Published online Jun 28, 2008. doi: 10.3748/wjg.14.3903
Revised: February 16, 2008
Accepted: February 23, 2008
Published online: June 28, 2008
AIM: To explore the method of isolation and biological analysis of tumor stem cells from pancreatic adenocarcinoma cell line PANC-1.
METHODS: The PANC-1 cells were cultured in Dulbecco modified eagle medium F12 (1:1 volume) (DMEM-F12) supplemented with 20% fetal bovine serum (FBS). Subpopulation cells with properties of tumor stem cells were isolated from pancreatic adenocarcinoma cell line PANC-1 according to the cell surface markers CD44 and CD24 by flow cytometry. The proliferative capability of these cells in vitro were estimated by 3-[4,5-dimehyl-2-thiazolyl]-2, 5-diphenyl-2H-tetrazolium bromide (MTT) method. And the tumor growth of different subpopulation cells which were injected into the hypodermisof right and left armpit of nude mice was studied, and expression of CD44 and CD24 of the CD44+CD24+ cell-formed nodules and PANC-1 cells were detected by avidin-biotin-peroxidase complex (ABC) immunohistochemical staining.
RESULTS: The 5.1%-17.5% of sorted PANC-1 cells expressed the cell surface marker CD44, 57.8% -70.1% expressed CD24, only 2.1%-3.5% of cells were CD44+ CD24+. Compared with CD44-CD24- cells, CD44+CD24+ cells had a lower growth rate in vitro. Implantation of 104 CD44-CD24- cells in nude mice showed no evident tumor growth at wk 12. In contrast, large tumors were found in nude mice implanted with 103 CD44+CD24+ cells at wk 4 (2/8), a 20-fold increase in tumorigenic potential (P < 0.05 or P < 0.01). There was no obvious histological difference between the cells of the CD44+CD24+ cell-formed nodules and PANC-1 cells.
CONCLUSION: CD44 and CD24 may be used as the cell surface markers for isolation of pancreatic cancer stem cells from pancreatic adenocarcinoma cell line PANC-1. Subpopulation cells CD44+CD24+ have properties of tumor stem cells. Because cancer stem cells are thought to be responsible for tumor initiation and its recurrence after an initial response to chemotherapy, it may be a very promising target for new drug development.
- Citation: Huang P, Wang CY, Gou SM, Wu HS, Liu T, Xiong JX. Isolation and biological analysis of tumor stem cells from pancreatic adenocarcinoma. World J Gastroenterol 2008; 14(24): 3903-3907
- URL: https://www.wjgnet.com/1007-9327/full/v14/i24/3903.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.3903
Pancreatic carcinoma is an obstinate disease that is difficult to deal with. Though pancreatic cancer accounts for only 2%-3% of all cancers, it is the fourth most frequent cause of cancer death in industrialized countries[1]. It is estimated in the United States in 1998 that at least 29 000 new cases of pancreatic cancer will be diagnosed[2]. Unfortunately, only 18% will survive one year after diagnosis, the five-year survival rate is 4%. This is because by the time a patient exhibits symptoms, and the cancer is diagnosed, it is no longer in its early stage[3–5]. The main conventional treatments for pancreatic cancer are surgery, radiation therapy and chemotherapy. Despite advances in surgical and medical therapy, little effect has been made on the mortality rate of this disease. According to Bjerkvig et al[6], the capacity of a tumor to grow and propagate is dependent on a small subset of cells (so-called tumor stem cells), tumor stem cells are immature cells that can replicate or self-renew, and are able to differentiate or grow into all the cells that an organism or particular organ system need. It has profound implications to understand how tumors evolve and how we treat tumors. If we can destroy these tumor stem cells, it will be possible to treat the patients successfully. However, it is difficult to purify tumor stem cells because of lack of specific cell surface markers in solid tumors. Recently, it was reported that cancer stem cells existed in some solid malignancies, including breast[7], brain[89], prostate[10], and lung cancers[11]. Thus, we deduced that pancreatic cancer might contain its own stem cells responsible for its metastasis and recurrence. To prove this hypothesis, we isolated subpopulation cells that have characteristics of tumor stem cells according to markers CD44 and CD24 by flow cytometry from pancreatic adenocarcinoma cell line PANC-1, and explore their biological characteristics. This study was to identify the method of isolation of pancreatic tumor stem cells and the ability of propagation of the tumor stem cells in vitro and in vivo.
Male nude mice, aged 6-8 wk and weighing 20 ± 2 g, were provided by the Experimental Animal Center, Hubei Center for Disease Control and Prevention, China. The nude mice were caged individually under specific pathogen free (SPF) conditions. Human pancreatic adenocarcinoma cell line PANC-1 was obtained from American Type Culture Collection, Manassas, Virginia, the Dulbecco modified eagle medium F12 (1:1 volume) (DMEM-F12) from Hyclone, Wuhan, China, the fetal bovine serum from Sijiqing, Hangzhou, China, trypsin from Sigma-Aldrich, Shanghai, China, the epidermal growth factor (EGF), basic fibroblast growth factor (b-FGF), insulin-transferrin-selenium solution (ITS) and trypsin from Sigma-Aldrich, Shanghai, China and PE anti-human CD44 and FITC anti-human CD24 were purchased from American Ancell.
The cells were cultured in incubator filled with 5% CO2 at 37°C. The PANC-1 cells were cultured in DMEM-F12 (1:1 volume) supplemented with 20% fetal bovine serum (FBS), penicillin (1 × 105 U/L) and streptomycin (100 mg/L).
Cells were dissociated by trypsin-EDTA solution (trypsin, 0.25%; EDTA, 0.02%) for 2-5 min at 37°C, transferred to a 5-mL tube, washed twice with PBS with 2% heat-inactivated calf serum (HICS; 5 min at 1000 r/min), resuspended in 100 &mgr;L (per 106 cells) of PBS, then were counted. PE anti-human CD44 and (or) FITC anti-human CD24 (appropriate dilution per antibody) were added and incubated for 30 min at 4°C, and then washed twice with PBS. Flow cytometry was performed on a FACS, and data were analyzed with the Cell Quest software (B.D., America). Using forward and side scatter profile, debris and dead cells were gated out. Cells were routinely sorted twice, and reanalyzed for purity. Then CD44+, CD44- cells, CD44+CD24+ and CD44-CD24-and unsorted cells were obtained.
The CD44+CD24+, CD44-CD24- and unsorted cells were diluted to a density of about 104 cells/mL with serum-free medium (SFM), a mixture of DMEM-F12 containing 10 ng/mL fibroblast and 20 ng/mL epidermal growth factors, 5 kg/mL insulin, 2.75 mg/mL transferrin, 2.75 ng/mL selenium (insulin-transferrin-selenium solution), penicillin (1 × 105 U/L) and streptomycin (100 mg/L). The 200-&mgr;L/well diluted cell suspension was plated to 96-well culture dishes. The wells with 2 × 103 cells were observed everyday under an Olympus CKX41 microscope; the images were captured using an Olympus C5050Z camera. Each group was set up with five duplicate holes. Their OD values were measured with spectrophotometer at 490 nm by 3-[4,5-dimehyl-2-thiazolyl]-2, 5-diphenyl-2H-tetrazolium bromide (MTT) method, and a 96-well plate was determined every 24h. The mean value was obtained and a growth curve was drawn.
After resuspension, CD44+, CD44-, CD24+, CD24-, CD44+CD24+, CD44-CD24- and unsorted cells were diluted to a density of about 5 × 106 to 5 × 103 cells/mL with SCM. The cells (0.1 mL) were injected into the hypodermis of right and left armpit of nude mice. The mice were maintained in a specific pathogen-free room under constant temperature and humidity.
All samples of the CD44+CD24+ cell-formed nodules were placed into 10% formalin immediately, processed with routine histological procedures, and embedded in paraffin. Serial sections were cut 5 &mgr;m thick, and parts of them were stained with hematoxylin and eosin for routine histological observation under light microscope. The others were used for immunohistochemical examination for the CD44 and CD24. After deparaffinization (hydration), sections were treated sequentially with normal goat serum, anti-human CD44 polyclonal antibody (1:200) or anti-human CD24 polyclonal antibody (1:200), biotin-labeled goat anti-mouse IgG, and avidin-biotin-peroxidase complex (ABC). The sites of peroxidase binding were demonstrated by the diaminobenzidine method. Sections were then counterstained with hematoxylin for microscopic examination. Similar procedures were done for the PANC-1 cells. The numbers and areas of CD44-positive and CD24-positive foci > 0.2 mm in diameter and the total areas of the examined sections were measured using a Olympus C5050Z digital camera, Adobe Photoshop version 7.0, and Image-Pro Plus version 6.0.
Data were expressed as means ± SD, and were analyzed with SPSS 12.0, P < 0.05 was considered significant in difference.
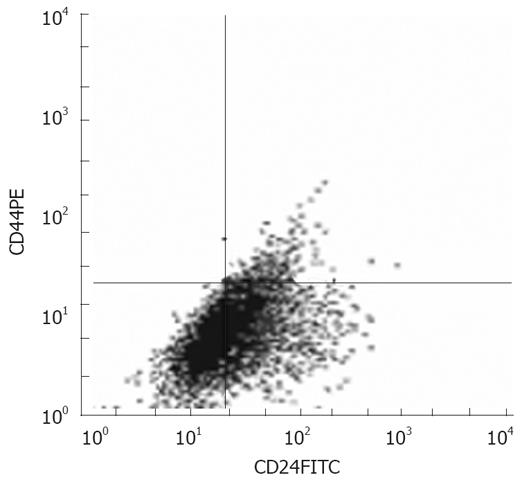
To determine the presence of CD44 and CD24 on the cell surface of the PANC-1 cells, flow cytometric analysis was made. The cell surface markers CD44 and CD24 were chosen as a starting point based on prior work on breast cancer stem cells, in which CD44+CD24-/lowLineage tumorigenic cells generated tumors histologically similar to primary breast tumors when as few as 100 cells were transplanted, whereas tens of thousands of bulk unsorted cancer cells were needed to form tumors in NOD/SCID mice[7]. CD44 and CD24 have been identified as the stem cell surface markers, which act as adhesive molecules with multiple signaling functions[12–14]. As shown in Figure 1, 5.1%- 17.5% of sorted PANC-1 cells expressed the cell surface marker CD44, and 57.8%-70.1% expressed CD24. When expression of multiple surface markers was examined, only 2.1%-3.5% of cells were CD44+ CD24+ (Figure 1).
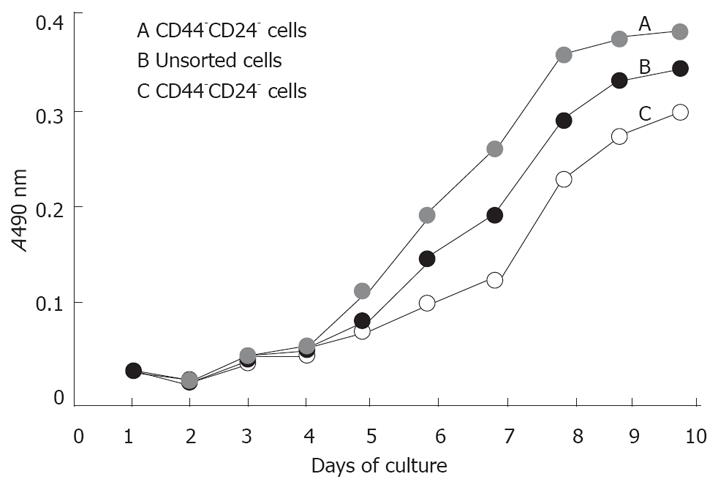
To evaluate the proliferation ability of cells in vitro, the CD44+CD24+, CD44-CD24- and unsorted cells were cultured in SCM in 96-well culture dishes, their OD values were measured with spectrophotometer at 490nm by MTT method. Compared with CD44-CD24- cells, CD44+CD24+ cells had a lower growth rate and longer doubling time in vitro. For the former, the index growth trend appeared at the 5th day, while the latter appeared at the day 7 (Figure 2).
To test the capability of tumor initiation, we injected cells into the hypodermis of right and left armpit of nude mice. When unsorted PANC-1 cells (5 × 103) were injected, no tumor growth was found at wk 12 while 104 cells were injected, one of six mice developed tumors. For cancer cells sorted for the markers CD44 and CD24, expression of individual markers identified cell populations with enhanced tumorigenic potential. For example, injection of 5 × 103 CD44+ cells would occasionally form a tumor (1 of 6 animals), whereas no tumor was observed with CD44- cells until at least 5 × 104 cells were injected (1 of 10 animals). Six of 10 animals developed tumors when injected with 5 × 104 CD44+ cells, representing a 10-fold increase in tumorigenic potential compared with marker negative cells (P = 0.029). Similar results were obtained with CD24+. Injection of CD44+CD24+ cells resulted in an enhanced tumorigenic potential compared with single marker sorted cells. More tumors formed with injection of as few as 103 cells, and no tumor formed in marker-negative cells until at least 5 × 104 cells were injected. The sorted cell population with the highest tumorigenic potential was those expressing CD44 and CD24. For example, injection of 104 CD44-CD24- cells in nude mice found no tumor growth at wk 12. In contrast, nude mice injected with 103 CD44+CD24+ cells had large tumors at wk 4 (2 of 8), a 20-fold increase in tumorigenic potential (P < 0.05 or P < 0.01) (Table 1). There was no obvious histological difference between the CD44+CD24+ cell-formed nodules and PANC-1 cells.
| Groups | 5×105 | 105 | 5×104 | 104 | 5×103 | 103 | 102 |
| Unsorted | 6/6 | 5/6 | 3/6 | 1/6 | 0/6 | 0/0 | 0/0 |
| CD44+ | 0/0 | 9/10 | 6/10 | 3/10 | 1/6 | 0/4 | 0/0 |
| CD44 | 0/0 | 2/10 | 1/10 | 0/4 | 0/0 | 0/0 | 0/0 |
| P | 0.0027 | 0.0286 | 0.3297 | ||||
| CD24+ | 0/0 | 7/8 | 4/8 | 3/8 | 0/4 | 0/0 | 0/0 |
| CD24 | 0/0 | 2/8 | 1/8 | 0/8 | 0/0 | 0/0 | 0/0 |
| P | 0.0203 | 0.1410 | 0.1000 | ||||
| CD44+ CD24+ | 0/0 | 8/8 | 7/8 | 6/8 | 4/8 | 2/8 | 0/4 |
| CD44 CD24 | 0/0 | 1/8 | 1/8 | 0/8 | 0/8 | 0/8 | 0/0 |
| P | 0.0007 | 0.0051 | 0.0035 | 0.0385 | 0.2333 |
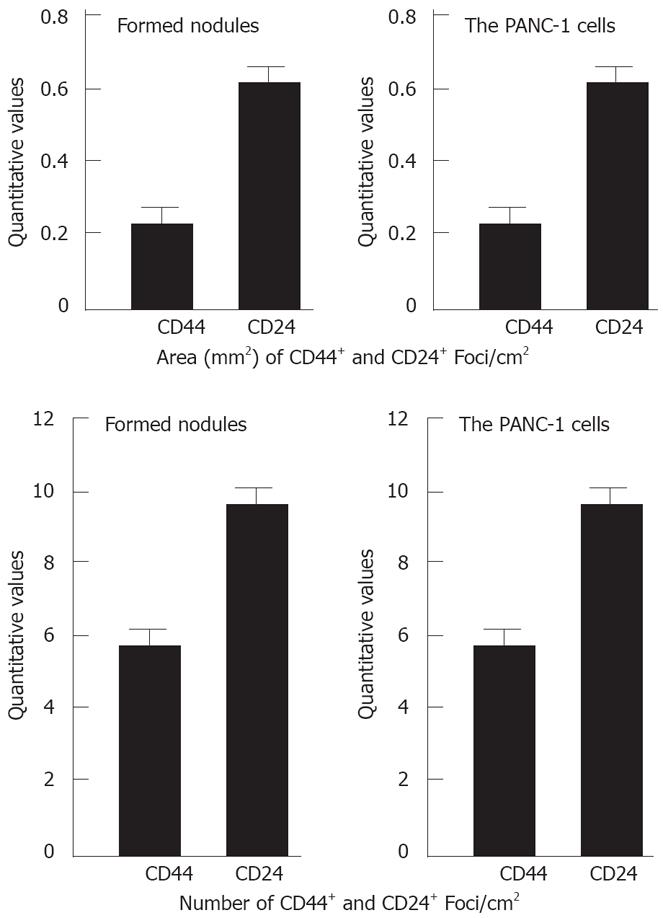
For sections stained with hematoxylin and eosin, tumor cells with variable shape from polygon, spindle to irregular were seen under light microscope. The total CD44- positive and CD24- positive numbers and areas in the examined sections were measured using an Olympus C5050Z digital camera, Adobe Photoshop version 7.0, and Image-Pro Plus version 6.0. There was no significant difference in quantitative values of CD44+ and CD24+ cells between the formed nodules and the PANC-1 cells (P > 0.05) (Figure 3).
The theory of tumor stem cells[1516] indicates that tumor cells have heterogeneity, i.e., the majority of cells in the tumor have lost the growth potential, only a small subset of cells have the capability of the infinite proliferation, the differentiation and the formation of cloning in vitro. The initial isolation and identification of tumor stem cells was first proved in hematological malignancies. The CD34+CD38- phenotype cells (5% of the cancer cells) with obvious proliferation, differentiation and self-renewal ability were purified from the blood of the patients with acute myeloid leukemia[1718]. In 2003, researchers found that only a small subset of human breast cancer cells, with the phenotype CD44+CD24-, formed new tumors in NOD/SCID mice[7]. These breast cancer-initiating cells can be isolated and propagated in vitro as extensively proliferating, clonal, nonadherent spherical clusters are able to differentiate along different mammary epithelial lineages[19]. A small population of cancer-initiating cells (also called cancer stem cells) was later found in several malignancies, including brain[8], prostate[10], liver[2021], lung[22], melanoma[23], and colon tumors[2425].
Although there is increasing evidence that a rare population of undifferentiated cells is responsible for tumor formation and maintenance, little work has been done on the identification of pancreatic cancer special surface markers or on isolation of pancreatic tumor-initiating cells. Based on studies in breast cancer[7] and pancreatic adenocarcinoma[16], we identified cells with the characteristics of tumor stem cells according to the cell surface markers CD44 and CD24 by flow cytometry from pancreatic adenocarcinoma cell line PANC-1. Tumor stem cells have the capability to maintain themselves in culture in an undifferentiated state, initiate tumor growth after xenotransplantation in mice, and differentiate into cancers that are phenotypically indistinguishable from the original tumor. We found that 5.1%-17.5% of sorted PANC-1 cells expressed the cell surface marker CD44, 57.8%-70.1% expressed CD24, and only 2.1%-3.5% of cells were CD44+CD24+. To take a small subset of cells and put it in the organism and see if it regenerates the original tissues is the classic definition of a stem cell. We injected cells into the hypodermis of the right and left armpit of nude mice to test the capability of tumor initiation. When 5 × 103 unsorted PANC-1 cells were injected into nude mice, no tumor grew at wk 12 unless at least 104 cells were injected. For cancer cells sorted for the markers CD44 and CD24, injection of 5 × 103 CD44+ cells would form a tumor, whereas no tumor was observed with CD44-cells until at least 5 × 104 cells were injected. Similar results were obtained with CD24+. The sorted cell population with the highest tumorigenic potential was those cells expressing CD44 and CD24. For instance, injection of 104 CD44-CD24- cells into nude mice, no tumor growth was evident at wk 12. In contrast, nude mice injected with 103 CD44+CD24+ cells had large tumors at wk 4. Moreover, the CD44+CD24+ cells maintained the ability to engraft and reproduce the same histological and antigenic pattern of the PANC-1. In addition, compared with CD44-CD24- cells in vitro, CD44+CD24+ cells had a lower growth rate. The reason is that tumor stem cells are similar to stem cells, which is in relatively static group of cells, and besides other primates, the stem cell pool proliferates once a year[26]. For the CD44+CD24+ cells, there were biological behaviors of the lower proliferative index and the faster tumor growth rate in vivo. It is self-contradictory. The reason awaits further studies. In addition, there was no obvious histological difference between the CD44+CD24+ cell-formed nodules and PANC-1 cells.
The above results showed that CD44 and CD24 may be used as markers for isolation of pancreatic cancer stem cells from pancreatic adenocarcinoma cell line PANC-1, subpopulation cells CD44+CD24+ have the characteristics of tumor stem cells. The purification and other biological behaviors of pancreatic adenocarcinoma stem cells need to be further studied in the future.
Pancreatic carcinoma is an obstinate disease that is difficult to deal with. Though pancreatic cancer accounts for only 2%-3% of all cancers, it is the fourth most frequent cause of cancer deaths in industrialized countries. Unfortunately, only 18% will survive one year after diagnosis, the five-year survival rate is only 4%. Conventional main treatments for pancreatic cancer are surgery, radiation therapy and chemotherapy. Despite advances in surgical and medical therapy, little effect has been achieved on the mortality rate of this disease.
The initial isolation and identification of tumor stem cells was first proved in hematological malignancies. The CD34+CD38- phenotype cells (5% of the cancer cells) with obvious proliferation, differentiation and self-renewal ability had been purified from the blood of the patients with acute myeloid leukemia. Researchers have discovered a small population of cancer-initiating cells (also called cancer stem cells) in several malignancies, including brain, prostate, liver, lung, melanoma, and colon tumors.
The authors isolated pancreatic adenocarcinoma cell line PANC-1 according to the cell surface markers CD44 and CD24 by flow cytometry, obtained subpopulation cells which have properties of tumor stem cells, and identified the ability of propagation of the tumor stem cells in vitro and in vivo.
Because cancer stem cells are thought to be responsible for tumor initiation and its recurrence after an initial response to chemotherapy, it may be a very promising target for new drug development.
This study corroborates a recent publication in the pancreas reporting that a subpopulation of Panc1 cells can propagate to form spheres and that these cells express stem cell markers such as CD44. The study is very interesting.
We thank our colleagues from the Research Laboratory of General Surgery, Union Hospital, Wuhan, for their technical assistance.
| 1. | Bardeesy N, DePinho RA. Pancreatic cancer biology and genetics. Nat Rev Cancer. 2002;2:897-909. |
| 2. | Murphy SL. Deaths: final data for 1998. Natl Vital Stat Rep. 2000;48:1-105. |
| 3. | Cameron JL, Crist DW, Sitzmann JV, Hruban RH, Boitnott JK, Seidler AJ, Coleman J. Factors influencing survival after pancreaticoduodenectomy for pancreatic cancer. Am J Surg. 1991;161:120-124; discussion 124-125. |
| 4. | Niederhuber JE, Brennan MF, Menck HR. The National Cancer Data Base report on pancreatic cancer. Cancer. 1995;76:1671-1677. |
| 5. | Jemal A, Thomas A, Murray T, Thun M. Cancer statistics, 2002. CA Cancer J Clin. 2002;52:23-47. |
| 6. | Bjerkvig R, Tysnes BB, Aboody KS, Najbauer J, Terzis AJ. Opinion: the origin of the cancer stem cell: current controversies and new insights. Nat Rev Cancer. 2005;5:899-904. |
| 7. | Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983-3988. |
| 8. | Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821-5828. |
| 9. | Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, Fiocco R, Foroni C, Dimeco F, Vescovi A. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011-7021. |
| 10. | Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65:10946-10951. |
| 11. | Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, Jacks T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823-835. |
| 12. | Litvinov SV, Velders MP, Bakker HA, Fleuren GJ, Warnaar SO. Ep-CAM: a human epithelial antigen is a homophilic cell-cell adhesion molecule. J Cell Biol. 1994;125:437-446. |
| 13. | Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol. 2003;4:33-45. |
| 14. | Weichert W, Denkert C, Burkhardt M, Gansukh T, Bellach J, Altevogt P, Dietel M, Kristiansen G. Cytoplasmic CD24 expression in colorectal cancer independently correlates with shortened patient survival. Clin Cancer Res. 2005;11:6574-6581. |
| 15. | Bjerkvig R, Tysnes BB, Aboody KS, Najbauer J, Terzis AJ. Opinion: the origin of the cancer stem cell: current controversies and new insights. Nat Rev Cancer. 2005;5:899-904. |
| 16. | Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030-1037. |
| 17. | Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730-737. |
| 18. | Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645-648. |
| 19. | Ponti D, Costa A, Zaffaroni N, Pratesi G, Petrangolini G, Coradini D, Pilotti S, Pierotti MA, Daidone MG. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005;65:5506-5511. |
| 20. | Chiba T, Kita K, Zheng YW, Yokosuka O, Saisho H, Iwama A, Nakauchi H, Taniguchi H. Side population purified from hepatocellular carcinoma cells harbors cancer stem cell-like properties. Hepatology. 2006;44:240-251. |
| 21. | Suetsugu A, Nagaki M, Aoki H, Motohashi T, Kunisada T, Moriwaki H. Characterization of CD133+ hepatocellular carcinoma cells as cancer stem/progenitor cells. Biochem Biophys Res Commun. 2006;351:820-824. |
| 22. | Dome B, Timar J, Dobos J, Meszaros L, Raso E, Paku S, Kenessey I, Ostoros G, Magyar M, Ladanyi A. Identification and clinical significance of circulating endothelial progenitor cells in human non-small cell lung cancer. Cancer Res. 2006;66:7341-7347. |
| 23. | Grichnik JM, Burch JA, Schulteis RD, Shan S, Liu J, Darrow TL, Vervaert CE, Seigler HF. Melanoma, a tumor based on a mutant stem cell? J Invest Dermatol. 2006;126:142-153. |
| 24. | O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106-110. |
| 25. | Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111-115. |
| 26. | Dunnwald M, Chinnathambi S, Alexandrunas D, Bickenbach JR. Mouse epidermal stem cells proceed through the cell cycle. J Cell Physiol. 2003;195:194-201. |