Published online Jun 21, 2008. doi: 10.3748/wjg.14.3729
Revised: March 19, 2008
Accepted: March 26, 2008
Published online: June 21, 2008
AIM: To examine the possible effects of honey supplementation on hepatic damage due to obstruction of the common bile duct in an experimental rat model.
METHODS: The study was performed with 30 male rats divided into three groups: a sham group, an obstructive jaundice group, and an obstructive jaundice plus honey group. At the end of the study period, the animals were sacrificed, and levels of nitric oxide (NO), and NO synthase (NOS) activities were measured in liver tissues, and levels of adenosine deaminase (ADA) and alanine transaminase (ALT) activities were measured in serum.
RESULTS: Blood ALT and ADA activities were significantly elevated in the jaundice group as compared to those of the sham group. In the obstructive jaundice plus honey group, blood ALT and ADA activities were significantly decreased as compared to those of the jaundice group. In erythrocytes and liver tissues, NO levels were found to be significantly higher in the obstructive jaundice plus honey group compared to those of the sham group. Additionally, NO levels were found to be significantly higher in liver tissues from the animals in the obstructive jaundice plus honey group than those of the jaundice group.
CONCLUSION: Honey was found to be beneficial in the prevention of hepatic damage due to obstruction of the common bile duct.
- Citation: Erguder BI, Kilicoglu SS, Namuslu M, Kilicoglu B, Devrim E, Kismet K, Durak I. Honey prevents hepatic damage induced by obstruction of the common bile duct. World J Gastroenterol 2008; 14(23): 3729-3732
- URL: https://www.wjgnet.com/1007-9327/full/v14/i23/3729.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.3729
Honey is produced by honeybees. They obtain nectar from various flowers, and digest it in their bodies, enrich it with their salivary and enzymatic secretions, and put it in honeycombs, so that ripe honey is formed[1]. Since ancient times, honey has been known as both flavorful food and a traditional therapeutic material. It has rich flavonoid components, such as luteolin, quercetin, apigenin, fisetin, kaempferol, isorhamnetin, acacetin, tamarixetin, chrysin, and galangin, and therefore, exhibits antioxidant activity. Additionally, honey provides antibacterial, anti-inflammatory, immune-stimulant, anti-ulcer and wound/burn healing (regenerative) effects[2].
Free radicals lead to oxidative damage in many molecules, such as lipids, proteins and nucleic acids. Many complications have been attributed to oxidative damage, including atherosclerosis, aging, and cancerous diseases. Antioxidant foods that are rich in flavonoids are protective agents against these ailments[3].
Obstructive jaundice leads to oxidative injury and inflammation in hepatocytes[45]. Over production of hydroxyl radicals in blood and liver from rats with obstructive jaundice has been reported[6].
Nitric oxide synthase (NOS) converts arginine to citrulline and nitric oxide (NO). Nitric oxide leads to activation of guanylyl cyclase, formation of cyclic guanosine 3′,5′-monophosphate (cGMP), stimulation of cGMP-protein kinases, and subsequent relaxation in smooth muscle. It has been reported that NOS gene knockout in mice causes an elevation in blood pressure and increased synthesis of cGMP prevents platelet aggregation[7].
Adenosine deaminase (ADA; E.C. 3.5.4.4) is an enzyme that catalyses conversion of adenosine to inosine and ammonia[8]. ADA activity has been found to increase in cirrhotic patients, and increased ADA activity has been suggested as a nonspecific hepatic marker for disease with liver damage[9].
Alanine transaminase (ALT; E.C. 2.6.1.1) is a transaminase enzyme that catalyzes the inter-conversion of the amino acid L-alanine to L-glutamate and vice versa. In liver diseases associated with hepatic necrosis, ALT levels characteristically are elevated[10].
In this study, we investigated the effects of honey on the NO pathway and ADA enzyme activity in rats that had induced obstructive jaundice.
Thirty male Wistar albino type rats of 12 wk old (250 ± 25 g in weight) were housed individually in wire cages under constant temperature (21°C ± 2°C) with a 12 h light-dark cycle. Twelve hours before anesthesia animals were deprived of food, but had free access to water until 2 h before anesthesia. No enteral or parenteral antibiotics were administered at any time. The animals were divided randomly into 3 groups of 10 rats each: the sham group (group I), the obstructive jaundice group (group II) and the obstructive jaundice plus honey group (group III). The animals were anesthetized by intramuscular injection of 30 mg/kg ketamine hydrochloride (Ketalar; Parke-Davis, Istanbul, Turkey) and 5 mg/kg xylasine (Rompun, Bayer, Istanbul, Turkey). Midline laparotomy was performed under sterile conditions. In group I, the common bile duct (CBD) was freed from the surrounding soft tissue, and was manipulated without ligation and transection. In groups II and III, the CBDs of the rats were identified, double ligated with 5-0 silk, and divided between the ligatures. Group III was nourished with honey 10 mg/kg per day by using a nasogastric tube that was inserted daily and removed after honey supplementation (Balparmak LTD, Istanbul, Turkey). The animals were sacrificed on postoperative day 7 with high-dose diethyl ether inhalation. Subsequently, their liver tissues were removed and blood samples were obtained. The blood samples were put in tubes, and then centrifuged at 2000 g for 5 min. Upper clear supernatant (serum) was taken and used in the enzymatic analyses. The liver tissues were first homogenized in physiologic saline (1 g in 5 mL) and then were centrifuged at 4000 g for 20 min. Upper clear supernatants were removed to use in the analyses. Protein levels of the supernatants were determined using Lowry’s method[11] were adjusted to equal concentrations before analyses.
NO levels and NOS enzyme activities were measured in liver tissues, and ADA and ALT enzymes activities were measured in serum.
The level of NO was estimated by the method based on the diazotization of sulfanilic acid by NO at acid pH, and subsequent coupling to N-(1-naphthyl-ethylene diamine) (Griess reaction) as described previously[12]. Since nitrate anion does not give a diazotization reaction with sulfanilic acid, the samples were treated by cadmium (a reducing agent) to reduce nitrate anions into nitrite anions before the NO estimation[13]. The results were expressed as &mgr;moL/mg protein. The total NOS activity (IU/mL) method is based on the Griess reaction[12]. The results were expressed as IU/mg protein.
Adenosine deaminase activity was studied by the method of Guisti based on spectrophotometric detection of ammonia formation[8]. The results were expressed as IU/L.
Serum ALT levels were determined by using a spectrophotometric method[14]. The results were expressed as U/L.
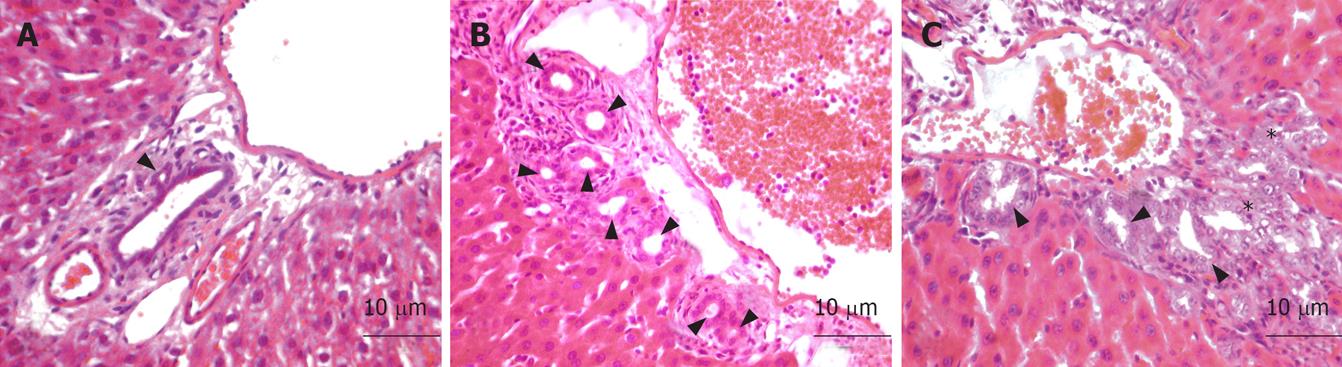
The histopathological analyses were carried out in the Histology and Embryology Department of Ankara University School of Medicine. Histopathological examination was performed by using light microscopic analyses. The samples were obtained from the liver and fixed in 10% neutral buffered formalin solution for 2 d. Tissues were washed in flowing water, and were dehydrated with rising concentrations of ethanol (50%, 75%, 96% and 100%). After dehydration, specimens were put into xylene to obtain transparency and were then infiltrated with, and embedded in paraffin. Embedded tissues were cut into sections of 5 &mgr;m thicknesses by Leica RM 2125 RT and were then stained with hematoxylin and eosin. Histopathologic examinations were performed and photographed by Nikon Eclipse E 600.
In the statistical evaluation of the results, one-way ANOVA, and post hoc LSD tests were used. P values of less than 0.05 were considered as significant.
The results are given in Table 1. Serum ALT and ADA enzymes activities were significantly elevated in group II compared to those of the sham group. In group III, serum ALT and ADA enzymes activities were found to be significantly decreased compared to those of group II. In liver tissues, NO levels were found to be higher in group III than those of the sham group. Additionally, NO levels were found to be significantly higher in liver tissues of group III compared to those of group II. There were no significant differences between groups for NOS activities.
In group I, there were no morphological alterations in the portal tract and whole liver tissue (Figure 1A). Group II tissues displayed some histopathological changes in the portal tract, such as proliferation of the duct epithelial cells, and looping and reduplication of the ducts and ductules. The surrounding hepatocytes were enlarged (Figure 1B). Histopathological evidence showing bile ductule proliferation was markedly reduced in group III. Regression of the bile duct epithelial cells, and phagocytosis of the debris from dying bile duct epithelial cells were observed. We also examined the conspicuous reduction in the size of enlarged hepatocytes (Figure 1C).
Since ancient times, honey has been known to have antibacterial and antioxidant properties due to its phenolic compounds[3]. It has been emphasized that honey has a rapid wound healing property[15]. Gethin et al demonstrated that honey has repairing potential in leg ulceration when wounds are dressed with honey[16]. The antimicrobial property of honey on some microbial isolates has also been reported[17]. Moreover, the antibacterial effect of honey on ocular flora has been displayed[18]. Furthermore, the scolicidal efficacy of propolis, which is a resinous material obtained by honey bees from plants or flowers, has been shown in cystic hydatid disease[19]. Recently, it has been found that honey leads to increased levels of NO in biological fluids and to reduced liver enzymes, such as AST and ALT, in blood[20].
In the obstructive jaundice group in our study, elevated serum ALT and ADA activities indicated liver damage. Additionally, reduced serum ALT and ADA activities in the obstructive jaundice plus honey group showed significant improvement in liver tissues. Histopathological examination also showed damage in the obstructive jaundice group with improvement in the obstructive jaundice plus honey group.
Our results showed the protective potential of honey with liver damage. It is possible that NO levels increased in the liver tissue due to the rich NO content of the honey itself, which is supported by our finding of unchanged NOS activity in liver tissue. Increased NO levels in the obstructive jaundice plus honey group may contribute to the protective result possibly through the elimination of toxic free radicals by NO.
In conclusion, we suggest that honey suppleme-ntation may give beneficial results in the prevention of hepatic damage induced by obstruction of the common bile duct.
In liver diseases associated with hepatic necrosis, the enzyme level characteristically is elevated. In this study, it was aimed to investigate the effects of honey on NO pathway, and adenosine deaminase (ADA) enzyme activity in rats which have induced obstructive jaundice.
The authors suggest that honey supplementation may give beneficial results to prevent the hepatic damage induced by obstruction of common bile duct.
This study tries to elucidate possible mechanism for honey supplementation in the hepatic damage induced by obstruction of common bile duct.
Histopathological examination was performed by using light microscopic analyses.
This is a valuable study indicating hepatocellular damage in obstructive jaundice group and protective potential of honey in this process. It’s a well-designed and important paper.
| 1. | Naef R, Jaquier A, Velluz A, Bachofen B. From the linden flower to linden honey-volatile constituents of linden nectar, the extract of bee-stomach and ripe honey. Chem Biodivers. 2004;1:1870-1879. |
| 2. | Fiorani M, Accorsi A, Blasa M, Diamantini G, Piatti E. Flavonoids from italian multifloral honeys reduce the extracellular ferricyanide in human red blood cells. J Agric Food Chem. 2006;54:8328-8334. |
| 3. | Perez E, Rodriguez-Malaver AJ, Vit P. Antioxidant capacity of Venezuelan honey in wistar rat homogenates. J Med Food. 2006;9:510-516. |
| 4. | Celebi F, Yilmaz I, Aksoy H, Gumus M, Taysi S, Oren D. Dehydroepiandrosterone prevents oxidative injury in obstructive jaundice in rats. J Int Med Res. 2004;32:400-405. |
| 5. | Takaoka M, Kubota Y, Tsuji K, Yamamoto S, Ogura M, Yanagitani K, Shimatani M, Shibatani N, Inoue K. Human neutrophil functions in obstructive jaundice. Hepatogastroenterology. 2001;48:71-75. |
| 6. | Liu TZ, Lee KT, Chern CL, Cheng JT, Stern A, Tsai LY. Free radical-triggered hepatic injury of experimental obstructive jaundice of rats involves overproduction of proinflammatory cytokines and enhanced activation of nuclear factor kappaB. Ann Clin Lab Sci. 2001;31:383-390. |
| 7. | Murray RK. Muscle and the cytoskeleton. Eds. Harper’s Biochemistry. Stamford: Appleton & Lange 2000; 729-730. |
| 8. | Guisti G. Enzyme activities. Methods of enzymatic analysis. Weinheim Bergest: Verlag Chemia 1974; 1087-1091. |
| 9. | Fernandez E, Rodrigo L, Riestra S, Carcia S, Gutierrez F, Ocio G. Adenosine deaminase isoenzymes and neopterin in liver cirrhosis. J Clin Gastroenterol. 2000;30:181-186. |
| 10. | Moss DW, Henderson AR. Clinical Enzymology. Tietz textbook of Clinical Chemistry. Philadelphia, Pennsylvania: W.B. Saunders Company 1999; 652-654. |
| 11. | Lowry O, Rosebrough N, Farr L, Randall R. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265-275. |
| 12. | Durak I, Kavutcu M, Kacmaz M, Avci A, Horasanli E, Dikmen B, Cimen MY, Ozturk HS. Effects of isoflurane on nitric oxide metabolism and oxidant status of guinea pig myocardium. Acta Anaesthesiol Scand. 2001;45:119-122. |
| 13. | Ridnour LA, Sim JE, Hayward MA, Wink DA, Martin SM, Buettner GR, Spitz DR. A spectrophotometric method for the direct detection and quantitation of nitric oxide, nitrite, and nitrate in cell culture media. Anal Biochem. 2000;281:223-229. |
| 14. | Reitman S, Frankel S. A colorim method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957;28:56-63. |
| 15. | Henriques A, Jackson S, Cooper R, Burton N. Free radical production and quenching in honeys with wound healing potential. J Antimicrob Chemother. 2006;58:773-777. |
| 16. | Gethin G, Cowman S. Case series of use of Manuka honey in leg ulceration. Int Wound J. 2005;2:10-15. |
| 17. | Al-Waili NS, Akmal M, Al-Waili FS, Saloom KY, Ali A. The antimicrobial potential of honey from United Arab Emirates on some microbial isolates. Med Sci Monit. 2005;11:BR433-BR438. |
| 18. | Albietz JM, Lenton LM. Effect of antibacterial honey on the ocular flora in tear deficiency and meibomian gland disease. Cornea. 2006;25:1012-1019. |
| 19. | Kismet K, Kilicoglu B, Koru O, Tanyuksel M, Oruc MT, Sorkun K, Salih B, Akkus MA. Evaluation on scolicidal efficacy of propolis. Eur Surg Res. 2006;38:476-481. |
| 20. | Al-Waili NS, Saloom KY, Akmal M, Al-Waili F, Al-Waili TN, Al-Waili AN, Ali A. Honey ameliorates influence of hemorrhage and food restriction on renal and hepatic functions, and hematological and biochemical variables. Int J Food Sci Nutr. 2006;57:353-362. |