Published online Jun 21, 2008. doi: 10.3748/wjg.14.3759
Revised: April 30, 2008
Accepted: May 7, 2008
Published online: June 21, 2008
Neurenteric cysts are extremely rare congenital anomalies, often presenting in the first 5 years of life, and are caused by an incomplete separation of the notochord from the foregut during the third week of embryogenesis. They are frequently accompanied with spinal or gastrointestinal abnormalities, but the latter may be absent in adults. Although usually located in the thorax, neurenteric cysts may be found along the entire spine. We present a 24-year-old woman admitted for epigastric pain, nausea, vomiting, low grade fever and leucocytosis. She underwent cystogastrostomy for a loculated cyst of the distal pancreas at the age of 4 years, which recurred when she was at the age of 11 years. Ultrasound and computer tomography (CT) scan revealed a 16 cm × 15 cm cystic mass in the body and tail of pancreas, with a 6-7 mm thickened wall. Laboratory data and chest X-ray were normal and spinal radiographs did not show any structural abnormalities. The patient underwent a complete cyst excision, and after an uneventful recovery, remained symptom-free without recurrence during the 5-year follow-up. The cyst was found to contain 1200 mL of pale viscous fluid. It was covered by a primitive single-layered cuboidal epithelium, along with specialized antral glandular parenchyma and hypoplastic primitive gastric mucosa. Focal glandular groups resembling those of the body of the stomach were also seen. In addition, ciliary respiratory epithelium, foci of squamous metaplasia and mucinous glands were present. The wall of the cyst contained a muscular layer, neuroglial tissue with plexogenic nerve fascicles, Paccini corpuscle-like structures, hyperplastic neuro ganglionar elements and occasional psammomatous bodies, as well as fibroblast-like areas of surrounding stroma. Cartilagenous tissue was not found in any part of the cyst. Immunohistochemistry confirmed the presence of neurogenic elements marked by S-100, GFAP, NF and NSE. The gastric epithelium showed mostly CK7 and EMA immunoexpression, and the respiratory epithelium revealed a CK8 and CK18 immunoprofile without CK 10/13 positive elements, though neither CEA or AFP positive cells were found. To our knowledge, this is the first reported case of an abdominally located neurenteric cyst with no associated spinal anomalies.
- Citation: Colović R, Micev M, Jovanović M, Matić S, Grubor N, Atkinson HDE. Abdominal neurenteric cyst. World J Gastroenterol 2008; 14(23): 3759-3762
- URL: https://www.wjgnet.com/1007-9327/full/v14/i23/3759.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.3759
Foregut duplications can be classified into three groups: enteric cysts (lined with intestinal epithelium), bronchogenic cysts (lined with respiratory epithelium), and neurenteric cysts (where enteric cysts are associated with vertebral anomalies or communications with the nervous system)[12].
Neurenteric cysts are extremely rare congenital anomalies, usually diagnosed in infancy[34] and tend to be located in the right upper posterior mediastinum, but can be found anywhere along the spine or even intracranially[156]. They may be associated with spinal anomalies such as hemivertebrae and anterior spina bifida[5], and esophageal atresia[78], though these associated abnormalities may be absent in adults[5].
We describe the first reported case of an abdominal neurenteric cyst, with no associated spinal anomalies.
A 24-year-old woman presented to our unit in 2001 with epigastric pain, nausea, vomiting, low grade fever and leucocytosis.
Her medical history and case notes showed that she underwent surgery for an 8 cm multi-loculated cyst in the region of the distal pancreas at the age of 4 years, and this was anastomosed to the posterior wall of the stomach. At the age of 11 years, a 2.5 cm recurrent cyst was found, though no significant enlargement was noted over the following decade, and she was largely symptom-free in almost 20 years.
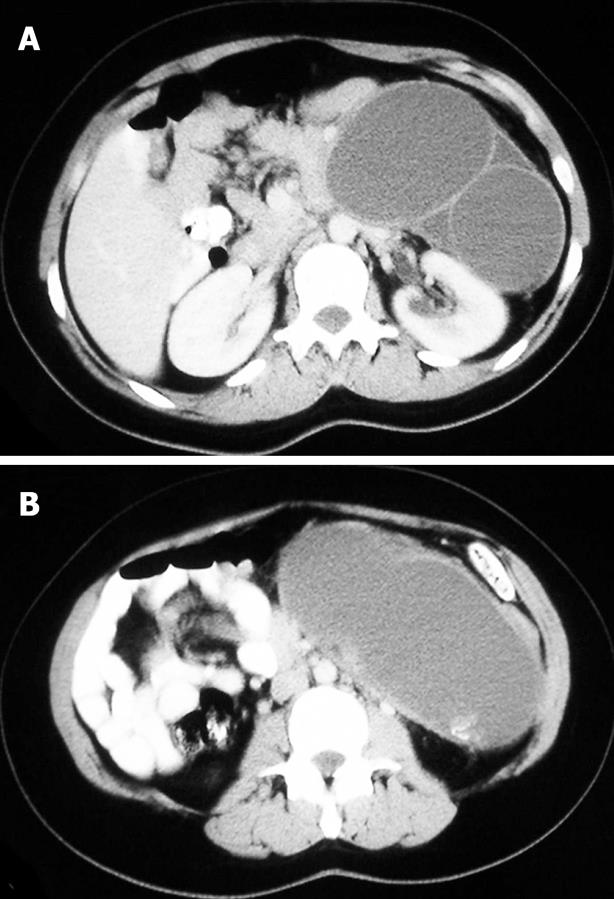
On examination she was found to have a tender palpable mass in the upper left epigastrium. Ultrasonography (US) and computer tomography (CT) scan revealed a cystic mass in the region of the body and tail of the pancreas, measuring 16 cm × 15 cm, with a 6-7 mm thickened wall, and filled with dense fluid (Figure 1). Laboratory data and chest X-ray were normal and spinal radiographs did not show any structural abnormalities. Barium swallow revealed that the stomach displaced to the right and anteriorly, and a gastroscopy did not show any sign of the previous cystogastrostomy on the posterior wall of the stomach.
With a working diagnosis of a mucinous cystadenoma of the pancreas, the patient underwent laparotomy. The cystic tumor was located in the body and tail of the pancreas, and was adherent to the stomach, splenic vessels, prevertebral fascia, and spine. The pancreatic parenchyma in the region of the cyst was completely atrophied, and the previously performed anastomosis was obliterated. The cyst was completely excised, and was found to contain 1200 mL of pale viscous fluid. Laboratory analyses did not show any elevation of amylase or polymorphonuclears cells, and no growth occurred on microbiological culture.
The postoperative recovery was uneventful, the preoperative symptoms completely resolved, and the patient remained symptom-free, without recurrence during the 5-year follow-up.
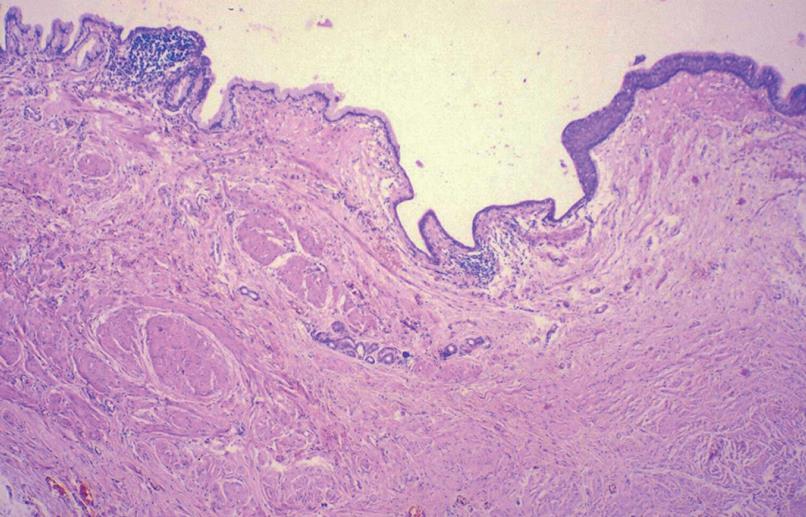
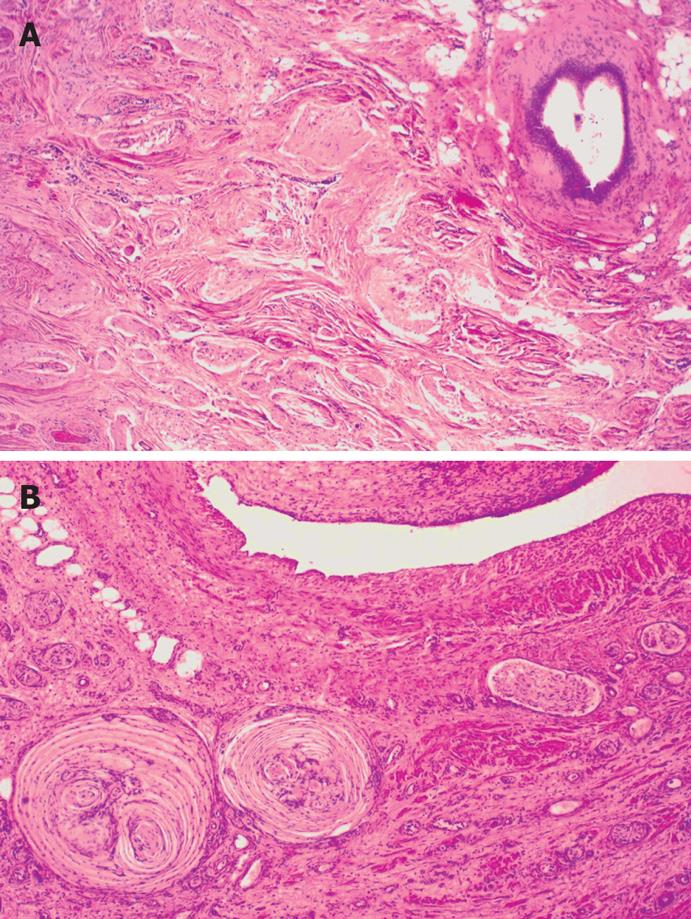
Macroscopically, the cyst wall, dark brown in color, was up to 14 mm in thickness and partly hyalinized. The inner surface of the cyst was smooth with some coarse sections, and some areas of the cyst wall also contained smaller cysts. Microscopic examination showed organoid organization resembling tissues, and organs similar to the embryonic foregut, including intestinal wall epithelial formation, partly covered by a primitive single-layered cuboidal epithelium, along with specialized antral glandular parenchyma, and hypoplastic primitive gastric mucosa. Focal glandular groups resembling those of the body of the stomach were also seen. In addition, ciliary respiratory epithelium, foci of squamous metaplasia, and mucinous glands were present. The wall of the cyst contained a muscular layer, neuroglial tissue with plexogenic nerve fascicles, Paccini corpuscle-like structures, hyperplastic neuro ganglionar elements, and occasional psammomatous bodies, as well as fibroblast-like areas of surrounding stroma (Figures 2 and 3).
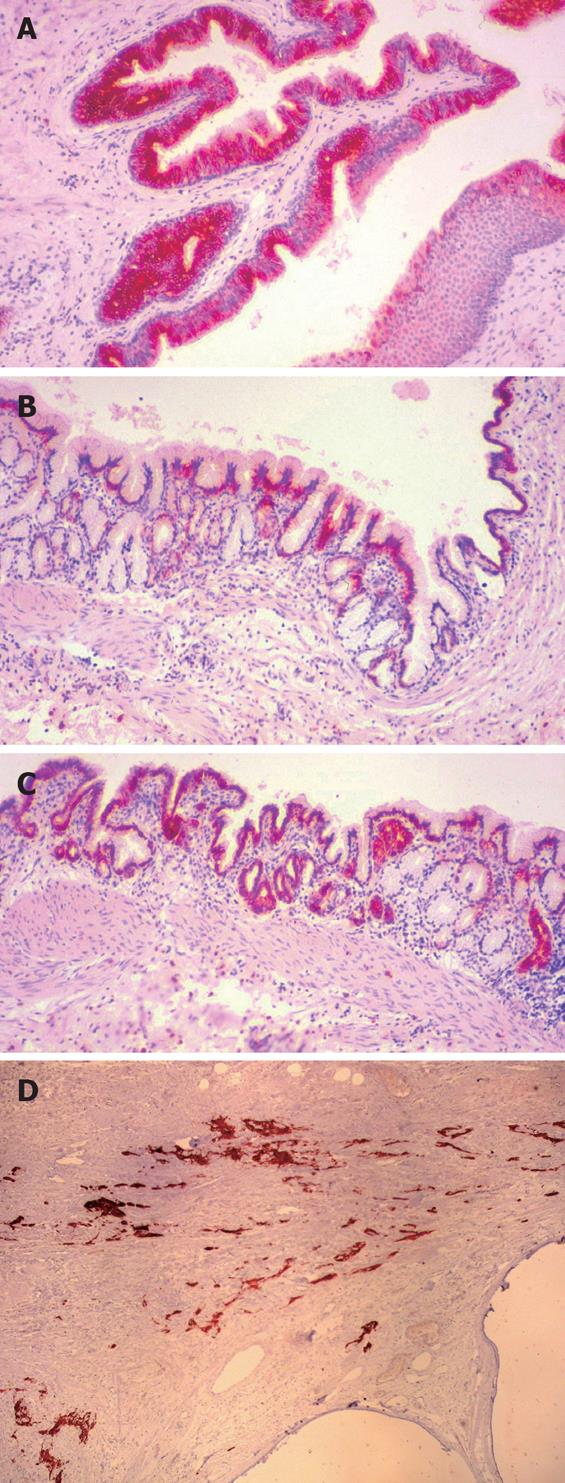
Immunohistochemistry confirmed the presence of neurogenic elements marked by S-100, GFAP, NF and NSE. The gastric epithelium showed mostly CK7 and EMA immunoexpression, and the respiratory epithelium revealed a CK8 and CK18 immunoprofile without CK 10/13 positive elements, though neither CEA or AFP positive cells were found (Figure 4A-D).
Neurenteric cysts may appear at any age, but are usually discovered during the first five years of life[49]. They are thought to develop early in the course of embryogenesis either due to incomplete separation of the notochord from the embryonic foregut (which are apposed), or due to herniation of the endoderm of the embryonic foregut into the dorsal ectoderm[1011]. This attachment of the cyst to the notochord may prevent fusion of the vertebral bodies and lead to spinal anomalies[15], indeed one third of patients have associated anomalies of the central nervous system or gastrointestinal tract[5].
Neurenteric cysts may be multiloculated or septate, lined with ciliated, non-ciliated, columnar or cuboidal epithelium, and may resemble intestinal, duodenal or gastric mucosa. These cells are usually PAS-positive and can contain mucus and globules, with occasional squamous cell metaplasia. A basal membrane is always present and the capsule consisting of fibrovascular tissue is fragile. The wall of the cyst may contain ganglionar cells, lymphatic tissue, pancreatic tissue, salivary glands or muscular tissue without serosa[1]. Cartilaginous tissue is never present[6]. The cysts usually contain clear, pale, yellowish or green viscous or mucinous fluid, depending on the presence or absence of previous hemorrhage[5].
They are classified into Types A-C according to the histology. Type A cysts, the most described in the literature[5], consist of simple lined cuboidal or columnar epithelium with or without cilia. Type B cysts include more complex gastrointestinal or tracheobronchial elements, including mucous glands and smooth muscle cells in the cyst walls. In addition to these elements, type C cysts also contain ependymal and/or glial tissue[5]. Our patient had a septated type C cyst containing respiratory epithelium, gastric epithelium, and ependymal and glial tissue.
The clinical presentation usually depends on the size and location of the cyst. The patient, typically a small infant, often presents with respiratory distress, dyspnoea, stridor or a persistent cough, caused by pressure of the cyst on the lung. Respiratory distress is usually found with a mediastinal mass, and a vertebral anomaly coexisting in more than 70% of pediatric patients with neurenteric cysts[39]. When located intraspinally, the cysts may cause motor and sensory neurological disturbance[9]. Chest pain, cardiac arrhythmias and dysphagia can also present, and in the rare cases of ulcer formation in ectopic gastric mucosa in the cyst wall, the patient can present with melaena or perforation of the cyst[112].
The diagnosis is usually made by US, which characterizes the cyst and can evaluate for chest masses, and with the advent of high resolution US, these cysts can be detected as early as 18 wk gestation[1513]. CT and nuclear magnetic resonance imaging (MRI) also have their place[14], though the final diagnosis is based on histopathological examination.
Complete cyst excision is the recommended treatment[615], with some patients requiring a simultaneous laminectomy[16]. If the cyst consists of two components, the symptomatic cyst should be excised first. If there is a combination of asymptomatic intraspinal and extraspinal cysts, the spinal cyst should be excised first in order to avoid any neurological deterioration which might occur during mediastinal cyst excision[6].
Our patient underwent a cystogastrostomy at the age of 4 years, presumably with an underlying misdiagnosis of a pancreatic pseudocyst. However, the cyst was covered with an epithelial layer, and recurred. This case nicely demonstrates that the cyst should have previously been completely excised, and when this was finally done, the patient’s symptoms resolved, with no cyst recurrence.
| 1. | Singh D, Singh S, Kiraw R, Bhagwat SS. Neurenteric Cyst 2003. Available from URL: http://www.bhj.org/journal/2003_4502_april/neurentericcyst_373.htm. |
| 2. | Ravitch MM. Mediastinal cysts and tumours. Pediatric Surgery Chicago: Year Book Medical Publishers 1986; 606-614. |
| 3. | Fernandes ET, Custer MD, Burton EM, Boulden TF, Wrenn EL Jr, Whittle AP, Edwards OP. Neurenteric cyst: surgery and diagnostic imaging. J Pediatr Surg. 1991;26:108-110. |
| 4. | Viladevall H. Primitive gut morphogenesis. 2004;2006 Available from URL: http://sprojects.mmi.mcgill.ca/embryology/gi/pgm.htm. |
| 5. | Olavarria SA, Diaz Guerrero DL, Yanis A. Neurenteric cyst. 2000;2006 Available from URL: http://www.thefetus.net/page.php?id=230. |
| 6. | Birmole BJ, Kulkarni BK, Vaidya AS, Borwankar SS. Intrathoracic enteric foregut duplication cyst. J Postgrad Med. 1994;40:228-230. |
| 7. | Kapouleas GP, Keramidas DC, Soutis M. Bochdalek's hernia combined with agenesis of the pericardium and intrathoracic solitary cyst of the liver. Z Kinderchir. 1989;44:377-378. |
| 8. | Hemalatha V, Batcup G, Brereton RJ, Spitz L. Intrathoracic foregut cyst (foregut duplication) associated with esophageal atresia. J Pediatr Surg. 1980;15:178-180. |
| 9. | Mancini M, Eggerstedt IM. Mediastinal Cysts. 2006;2006 Available from URL: http://www.emedicine.com/med/topic2985.htm. |
| 10. | Bourne A. Congenital and developmental abnormalities. Gastrointestinal and esophageal pathology New York: Churchill Livingstone 1995; 275-301. |
| 11. | Sen S, Bourne AJ, Morris LL, Furness ME, Ford WD. Dorsal enteric cysts--a study of eight cases. Aust N Z J Surg. 1988;58:51-55. |
| 12. | Cohen SR, Geller KA, Birns JW, Thompson JW, Meyer BW, Lindesmith GG. Foregut cysts in infants and children. Diagnosis and management. Ann Otol Rhinol Laryngol. 1982;91:622-627. |
| 13. | Perera GB, Milne M. Neurenteric cyst: antenatal diagnosis by ultrasound. Australas Radiol. 1997;41:300-302. |
| 14. | Rattan KN, Magu S, Rohilla S. Mediastinal foregut duplication cysts. Indian J Pediatr. 2004;71:103-105. |
| 15. | Cahill JF. An unusual cause of neonatal respiratory distress. RDS in a neonate with a neuro-enteric cyst. Anaesthesia. 1981;36:790-794. |
| 16. | Bilik R, Ginzberg H, Superina RA. Unconventional treatment of neuroenteric cyst in a newborn. J Pediatr Surg. 1995;30:115-117. |