainly in epithelial cells of the crypts. Immunoreaction was also found in lamina propria cells, where the positive staining was located in lymphocytes and phagocytes. Endocrine cells were negative. Electron microscope immunohistochemistry revealed that between different cell types the highest intensity stain was in the columnar epithelial cells.
Other investigators have shown cell-type specific expression of the isoenzyme in the human gastrointestinal tract[1520] in normal[16] and cancerous tissues[5]. This expression of GST pi has been associated with the progression of cancer after exposure to carcinogens[21]. In other studies, GST-pi is regarded as a marker in evaluating the effect of tumorectomy or in predicting the drug resistance of tumor cells[22–24].
Our investigation revealed that the immunoreaction varied from weak to strong, according to the degree of adenoma and the ultrastructural changes. The different density of GST-pi immunoreaction was weak in low-grade dysplasia to strong in high-grade dysplasia. There was an exception in this rule. We observed significant ultrastructural changes in some cases histologically characterized as mild-grade dysplasia. These cases, as high-grade dysplasia cases, revealed a contrast with the pattern of progressive differentiation seen in low-grade dysplasia tissues. We found immature undifferentiated cells showing a fast growing population, with many free polyribosomes and enlarged nucleus and nucleoli increased in size and number, showing increased protein synthetic activity. These cases also exhibited strong GST-pi immunoreactivity similar to those of high-grade dysplasia.
In our study, immunostaining was also observed in specific cellular structures. We found that GST-pi was located in the cytoplasm and especially in the nucleus adjacent to the nuclear membrane of colorectal dysplasia cells. This nuclear staining of GST-pi as found here also has been investigated in other studies. It has been reported previously that GST-pi was located in healthy and diseased stomach[25], esophagus[1926] and uterine cervix[27]. It has been postulated that nuclear GSTs are involved in RNA processing[28]. GST-pi also may induce apoptosis in premalignant cases and may play a pivotal role in early colon carcinogenesis. Other studies revealed lower percentages of apoptotic cells in premalignant cases than in healthy epithelium[29].
Glutathione-pi analog has been synthesized recently. Clinical trials have shown that GST-pi inhibitor induces the apoptosis in the precancerous lesions. It is expected to be promising in future carcinogenesis preventive medicine[3031].
The combination of immunohistochemical and ultrastructural analysis of GST-pi in polyp tissues and the variation of the isoenzyme expression in adenoma of different degree of dysplasia ultimately may lead to a better understanding of its role in carcinogenesis. These findings also may contribute to a better treatment of colorectal polyps in the future.
Placental Glutathione S-transferase (GST-pi) is an enzyme that plays an important role in the removal of toxic, probably carcinogenic, agents from the cell. GST-pi is extremely increased in colon adenocarcinoma in comparison to normal samples. The authors performed a combined study to explore the distribution of GST-pi in colon polyps, as they had no data for these tissues. They used the power of morphologic evaluation that is obtained through the use of light and electron microscopy with the detection of GST-pi in tissue and in subcellular structures of colorectal polyp tissues.
Clinical outcome of colon polyps is under consideration. Human placental GST-pi concentrations are increased in colon tumors even in premature stages, as well as in precancerous lesions. GST-pi protects cancer cells from cytostatic compounds and thereby apoptosis. The increased GST-pi levels contribute to the relatively high resistance to anti-cancer drugs, such as mitomycin C.
Investigations revealed that GST-pi was widely distributed in the human gastrointestinal tract. GST-pi may be over-expressed in an early phase of malignant transformation in premalignant and malignant cells. Nevertheless, the description of the immunohistochemical and the immunoelectron microscopical localization of GST-pi in human polyps was not available.
The combination of immunohistochemical and ultrastructural analysis of GST-pi in polyp tissues and the variation of the isoenzyme expression in adenoma of different degree of dysplasia ultimately may lead to a better understanding of its role in carcinogenesis. These findings also may contribute to a better treatment of colorectal polyps in the future, as Glutathione-pi analog has been synthesized recently. Clinical trials have shown that GST-pi inhibitor induces the apoptosis in the precancerous lesions. It is expected to become promising in future carcinogenesis preventive factors.
This is an interesting study. Authors give valuable data for colon polyps. Immunohistochemical localization of GST-pi in combination with ultrastructural changes indicates that GST-pi might be a sensitive agent for the detection of preneoplastic transformations in colon adenoma tissues.
| 1. | Peters WH, Nagengast FM, Wobbes T. Glutathione S-transferases in normal and cancerous human colon tissue. Carcinogenesis. 1989;10:2371-2374. |
| 2. | Peters WH, Wormskamp NG, Thies E. Expression of glutathione S-transferases in normal gastric mucosa and in gastric tumors. Carcinogenesis. 1990;11:1593-1596. |
| 3. | Tsuchida S, Sato K. Glutathione transferases and cancer. Crit Rev Biochem Mol Biol. 1992;27:337-384. |
| 4. | Peters WH, Kock L, Nagengast FM, Kremers PG. Biotransformation enzymes in human intestine: critical low levels in the colon? Gut. 1991;32:408-412. |
| 5. | de Bruin WC, Wagenmans MJ, Peters WH. Expression of glutathione S-transferase alpha, P1-1 and T1-1 in the human gastrointestinal tract. Jpn J Cancer Res. 2000;91:310-316. |
| 6. | Hayes JD, Pulford DJ. The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit Rev Biochem Mol Biol. 1995;30:445-600. |
| 7. | Tsutsumi M, Sugisaki T, Makino T, Miyagi N, Nakatani K, Shiratori T, Takahashi S, Konishi Y. Oncofetal expression of glutathione S-transferase placental form in human stomach carcinomas. Jpn J Cancer Res. 1987;78:631-633. |
| 8. | Moscow JA, Fairchild CR, Madden MJ, Ransom DT, Wieand HS, O’Brien EE, Poplack DG, Cossman J, Myers CE, Cowan KH. Expression of anionic glutathione-S-transferase and P-glycoprotein genes in human tissues and tumors. Cancer Res. 1989;49:1422-1428. |
| 9. | Sato K, Kitahara A, Satoh K, Ishikawa T, Tatematsu M, Ito N. The placental form of glutathione S-transferase as a new marker protein for preneoplasia in rat chemical hepatocarcinogenesis. Gann. 1984;75:199-202. |
| 10. | Tsuchida S, Sekine Y, Shineha R, Nishihira T, Sato K. Elevation of the placental glutathione S-transferase form (GST-pi) in tumor tissues and the levels in sera of patients with cancer. Cancer Res. 1989;49:5225-5229. |
| 11. | Cairns J, Wright C, Cattan AR, Hall AG, Cantwell BJ, Harris AL, Horne CH. Immunohistochemical demonstration of glutathione S-transferases in primary human breast carcinomas. J Pathol. 1992;166:19-25. |
| 12. | Hamada S, Kamada M, Furumoto H, Hirao T, Aono T. Expression of glutathione S-transferase-pi in human ovarian cancer as an indicator of resistance to chemotherapy. Gynecol Oncol. 1994;52:313-319. |
| 13. | Kodate C, Fukushi A, Narita T, Kudo H, Soma Y, Sato K. Human placental form of glutathione S-transferase (GST-pi) as a new immunohistochemical marker for human colonic carcinoma. Jpn J Cancer Res. 1986;77:226-229. |
| 14. | Guo WJ, Zhou GD, Wu HJ, Liu YQ, Wu RG, Zhang WD. Ultrastructural localization of glutathione S-transferase-pi in human colorectal cancer cells. World J Gastroenterol. 2000;6:454-455. |
| 15. | Ranganathan S, Tew KD. Immunohistochemical localization of glutathione S-transferases alpha, mu, and pi in normal tissue and carcinomas from human colon. Carcinogenesis. 1991;12:2383-2387. |
| 16. | Terrier P, Townsend AJ, Coindre JM, Triche TJ, Cowan KH. An immunohistochemical study of pi class glutathione S-transferase expression in normal human tissue. Am J Pathol. 1990;137:845-853. |
| 17. | Sato K. Glutathione transferases as markers of preneoplasia and neoplasia. Adv Cancer Res. 1989;52:205-255. |
| 18. | Tew KD. Glutathione-associated enzymes in anticancer drug resistance. Cancer Res. 1994;54:4313-4320. |
| 19. | van Lieshout EM, van Haelst UJ, Wobbes T, Peters WH. Immunohistochemical localization of glutathione S-transferase alpha and pi in human esophageal squamous epithelium, Barrett’s epithelium and carcinoma. Jpn J Cancer Res. 1999;90:530-535. |
| 20. | Hayes PC, Harrison DJ. Immunohistochemical analysis of pancreas and gastrointestinal tract in man. In : Hays JD, Pickett CB, Mantle TJ, editors. Glutathione S-Transferases and Drug Resistance. London: Taylor and Francis 1990; 441-450. |
| 21. | Farber E. Cellular biochemistry of the stepwise development of cancer with chemicals: G. H. A. Clowes memorial lecture. Cancer Res. 1984;44:5463-5474. |
| 22. | Katagiri A, Tomita Y, Nishiyama T, Kimura M, Sato S. Immunohistochemical detection of P-glycoprotein and GSTP1-1 in testis cancer. Br J Cancer. 1993;68:125-129. |
| 23. | Yang CR, Ou YC, Kuo JH, Kao YL, Chen CL, Yean SY, Horng YY, Yang CS. Intracellular glutathione content of urothelial cancer in correlation to chemotherapy response. Cancer Lett. 1997;119:157-162. |
| 24. | Pendyala L, Velagapudi S, Toth K, Zdanowicz J, Glaves D, Slocum H, Perez R, Huben R, Creaven PJ, Raghavan D. Translational studies of glutathione in bladder cancer cell lines and human specimens. Clin Cancer Res. 1997;3:793-798. |
| 25. | Schipper DL, Wagenmans MJ, Van Haelst U, Peters WH, Wobbes T, Verhofstad AA, Lange WP, Wagener DJ. Immunohistochemical determination of glutathione S-transferases in gastric carcinomas and in adjacent normal gastric epithelium. Anticancer Res. 1996;16:565-571. |
| 26. | van Lieshout EM, Tiemessen DM, Witteman BJ, Jansen JB, Peters WH. Low glutathione and glutathione S-transferase levels in Barrett’s esophagus as compared to normal esophageal epithelium. Jpn J Cancer Res. 1999;90:81-85. |
| 27. | Carder PJ, al-Nafussi A, Rahilly M, Lauder J, Harrison DJ. Glutathione S-transferase detoxication enzymes in cervical neoplasia. J Pathol. 1990;162:303-308. |
| 28. | Hayes PC, Harrison DJ, Bouchier IA, McLellan LI, Hayes JD. Cytosolic and microsomal glutathione S-transferase isoenzymes in normal human liver and intestinal epithelium. Gut. 1989;30:854-859. |
| 29. | Nobuoka A, Takayama T, Miyanishi K, Sato T, Takanashi K, Hayashi T, Kukitsu T, Sato Y, Takahashi M, Okamoto T. Glutathione-S-transferase P1-1 protects aberrant crypt foci from apoptosis induced by deoxycholic acid. Gastroenterology. 2004;127:428-443. |
| 30. | Nakajima T, Takayama T, Miyanishi K, Nobuoka A, Hayashi T, Abe T, Kato J, Sakon K, Naniwa Y, Tanabe H. Reversal of multiple drug resistance in cholangiocarcinoma by the glutathione S-transferase-pi-specific inhibitor O1-hexadecyl-gamma-glutamyl-S-benzylcysteinyl-D-phenylglycine ethylester. J Pharmacol Exp Ther. 2003;306:861-869. |
| 31. | Nobuoka A, Takayama T, Miyanishi K, Sato T, Takanashi K, Hayashi T, Kukitsu T, Sato Y, Takahashi M, Okamoto T. Glutathione-S-transferase P1-1 protects aberrant crypt foci from apoptosis induced by deoxycholic acid. Gastroenterology. 2004;127:428-443. |