INTRODUCTION
Chronic pancreatitis (CP) is defined as a disease causing inflammation of the pancreas resulting in progressive and irreversible morphological and functional derangements with end-stage exocrine/endocrine failure. The most important symptom is abdominal pain that is present in 80%-90% of patients along evolution of the disease[1]. Pancreatic pain presents characteristically with severe dull epigastric pain, eventually radiating to the back. The pain is often recurrent, intense and long-lasting, and may be associated with malnutrition, narcotic addiction and major socio-economical problems. The pain mechanisms are incompletely understood and perhaps multifactorial. So far, the following causes have been suggested: (1) increased intrapancreatic pressure within the pancreatic duct or parenchyma ensuing tissue ischemia; (2) inflammation in the pancreas; (3) extrapancreatic causes of pain such as bile duct and duodenal stenosis due to extensive pancreatic fibrosis and inflammation, and (4) alterations in pancreatic nerves including an increase in nerve fibers and neurogenic inflammation[2]. Histological findings in the pancreas in patients with pain due to CP have revealed an increase in the number and diameter of pancreatic nerve fibers and in the amount of neurotransmitters. These findings are also seen after neuronal lesions in other tissues and have supported the theory that neurogenic inflammation plays a key role in the pain pathogenesis[3–5]. Supporting the neurogenic pain theory, the pain in CP is often described as shooting and lancinating. Therefore, it may clinically resemble that of neuropathic pain typically presenting as stimulus-independent shooting or burning pain together with stimulus-evoked hyperalgesia[6].
Due to the difficulties to access the pancreas and potential harmful complications during the procedure, it is not possible directly to investigate whether, for example, allodynia or other characteristic features of neuropathic pain are present during manipulation of the organ. However, visceral pain is typically diffuse and difficult to localize, which to a large extend can be explained by the widespread termination of visceral afferents into 2nd order neurons in multiple segments of the spinal cord[7]. Due to this overlap in central termination of visceral afferents, disease mechanisms of the pancreas are typically also reflected during pain stimulation of the esophagus[8]. Hence, experimental models where the esophagus is involved will typically reflect any abnormal pain mechanisms relating to the pancreas. Previous studies have given evidence for neuroplastic changes and reorganization of the brain in patients with chronic and neuropathic pain[9], and in recent experiments done by our group such changes were also seen during pain stimulation of the esophagus in patients with CP[10]. An approach to detect characteristics of neuropathic pain in CP can, therefore, be related to analysis of the electroencephalographic (EEG) during pain stimulation of the esophagus. Is has been proposed that power in the theta (3.5-7.5 Hz) EEG band may be a hallmark of patients with different kinds of neuropathic pain[11–13]. Recently, Sarnthein and coworkers showed that patients with neuropathic pain disorders exhibited higher spectral power of the resting EEG especially in the theta range[14]. After treatment with a therapeutic lesion in the thalamus, the pain resolved together with normalization of the EEG abnormalities. Hence, EEG analysis may be a tool to diagnose neurogenic pain. Among the methods used for brain imaging EEG (and magnetoencephalography) has the highest temporal resolution reflecting the functions of the brain directly. However, the scalp EEG is a composite of oscillatory activity from deep neuronal sources reaching the scalp by volume conduction with major distortion of the signal. Furthermore, the overall signal is composed by infinity of electrical sources resulting in complex dynamic changes over short time periods. Therefore, mathematical models are needed to analyze the EEG. Power analysis using, for example, Fast Fourier Transform is a commonly used method, but it does not give reliable information about the frequency content in the very short time window of less than 0.5 s representing the exogenous pain component[15]. A relatively new approach for the analysis of biomedical signals is represented by adaptive methods[1617]. Recently, a new method called Topographic Matching Pursuit (TMP) was developed to analyze specific components of the EEG[18]. The method decomposes the multichannel EEG signal into a relatively small number of components (called atoms) chosen from a very big and redundant set (called dictionary). Such atoms are able to describe specific spatio-temporal evolution of EEG components in adaptive and very short time windows.
In the current study, TMP analysis was used to evaluate the EEG after experimental pain in the esophagus in patients with chronic pancreatitis and in healthy controls. We hypothesized that there would be differences in the theta band due to the neurogenic component involved in CP.
MATERIALS AND METHODS
Subjects
Eight patients, six men and two women, mean age 55 years (range, 53-62 years) with CP fulfilling the Marseille-Rome/Cambridge diagnostic criteria[19] were enrolled in the study. They all had intermittent pain attacks characteristic for CP and were only included if they had no present alcohol abuse or chronic intake of painkillers. The duration of the pancreatitis was mean 6.1 (range, 1-10) years and the mean frequency of the pain was 5.0 times a week (range, 2-7) with a mean duration of 3.9 h (range, 2-9). None of the patients had any other diseases associated with pain or any history of neurological diseases. Twelve volunteers (ten men and two women, median age 37.5 years (23-49) served as controls. They were all healthy without any gastrointestinal symptoms or disorders associated with pain. The study was conducted according to the Helsinki II Declaration and approved by the Ethical Committee of North Jutland (No. VN 2003/120 mch).
Experimental protocol and gut stimulation
The subjects were fasting 8 h before the experiment. The same method as previously described was used[20]. Briefly, intubation of the esophagus was done through the nose with a 6 mm endoscope [Ultra Slim Gastroscope (Pentax EG-1840)]. The device for bipolar electrical stimulation was inserted through the biopsy channel in the endoscope. The stimulator was positioned 5 cm above the gastro-esophageal junction. A constant current electrical stimulator (Noxitest, Aalborg, Denmark) was used to deliver the visceral stimuli. The intensity of the current was limited to 80 mA and the voltage to 200 V. Before all stimulations the inter-electrode impedance was measured to be < 2 kΩ, securing optimal electrode position throughout the experiment. The intensity of the current was gradually increased in steps of 0.1-0.5 mA until the sensation changed from unpleasantness to pain (pain detection threshold). During the experiment the subject rested quietly and relaxed with the eyes open and was asked to minimize blinking and focus on a fixed point. A single stimulus consisted of 5 fast rectangular constant current pulses at 200 Hz with duration of 1 ms[21]. Thirty-five single stimuli were applied with intensity at the pain threshold with an inter-stimulus interval of 5 s. During the stimulations the subjects were observed by a physician, and the electrocardiogram was continuously monitored.
Recordings
The EEG was recorded from 64 surface electrodes using a standard EEG cap (Quick-Cap International, Neuroscan, USA) following the extended international 10-20 system. The impedance of the electrodes was below 2 kΩ. In addition, two electrodes were placed at the right upper brow and the left external canthus to monitor eye movements. A linked-ears reference was used. EEG signals were recorded and sampled at 1000 Hz, and band-pass filtered between 0.05 Hz and 70 Hz (SynAmps, Neuroscan, USA).
EEG analysis using TMP
Following the recording, thirty-five single trials were visually examined for artifacts. The contaminated epochs were removed off-line. The data were then baseline corrected and corrected for linear trends (SynAmps, Neuroscan, USA). The artifact free epochs of 450 ms were then collapsed into a single average file. The averaged data from the patients and controls were submitted to TMP analysis. In summary, the TMP is an adaptive and iterative algorithm. It creates spatio-temporal data approximations using a relatively small number of simple components (called atoms) chosen from a very big and redundant set (called dictionary). The TMP method is based on the Matching Pursuit algorithm[22]. However, the standard “Gabor atoms” used in the Matching Pursuit describes only the temporal evolution of EEG components in a single channel or channel-averages. The TMP uses atoms, which are distributed in time and space enabling the analysis of multichannel data. The TMP atoms are very well localizable in time, frequency and space domains and are described by only a few parameters. This approach simplifies analysis of multichannel data and enables identification and extraction of spatio-temporal components with biological meaning as well as identification and separation of artifacts.
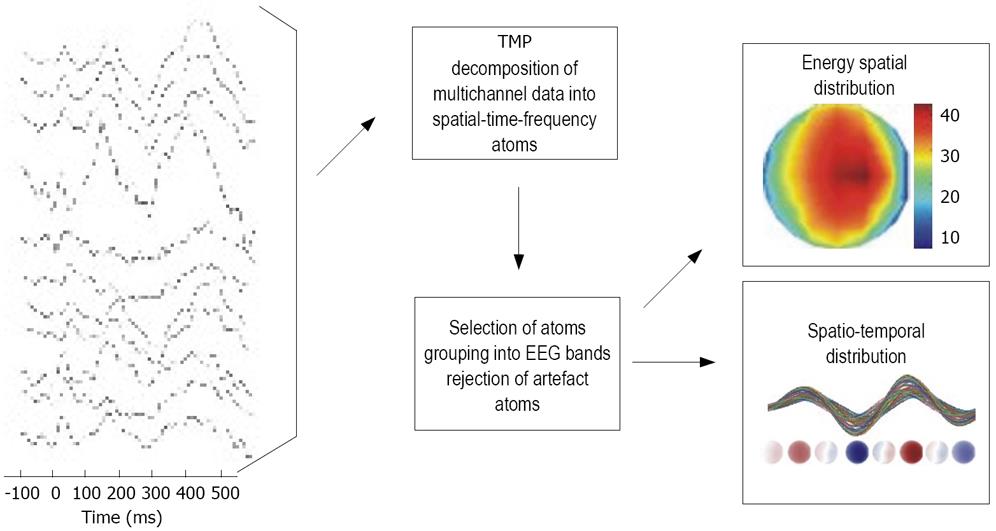
Figure 1 shows the analysis scheme used for the data in this study. First, the EEG data were decomposed into TMP atoms. Twenty atoms per subject were used. The computed atoms were grouped into frequency bands according to their modulation (frequency) parameter. This is an equivalent of a band-pass filtering into delta (0.5-3.5 Hz), theta (3.5-7.5 Hz), alpha1 (7.5-10 Hz), alpha2 (10-12.5 Hz), beta1 (12.5-18 Hz), beta2 (18-24 Hz) and beta3 (24-30 Hz). Typically, each frequency band was represented by one to three atoms. For each atom or group of atoms from a specific band, a spatial distribution of signal’s energy as well as spatio-temporal patterns was examined.
Figure 1 The analysis scheme used for the data investigation.
The EEG data were decomposed into a set of TMP atoms, which parameterized the data. Next, the atoms were grouped into bands according to their frequency parameter. This allowed convenient analysis of time and spatial properties of oscillations in all frequency bands.
RESULTS
All subjects reported painful sensations to the electrical stimulation of the esophagus. The mean current intensities (at the pain thresholds) for the controls and patients were 12.8 ± 3.9 mA and 10.0 ± 1.4 mA, respectively (P = 0.08).
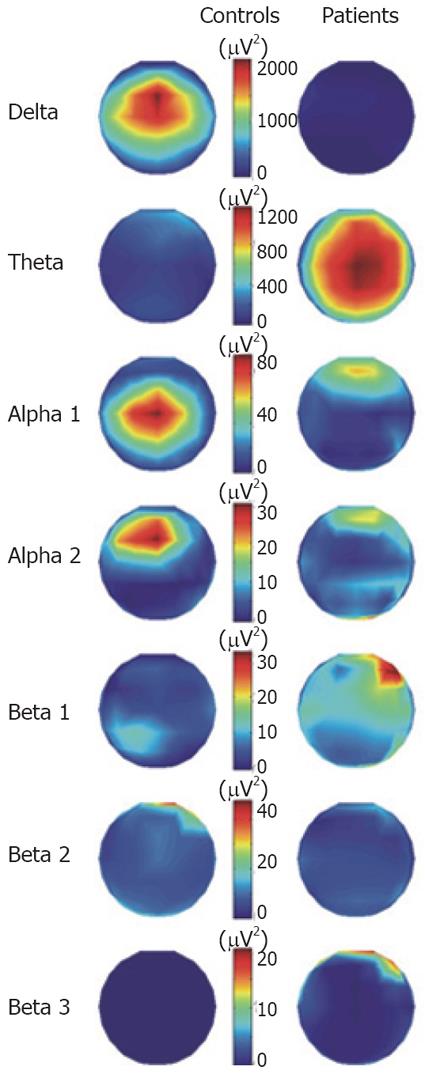
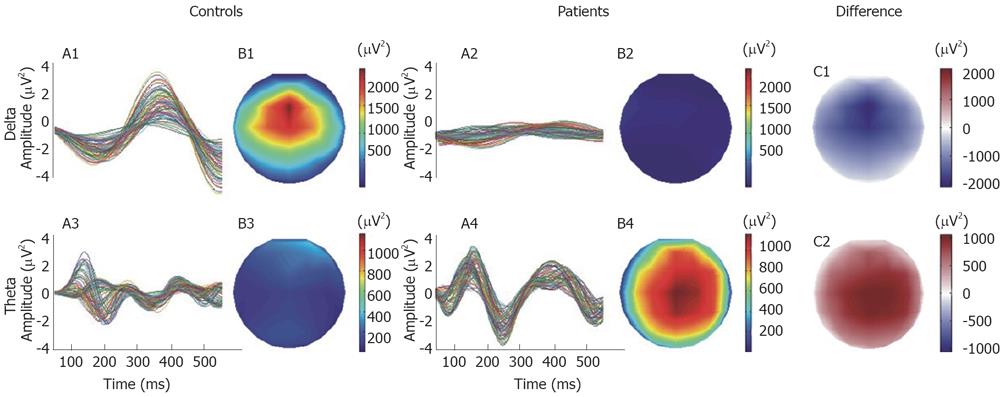
The grand mean averages of the spatial distributions of signal energy during the 450 ms analysis after the evoked esophageal pain for patients and controls are shown in Figure 2. The spatial distributions are shown separately for the delta, theta, alpha and beta bands. Clearly major differences between controls and patients were seen in the delta and theta bands, whereas there were only minor differences in the other frequency bands. In Figure 3, the temporal evolution together with the spatial distribution maps of energy for the delta and theta activities in the analyzed time period are shown in the two groups. The butterfly plots showing the individual channels show that the mean amplitude (energy) in the delta band was higher in the controls. The patients only showed scattered activity in this band without any consistency between the individual channels. In the theta band, the patients showed higher activity than controls and the theta activity persisted throughout the 450 ms of analysis, whereas in controls it was strongly attenuated after 250 ms. Finally, the theta activity had a lower frequency in the patients. The main theta components oscillated with about 4.4 Hz in the patients and with about 5.5 Hz in the controls. When the mean activity in the patients was subtracted from that in controls (difference in the Figure 3), there were major differences in energy. The delta activity in controls and theta activity in patients had similar amplitudes and topographic distributions. However, the delta oscillations in controls were slightly stronger in the frontal lobe, whereas the theta activity in patients was stronger in the parietal lobe (Figure 3B1 and 3B4).
Figure 2 The spatial distribution of energy divided into frequency bands for patients and controls throughout the 450 ms of analysis.
Note that a different color scaling for each frequency band was used.
Figure 3 The temporal evolution and the spatial distribution maps of energy of the delta and theta activities for the 450 ms of analysis.
In the right panel, the difference maps of energy were computed by subtracting the energy maps of controls from the patients.
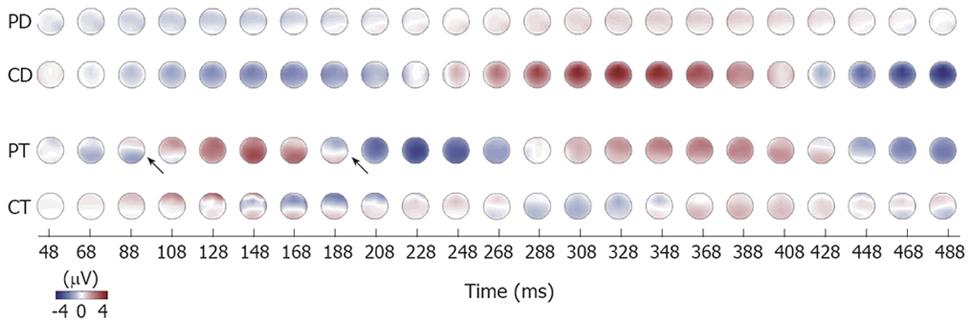
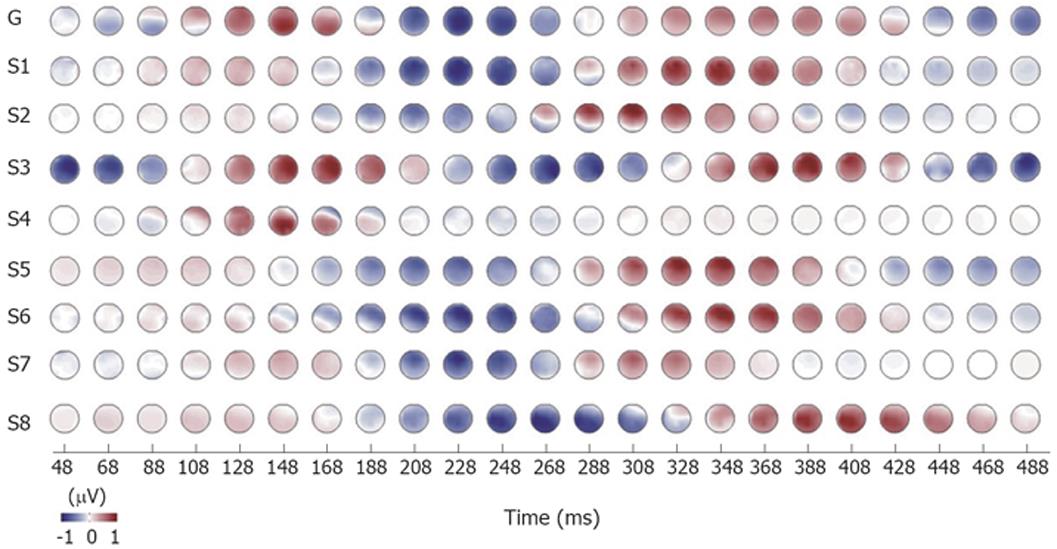
In Figure 4, the topography relating to the time evolution in the delta and theta bands are shown. The time evolution of the spatial distribution showed differences for patients and controls. The oscillations in the delta band for controls were synchronous with a stable monopolar spatial distribution. This means that there were only minor and constant phase differences between channels. In contrast, the patients exhibit slightly larger phase differences between different channels which desynchronized the oscillations (see arrows in Figure 4). The amplitude of the oscillations was also smaller for patients in the delta band. However, in the theta band the patients exhibit synchronous brain activation with almost constant and relatively small phase differences between the channels. The theta activity for controls, however, had inconstant phase differences between channels. The topographical EEG data evolution of the theta activity was rather consistent in all patients as shown in Figure 5.
Figure 4 The time evolution of amplitude distribution for delta and theta bands in patients and controls.
Note the stable, monopolar spatial distribution of delta activity in controls (CD). This is due to constant and small phase differences between measurement channels. The theta activity in patients has slight changes in phase and, therefore, the spatial distribution is not always stable (see arrows). PD: Patients delta; PT: Patients theta; CD: Controls delta; CT: Controls theta.
Figure 5 Normalized time evolution of amplitude distributions for theta bands in patients with chronic pancreatitis No.
1-8. G: Grand mean of all patients.
DISCUSSION
The topographic matching pursuit method allowed multichannel signal decomposition of EEG data and showed that during evoked visceral pain there are major differences in the delta and theta band between patients with chronic pancreatitis and healthy controls. The differences in the theta band were the most consistent and indicate, together with previous observations, that neuropathic pain mechanisms may be involved in chronic pancreatitis.
In the classical description of EEG, time and frequency distribution is computed using frequency analysis, but as the specific brain processing of phasic exogenous pain stimuli is very short lasting (about 100-200 ms)[15] ordinary signal analysis cannot be used (see below). To encompass the problems with time-resolution, some researchers have used a tonic pain stimulus[23]. However, tonic pain with the same intensity is difficult to evoke consistently in GI tract where, for example, long-lasting stimulation typically creates a waxing or waning response[24–26]. Furthermore, nausea and vomiting is often evoked interfering with the cortical response[27]. Hence, in experimental pain experiments of the gut phasic stimuli such as electrical stimulation of the mucosa are often used. Another way to overcome the problems with analysis of short time segments is to give a series of stimuli and compute the evoked brain response based on summation of the stimulus sequences[28]. However, such averaged evoked brain responses carry the risk for discarding much of the dynamic information contained in the original data[20]. To obtain detailed information of the EEG in individual channels, other methods where the signal is analyzed must, therefore, be added.
Common analysis schemes of multichannel bioelectromagnetic data involve analysis of selected single channels in the time or time-frequency domain. Typically, methods such as Wavelet Transform are used to extract the time-frequency information from the data. These represent signals by correlations with all their basis functions. This may result in “blurred” representations of data when some simple signal components are represented in terms of many basis functions and interpretation can be difficult. Further, they are only stable for rather long EEG segments and discrete changes relating to the exact time and duration of individual waveforms cannot be measured[18]. The TMP method represents a completely different approach to signal analysis. It is able to decompose the data under analysis into a relatively small number of simple components. Such a concise description of the data simplifies the analysis and interpretation of the results. The parameterization of signal components allows convenient further analysis. It enables identification and separation of signals with biological origin or removal of artifacts[18]. Each signal component is represented by a TMP atom that can be inspected individually in the time, frequency and space domains, and a topographic distribution of amplitude and phase differences between channels can be plotted. Additionally, temporal changes of amplitude topography can be analyzed. This allows identification of patterns and components in the time-frequency-space domain. The main disadvantage of the TMP algorithm is its high computation cost.
We used electrical stimulation of the esophagus to mimic the visceral pain response. Although the pain was not evoked in the pancreas per second, the visceral afferents terminate rather diffuse in the spinal cord affecting many neurons at different segmental levels[7]. Hence, the esophagus innervates neurons spreading from C5-L2 explaining the diffuse pain seen in the clinic[29]. The visceral afferents from different organs terminate often in the same functional neuron pool, resulting in viscero-visceral convergence (e.g. pain from one visceral can cause a change in the sensory response in another[30]). In accordance with the neurophysiologic findings we have previously shown that patients with pancreatitis have hypoalgesia to distension in both the esophagus and duodenum[8]. Furthermore, that the latency of the evoked brain potentials to electrical stimulation of both the esophagus and duodenum was decreased in these patients[10]. Thus it is most likely that any neuroplastic changes and abnormal mechanisms in the pain processing caused by the intermittent pancreatic pain will also affect visceral pain evoked in the nearby organs.
Neuropathic pain is typically described as stimulus-independent shooting, lancinating or burning pain[6] resembling the pain in CP. Recently, evidence for a neuropathic component of the pain in CP was provided by histological findings demonstrating an increase in the number and diameter of pancreatic nerves. Furthermore intensification of neurotransmitters, such as substance P, calcium gene-related peptide and interleukin 8, being markers of neuronal plasticity were found[5]. The growth-associated protein-43 is increased after neuronal lesions and has been found in the majority of pancreatic nerve fibers with a correlation with the pain scores[31]. As increased activity in the theta band seems to be a marker of neuropathic pain[14], the current findings strengthen this neuropathic pain hypothesis in CP. The increased theta activity may reflect a thalamocortical dysrhythmia, as shown with coherence between direct single-unit thalamic recordings in patients suffering from ongoing neuropathic pain and other neurological disorders[1213]. In these studies, it was hypothesized that the theta activity reflects a disturbance in the thalamocortical interplay needed for higher cognitive functions such as those engaged in pain. The normalization of the EEG after therapeutic lesions in the thalamus (and a 95% reduction of the pain), further support this hypothesis[14]. Our patients all suffered from intermittent pain and hence we studied evoked gut pain and not the resting EEG. Besides a demonstration of increased theta activity we were able to show that the activity persisted throughout the 450 ms of analysis, whereas it faded out in controls. Furthermore, the theta pattern was consistent in all patients.
The low activity in the delta band was consistent in all patients. However, there was a major spread between subjects and the features were more difficult to interpret. Increase in delta power, especially in the frontal derivations has been shown in some previous studies using experimental tonic pain[23], but the findings were not consistent[32–34]. Recently, we used independent component analysis of the EEG following esophageal stimulation in healthy subjects[20]. In this experiment, electrical pain evoked dominant synchronous activity in the delta band in several brain regions, especially in the components with dipoles in the thalamus and cingulate cortex. Whether the difference between controls and patients seen in the current study reflects the expected delta power increase during experimental pain in healthy volunteers, or whether it is a feature of chronic pain remains to be investigated in further detail.
The findings of the current study may have clinical implications. Treatment of chronic pancreatitis is difficult and it often becomes necessary to use strong analgesics such as opioids[1]. However, drugs shown to be effective in neuropathic pain also seem to be efficient. Hence, Wilder-Smith et al showed that tramadol, a weak opioid drug with several non-opioid effects in the central nervous system, in high doses was superior to traditional opioids in the treatment of pain in CP[35]. Tricyclic antidepressants and pregabalin, which are also effective in neuropathic pain, have in our experience been of value as adjuvant therapy in difficult patients. Obviously, it could be interesting to use the current method before and after effective therapy of pain in patients with CP.
The increased theta activity during experimental visceral pain in patients with chronic pancreatitis gives further evidence that the pain mechanisms in this disease share many features with those seen in neuropathic pain. This has important implications for the clinical understanding and treatment of pain in chronic pancreatitis, which may be directed against drugs with effects on neuropathic pain disorders.
COMMENTS
Background
The pain mechanisms in chronic pancreatitis are incompletely understood, but it has been suggested that neuropathic mechanisms (like in phantom pain) are important. The energy in the theta (3.5-7.5 Hz) band of the electroencephalogram may be a hallmark of patients with neuropathic pain, and we compared the energy in the theta band during painful stimulation of the esophagus in patients with chronic pancreatitis and in healthy controls.
Research frontiers
Histological findings in the pancreas in patients with pain due to chronic pancreatitis have mimicked those seen in patients with neurogenic inflammation. The neurogenic theory is also supported by clinical and experimental findings.
Innovations and breakthroughs
To assess the neurophysiological mechanisms standardization of the pain stimulus in experimental settings is necessary. During short-lasting experimental pain stimulation of the esophagus, as used in the paper, it is difficult to compute discrete electrophysiological signals using conventional methods. We, therefore, used a new method called “Topographic Matching Pursuit” and found a selective increase of the theta energy in the patients during the evoked esophageal pain.
Applications
The increased theta activity during experimental pain gives further evidence that the pain mechanisms in chronic pancreatitis share many features with neuropathic pain. This has important implications for the clinical understanding and treatment of pain in chronic pancreatitis.
Peer review
The differences in the theta band indicate that neuropathic pain mechanisms are involved in chronic pancreatitis. This has important implications for the understanding and treatment of pain in these patients, which should be directed against drugs with effects on neuropathic pain disorders.
APPENDIX
Topographic Matching Pursuit (TMP) is an adaptive method for sparse decomposition of multichannel signals. It is based on the Matching Pursuit (MP) algorithm[36–39]. It iteratively creates approximations of signals using relatively small number of simple components chosen from a very big and redundant set. The components are called atoms. A set of atoms is called dictionary. Typical atoms are scaled and modulated as translated Gaussian functions and are called Gabor atoms. The advantage of Gabor atoms is their optimal time-frequency resolution. MP has very interesting and useful properties. However, it can be directly used only for decomposition of single-channel data, because Gabor atoms are distributed only in time. Biomedical signals are typically measured with multiple sensors. The TMP algorithm is searching the standard MP dictionary to find a Gabor atom, which has the biggest similarity simultaneously to all measurement channels. A multichannel extension of the MP algorithm was discussed by Gribonval and applied to EEG data by Durka et al[40]. In the TMP version of multichannel MP the amplitude and the phase shift of the Gabor atom is optimized for each channel in order to obtain the highest correlation of the Gabor atom with the respective channel. The Gabor atom with the highest value of the sum of squared correlation coefficients with all measurement channels is chosen for the approximation in the first iteration: with Fi-i-th data channel, gγ-atom from the dictionary with index γ.
The correlation coefficients define a topographic distribution of amplitude over measurement channels. One can describe a TMP atom as a Gabor atom with an additional list of amplitudes and phase shifts for all channels (Figure Appendix1). A high amplitude value for a particular channel indicates strong presence of the component in this channel. In contrast, a low amplitude value denotes that the component is weakly present in this channel. Next, the TMP atom which has the biggest similarity with the multichannel data under analysis (as described above) is subtracted from the data. This creates the first order spatio-temporal residuum of the data. The data under analysis can be treated as the 0th order residuum. The further steps of the TMP algorithm can be summarized in the three iterative points: (1) Search the dictionary for the atom, which has the biggest similarity (correlation) with the previously computed spatio-temporal residuum. (2) Create the next order residuum by subtracting the chosen atom from the previously computed residuum. (3) Repeat steps 1 to 2 until the current residuum is sufficiently low.
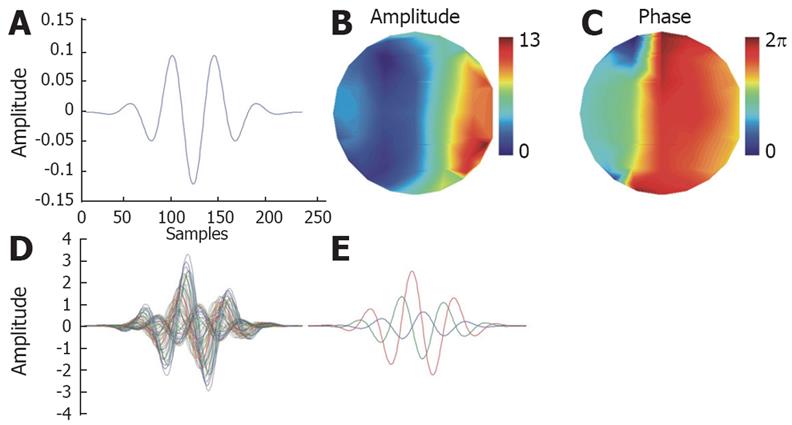
Figure Appendix1 Example of a TMP atom for a 61 channel EEG system.
A: Base Gabor atom; B: Spatial distribution of amplitude; C: Spatial distribution of phase; Overlay plots of the TMP atom: All 61 channels (D), channels 1, 10 and 30 (E).
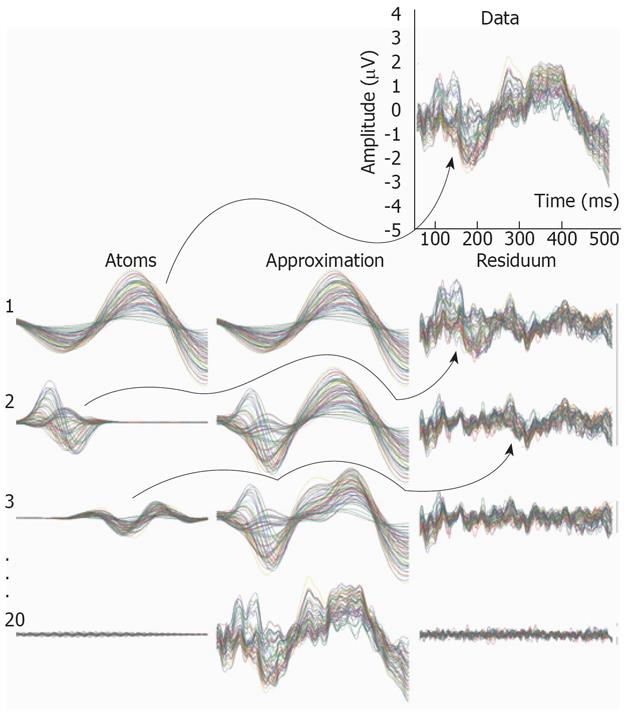
An example of decomposition of EEG data is presented in Figure Appendix2. The EEG data is presented in the top right corner. Each row in the figure represents one iteration of the TMP algorithm. The chosen atoms and computed approximations with their respective residues for four different iterations are presented. The approximations are computed by summing atoms from all previous and the present iteration. With increasing number of iterations, one can observe the convergence of the approximation to the EEG data. Simultaneously, the residuum, which is the difference between the data and the approximation, is vanishing.
Figure Appendix2 Example of decomposition of multichannel EEG data.
The data is presented in the top-right corner (61 channels overlay plot). In the following rows chosen atoms, approximations and residues for the 1st, 2nd, 3rd, and 20th iteration are shown. In each iteration the chosen atom is the most similar one with the previously computed residuum (see arrows). The residuum is created by subtracting the chosen atom from the residuum computed in the previous iteration. The signal itself can be treated as the 0th order residuum. The approximation in each iteration is the sum of all atoms chosen in the present and previous iterations. Hence, in the first iteration the chosen atom is simultaneously the first approximation of the data. Note that with the increasing number of iterations the approximation is approaching the data and the residuum is vanishing. All time plots of atoms, approximations and residues are 61-channel overlay plots shown in the same scale.
The result of the TMP algorithm is a set of TMP atoms. The sum of the atoms approximates the data under analysis. The TMP atoms are localized very well in the time, frequency and space domains. They have six parameters: scale (time length), modulation (frequency), translation (time localization), and-relating to the different channels-a spatial distribution of phase and amplitude. Thus, the choice of the atoms for the approximation parameterizes the multichannel data under analysis. This allows convenient analysis and processing of such parameterized data. For example it enables identification and separation of signal components with biological origin or removal of artifacts. Each signal component represented by a TMP atom can be inspected individually in the time, frequency and space domains. A topographic distribution of amplitude and phase differences between channels can be plotted. Additionally, temporal changes of amplitude topography can be analyzed. This allows identification of patterns and components in the time-frequency-space domain.
Supported by“Nordjyllands Amts Forskningslegat” and the Danish Technical Research Council