Published online Jun 14, 2008. doi: 10.3748/wjg.14.3554
Revised: September 19, 2007
Accepted: September 26, 2007
Published online: June 14, 2008
AIM: To prepare chitosan-polyaspartic acid-5-fluorouracil (CTS-Pasp-5Fu) nanoparticles and investigate its anti-carcinoma effect and toxicity.
METHODS: CTS-Pasp-5Fu nanoparticles were synthesized by ionic gelatification. Male BABL/c nude mice were injected with SGC-7901 gastric carcinoma cell line mass to establish a human gastric carcinoma model. They were randomly allocated into 4 groups: CTS-Pasp-5Fu (containing 5-Fu 1.25 mg/kg), 5-Fu (1.25 mg/kg), CTS-Pasp and normal saline groups. Tumor weight was measured and assay of colony forming unit-granulocyte and macrophage (CFU-GM) was performed. The structural change of cells and tissues was observed and the Bax and Bcl-2 genes were detected.
RESULTS: Compared with normal saline, the inhibition rates of tumor growth for the CTS-Pasp, 5-Fu and CTS-Pasp-5Fu groups were 5.58%, 58.69% and 70.82%, respectively. The tumor inhibition rates for the CTS-Pasp, 5-Fu and CTS-Pasp-5Fu groups were 5.09%, 65.3% and 72.79%, respectively. There was a significant decrease in the number of CFU-GM formation and increase of total bilirubin, and alanine aminotransferase in the 5-Fu group, but no change in those of the other three groups. There was no change in white blood cell count and creatinine among the four groups. Pathological section of liver and nephridial tissues showed that the damage in the 5-Fu group was more severe than that in the CTS-Pasp-5Fu group. 5-Fu and CTS-Pasp-5Fu groups could both down-regulate the Bcl-2 expression and up-regulate the Bax expression to different extent, and the accommodate effect of CTS-Pasp-5Fu was more obvious than 5-Fu.
CONCLUSION: The tumor inhibition rate of CTS-Pasp-5Fu nanoparticles is much higher than that of 5-Fu alone.
- Citation: Zhang DY, Shen XZ, Wang JY, Dong L, Zheng YL, Wu LL. Preparation of chitosan-polyaspartic acid-5-fluorouracil nanoparticles and its anti-carcinoma effect on tumor growth in nude mice. World J Gastroenterol 2008; 14(22): 3554-3562
- URL: https://www.wjgnet.com/1007-9327/full/v14/i22/3554.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.3554
As is well known, gastric carcinoma is one of the most familiar gastrointestinal malignant tumors. 5-fluorouracil (5-Fu) is universally used as an antineoplastic agent in gastrointestinal cancer[1]; however, it has a short plasma half-life period in vivo (only 5-10 min). This requires us to make continuous infusion (CI) schedules of 5-Fu in order to maintain an effective concentration in vivo[23]. The novel oral fluoropyrimidine appears to offer comparable efficacy while providing a more convenient schedule[4]. Chitosan (CTS) is the second most abundant polysaccharide and a cationic polyelectrolyte present in nature. CTS has shown a favorable biocompatibility[56] as well as the ability to increase membrane permeability both in vitro[78] and in vivo[9], which are degraded by lysozyme in serum. CTS has received more attention in the pharmaceutical field for a wide range of drug-delivery applications[10–14]. Polyaspartic acid is a kind of newly biodegradable, innocuous and friendly environmental bio-organic polymer, recognized as a green material, and widely applied in the areas such as agriculture, medicine, commodity, water treatment, etc. The synthesis and application of polyaspartic acid have been studied in many companies. However, little work focused on the complex of chitosan and polyaspartic acid. Our previous study showed that chitosan can encapsulate appreciable quantities of polyaspartic acid (Pasp) into stable nanoparticles, and the method of ion gelatification for preparing chitosan-polyaspartic acid-5-fluorouracil (CTS-Pasp-5Fu) nanoparticles is stable, simple and well biocompatible. Compared with 5-Fu, the Cmax of its nanoparticles is decreased, the AUC was increased and the T1/2 is prolonged obviously. The CTS-Pasp-5Fu nanoparticles are released controllably and could overcome some disadvantages of 5-Fu[15]. The major goal of the present work was to prepare CTS-Pasp-5Fu nanoparticles and investigate its anti-carcinogenic effect and toxicity.
Chitosan with a deacetylation degree (DD) of 95.3% and the molecular weight (Mw) of 6-270 kDa was purchased from Kabo Biochemical Company (Shanghai, China). Polyaspartic acid was prepared by the Department of Macromolecular Science, Key Laborotory of Molecular Engineering of Polymers of Chinese Ministry of Education, Fudan University (Shanghai) as previously described[16]. The average molecular weight of PAsp was 5.0 kDa. 5-fluorouracil was purchased from Donghai Pharmaceutical Company (Shanghai, China).The human gastric cancer cell line, SGC-7901, was serially subcultivated by the Department of Laboratory of Gastroenterology, Zhongshan Hospital. Nude male BALB/c mice (aged 35-42 d and weighing 20-23 g) and Kunming male mice (weight 5-20 g) were obtained from the Department of Laboratory Animals, Fudan University. Methyl cellulose M450 was purchased from China Medicine (group) Shanghai Chemical Reagent Corporation. Iscove’s modified Dulbecco’s medium was purchased from GIBCO Company (UK). Fetal bovine serum was purchased from Hangzhou Sijiqing Biological Engineering Materials Co, Ltd (Hangzhou, China). Antisubstance R-0023 was purchased from Changdao Biotechnology Ltd (Shanghai). All other chemicals were of analytical grade and used without further purification.
Chitosan nanoparticles were prepared as reported by Calvo et al[17] (1997) based on the ionic gelation of CS with TPP anions. Briefly, chitosan was dissolved in dilute acetic acid solution (10 g/L). The concentration of acetic acid in aqueous solution was the same as that of chitosan. Quantum satis 5-Fu was dissolved in this solution at room temperature. Afterwards, under magnetic stirring, the mixture solution of 5-Fu and CS was dropped into the Pasp solution at a rate of one drop/sec. Then, opalescent suspension was formed. The obtained suspension was filtered with a paper filter for use. Glutaraldehyde crosslinking nanoparticles were dropped in to drug-loaded CTS-Pasp suspension under magnetic stirring. This mixture was further stirred for three hours at room temperature. CTS-Pasp-5-Fu nanoparticles were separated from the aqueous suspension medium by ultra-centrifugation at 35 000 r/min for 30 min at 25°C, washed by dilute acetic acid (pH 5.0) solution and separated by three times ultracentrifugation. The sample was re-dispersed.
Dynamic light scattering (DLS) (Malvern, Autoszer 4700) was used to measure the hydrodynamic diameter and size distribution[18]. DLS measurement was done with a wave length of 532 nm at 25°C with an angle detection of 90°.
The morphology and dried TEM-assessed size measurement of the CTS-Pasp-5Fu nanoparticles were examined under transmission electron microscope (TEM; Hitachi, H-600). The sample was dried at room temperature and examined using a TEM without staining.
The encapsulation efficiency and loading capacity of nanoparticles were determined by separation of nanoparticles from the aqueous medium containing non-associated 5-Fu by ultracentrifugation at 35 000 r/min for 30 min at 14°C. The amount of free 5-Fu in the supernatant was measured by HPLC. HPLC was performed using a LC-4A HPLC system equipped with a LC-4A pump, and a SPD-10A UV detector (Shimadzu, Kyoto, Japan). The detective wavelength was set at 270 nm. HPLC analysis of samples was performed using a Science C18 column (4.6 × 250 nm, 5 &mgr;m, Japan) preceded by a C18 guard column(GL Science, Japan).The column temperature was maintained at 30°C. The mobile phase was a mixture of methanol/3.6% acetic acid. The flow rate was 1.0 mL/min. The 5-Fu encapsulation efficiency (EE) and the 5-Fu loading capacity (LC) of the nanoparticles were calculated as follows:
EE = (The amount of 5-Fu in the nanoparticles/Total amount of 5-Fu) × 100%
LC = (The amount of 5-Fu in the nanoparticles/Total amount of nanoparticles weight) × 100%
All measurements were performed in triplicate.
In vitro 5-Fu release profiles of chitosan-Pasp-5-Fu nanoparticles were determined as follows. The CTS-Pasp-5-Fu nanoparticles were separated from the aqueous suspension medium through ultra-centrifugation. CTS-Pasp-5-Fu nanoparticles and 5-Fu were re-dispersed in 4.0 mL of phosphate buffer saline (PBS), respectively, and placed into a dialysis membrane bag with a molecular weight cut-off of 10 kDa, tied and placed into 40.0 mL PBS medium. The entire system was kept at 37°C with continuous magnetic stirring. At 15 min, 1, 4, 8, 24, 48, 96, 144 or 192 h, 3 mL release medium was removed at each time point and 3 mL fresh medium PBS solution was added into the system. The amount of 5-Fu in the release medium was evaluated by HPLC. All measurements were performed in triplicate.
Kunming male mice (weight 20-25 g) were randomly divided into two groups. Each group was administrated with 5-Fu and CTS-Pasp nanoparticles. The plasma concentrations of 5-Fu were evaluated by HPLC after 15 min, 1, 2, 4, 6, 8, 12, 16, 24 and 48 h to compare their concentration curves[9].
Nude BALB/c male mice (aged 35-42 d and weighing 20-23 g) were inoculated subcutaneously near the nape with the transplanted human SGC-7901 gastric carcinoma cell line (1 × 107 cells per mouse). Two weeks later, the exuberantly proliferating tumor tissues were cut into 1.5 mm thick pieces and inoculated subcutaneously near nape of the 32 nude male BALB/c mice (aged 33-42 d and weighing 20-23 g) under aseptic conditions. The diameter of the tumor tissue transplanted into each nude mouse was measured with a slide caliper rule.
When the tumors grew to 100-300 nm3, the animals were randomly allocated into 4 groups with 8 mice in each group: CTS-Pasp-5Fu (containing 5-Fu 1.25 g/L), 5-Fu (1.25 g/L), chitosan-polyaspartic acid, and normal saline groups. Tumor weight was measured and the diameter measurement method was used to observe the dynamic changes in the antitumor response. Twenty-five mg/kg CTS-Pasp-5-Fu and 0.025 g/kg 5-Fu were given[19] each time whereas the control group was given an equal amount of normal saline or CTS-Pasp by gastric perfusion. The formula for calculating the tumor volume (TV) is: TV = 1/2 × a × b2, where a and b represent the length and width[20].
The inhibition rate of tumor growth (IR)[20] was calculated based on the results of the measurements. IR was calculated using the following formula: IR = (Tc-Tt)/Tc × 100%, where Tc represents control group and Tt represents treatment group.
The tumor was weighed when the experiment was over and the tumor inhibition rates (TIR) were calculated according to the following formula: TIR = (1-tumor weight of treatment group/tumor weight of control group) × 100%.
The white blood cell (WBC) count, total bilirubin (TB), alanine aminotransferase (ALT) and creatinine in the four groups were detected after 2 wk.
The nude mice were killed under aseptic conditions. The mice were sterilized thoroughly with 70% alcohol and the pelt was peeled off to fully expose the hip joint. Sterile sharp dissecting scissors were used to cut the knee joint and the femur near hip joint. The tissues were removed and bones were placed in DMEM culture solution. The ends of the bones were trimmed, a 1 mL sterile syringe needle was inserted into marrow shaft at the end of the femur and the marrow was flushed with Iscove’s MDM with 2% FBS in a sterile tube. Flushing was repeated and the number of nucleated cells were counted. The bone marrow cell suspension was done at a concentration of 2 × 105/mL and 0.3 mL of those cells was added into 3 mL MethoCultTM medium (prepared by Department of Gastroenterology, Zhongshan Hospital, Fudan University). The tubes were thoroughly mixed and let stand for 2-3 min to allow bubbles to rise to the top. MethoCultTM of 1.1 mL was dispensed into each 35 mm dish. Cultures were placed in a water-jacket at 37°C, 5% CO2, ≥ 95% humidity for 7 d. The growth of bone marrow cell colonies (≥ 30 nucleated cells constituted one colony) was observed[21].
The tumors, liver and nephridial tissues of the four groups were collected, fixed with 10% formalin, stained with hematoxylin and eosin, paraffin embedded and sectioned. The structural change of cells and tissues was observed. Four-grade histological denominator was used: +++, total necrosis; ++, necrosis areas exceed half of total areas; +, necrosis areas less than half of the total areas; -, no necrosis[22].
Tumor tissues from the four groups were paraffin embedded and shaken. The sections were deparaffinaged and douched with PBS 5 minutes after quenching the endogenous peroxidate with 3% hydrogen dioxide. They were repaired twice with citric acid fluid for 15 min, added with the first antisubstance (Bcl-2, 1:100; Bax, 1:100) and stayed overnight at 4°C. The second antisubstance with HRP was incubated for 30 min at 37°C and stained with a diaminobenzidine (DAB) developer. The sections were bleached with alcohol, cleared with methyl benzene, mounted with neutral balsam and photographed (ZEISS microscope, Germany). The ratio of positive expression area to the total image area was calculated.
The SPSS software package (SAS Institute Inc, North Carolina, USA) was used for analysis of variances. P < 0.05 was considered statistically different.
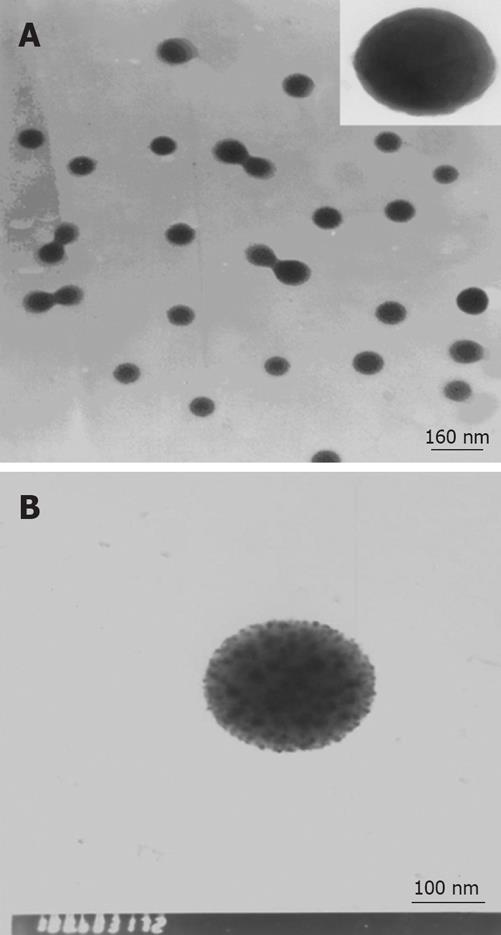
The mean hydrated size of particles and distribution of CTS-Pasp-5Fu nanoparticles were determined by DLS. The results indicated that the mean size and distribution of the samples were 206 nm and 0.14, respectively. The encapsulation efficiency (EE) of CTS-Pasp-5Fu was 40.2%, and the loading capacity was 34.9%. Figure 1 shows the morphological characteristics of CTS-Pasp-5Fu nanoparticles which were synthesized by ion gelatinization. The nanoparticles were spherical in shape and well distributed. The diameter of the particles ranged from 150 to 250 nm. 5-Fu was dispersed in nanoparticles by electrostatic interaction with particulate form.
Figure 2 shows the release profile of 5-Fu and CTS-Pasp-5-Fu. All the pure 5-Fu released rapidly from the dialysis bag within the first 1 h. CTS-Pasp-5Fu nanoparticles released 5-Fu gradually up to 100% in 100 h. Compared with the drug release of pure 5-Fu with 5-Fu loading CTS-Pasp nanoparticles, CTS-Pasp-5Fu nanoparticles might be used to provide a continuous release. We considered that 5-Fu embedded into the nanoparticles might be bound to PAsp by ionic reaction resulting in slow 5-Fu diffusion. 5-Fu released slowly and incompletely.
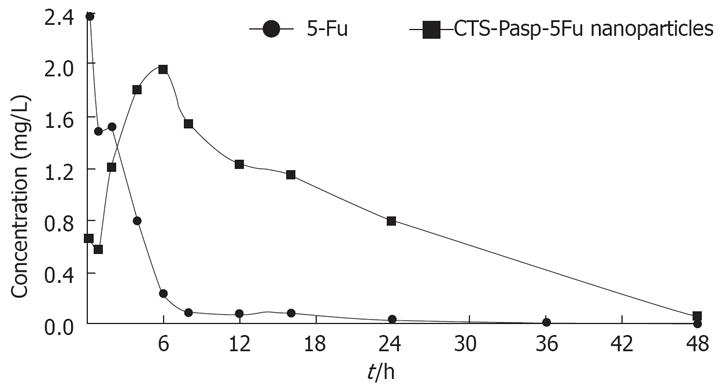
Figure 3 shows the concentration-time curves for the 5-Fu and CTS-Pasp-5Fu groups. The maximal concentration (Cmax) in the 5-Fu group occurred within 15 min and decreased rapidly, while that in the CTS-Pasp-5-Fu group occurred 6 h after the administration and the effective concentration lasted about 14 h. The plasma Cmax in the CTS-Pasp-5Fu group was lower than the 5-Fu group.
Before the treatment, the weight and tumor volume in four groups were not significantly different (P > 0.05). After the treatment, the mice in the 5-Fu group lost weight gradually (Table 1) and became hypokinetic and were in a poor general state. The other three groups were in a good general state. At the end of the experiment, 6 of 8 nude mice survived in the 5-Fu group and no mouse died in the other three groups. The inhibition rate of tumor growth (IR) for the 5-Fu and CTS-Pasp-5Fu groups was significantly higher than that for the NS group (58.69%, P = 0.004; 70.82%, P = 0.00015; Table 2). The IR for the CTS-Pasp-5Fu group was also enhanced but was not significantly different from that for the 5-Fu group (P > 0.05, P = 0.206; Table 2). There was no difference in the IR between CTS-Pasp and NS groups (P > 0.05, P = 0.607). The tumor size in the NS group was bigger than that in the 5-Fu and CTS-Psap-5FU groups (all P < 0.001, Table 3), and the tumor weight of CTS-Pasp and NS groups was not different (P > 0.05). Tumor inhibition rate (TIR) for the CTS-Pasp-5Fu and 5FU groups was significantly higher than that for the NS group (72.79% and 65.3%; Table 3). TIR for the CTS-Pasp-5Fu group was higher than that for the 5-Fu group, but the difference was not significant (Table 3, Figure 4).
| Groups | Before treatment | After treatment (d) | |||
| 3 | 7 | 10 | 14 | ||
| NS | 20.96 ± 0.82 | 22.35 ± 1.17 | 23.41 ± 1.53 | 24.28 ± 1.72 | 24.11 ± 1.73 |
| 5-Fu | 22.16 ± 2.83 | 22.20 ± 2.86 | 22.58 ± 2.80 | 20.86 ± 3.16 | 18.34 ± 3.69 |
| CTS-Pasp | 20.80 ± 1.19 | 22.96 ± 1.34 | 24.45 ± 1.26 | 24.14 ± 1.07 | 24.52 ± 1.75 |
| CTS-Pasp-5Fu | 22.01 ± 2.78 | 23.31 ± 2.39 | 25.15 ± 2.15 | 25.46 ± 2.08 | 25.53 ± 1.80 |
| Groups | Before treatment | After treatment | ||||||
| 3 d | 7 d | IR | 10 d | IR | 14 d | IR | ||
| NS | 0.11 ± 0.03 | 0.19 ± 0.05 | 0.33 ± 0.10 | 0.62 ± 0.28 | 0.80 ± 0.23 | |||
| 5-Fu | 0.12 ± 0.03 | 0.12 ± 0.04 | 0.24 ± 0.11 | 27.76% | 0.31 ± 0.19 | 50.27% | 0.33 ± 0.20 | 58.69% |
| CTS-Pasp | 0.12 ± 0.03 | 0.19 ± 0.04 | 0.32 ± 0.10 | 2.80% | 0.54 ± 0.10 | 13.49% | 0.76 ± 0.26 | 5.58% |
| CTS-Pasp-5Fu | 0.10 ± 0.01 | 0.11 ±0.04 | 0.13 ± 0.05 | 60.76% | 0.19 ± 0.05 | 68.99% | 0.23 ± 0.07 | 70.82% |
| Groups | Mean tumor weight (g) | TIR (%) |
| NS | 1.47 ± 0.18 | - |
| 5-Fu group | 0.51 ± 0.11 | 65.30 |
| CTS-Pasp group | 1.40 ± 0.13 | 5.09 |
| CTS-Pasp-5Fu group | 0.40 ± 0.13 | 72.79 |
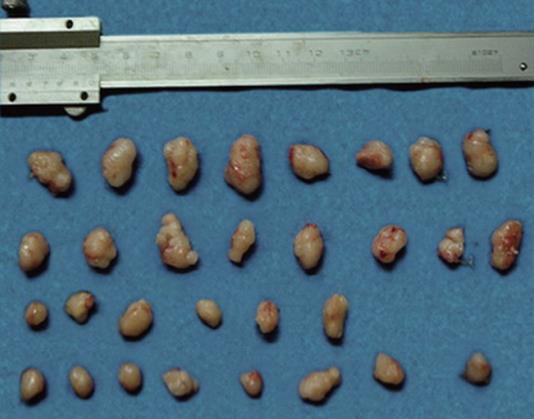
Figure 4 shows all the tumors of the four groups at the end of the experiment. There were only 6 tumors left in the 5-Fu group. The tumors of those of NS and CTS-Pasp groups were obviously bigger than the other two groups. The size of tumors in the 5-Fu and CTS-Pasp-5Fu groups was not significantly different, but TIR for the CTS-Pasp-5Fu group was higher than that for the 5-Fu group (72.79% vs 65.3%, Table 3).
The white blood cell count, total bilirubin and alanine aminotransferase (ALT) in the NS, CTS-Pasp and CTS-Pasp-5Fu groups were not significantly different (P > 0.05). Those in the 5-Fu group were higher than those in the other three groups (Table 4). There was no difference in creatinine among the four groups (P > 0.05).
| Groups | WBC(109/L) | TB(&mgr;mol/L) | ALT(U/L) | Creatinine (&mgr;mol/L) | CFU-GM (unit/plate) |
| NS | 1.48 ± 0.84 | 1.24 ± 0.19 | 67.44 ± 19.33 | 27.83 ± 6.95 | 247 ± 18.06 |
| 5-Fu | 1.42 ± 0.50 | 15.08 ± 4.32 | 145.67 ± 42.52 | 22.98 ± 8.33 | 120 ± 8.25 |
| CTS-Pasp | 1.50 ± 0.46 | 1.29 ± 0.67 | 65.43 ± 5.95 | 24.37 ± 4.89 | 241 ± 6.26 |
| CTS-Pasp-5Fu | 1.54 ± 0.23 | 1.26 ± 0.49 | 58.84 ± 4.36 | 22.18 ± 6.18 | 239.6 ± 7.40 |
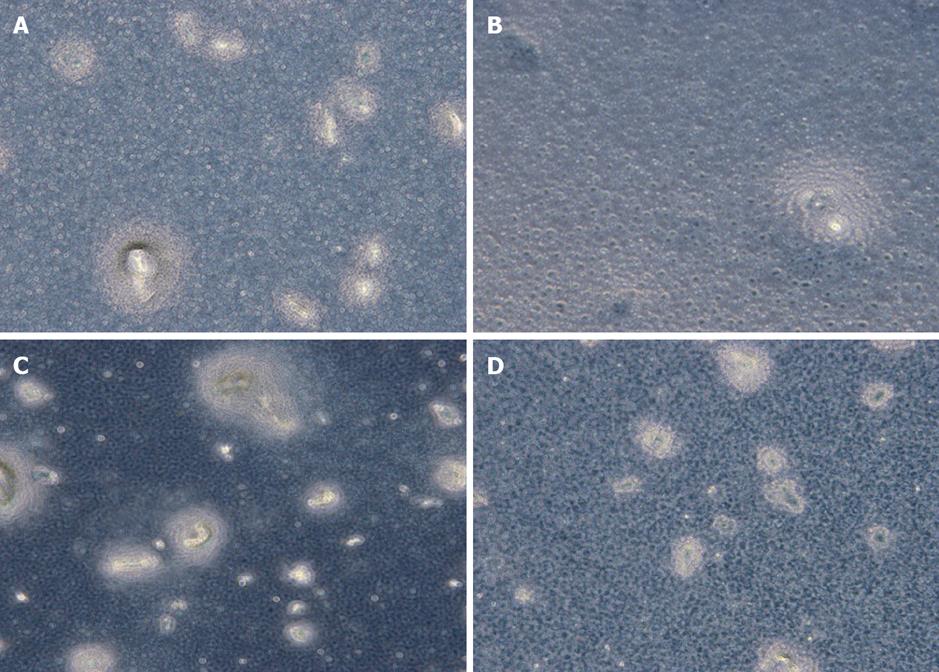
The bone marrow inhibition effect in the 5-Fu group was significantly different from that in the other three groups (P < 0.001, Table 4, Figure 5).
Nucleated cells ≥ 30 constituted one bone marrow cell colony[16] in the four groups (Figure 5). The growth of bone marrow cell colonies in the NS, CTS-Pasp and CTS-Pasp-5Fu groups was vigorous, while that in the 5-Fu group was sparse. CTS-Pasp-5Fu did not suppress the growth of bone marrow cells.
Table 5 shows the histological change in the four groups after treatment. The tumor cells in the NS and CTS-Pasp groups aligned regularly with slight inflammatory cell infiltration. The tumor cells in the CTS-Pasp-5Fu group were swollen with degeneration, necrosis and inflammatory cell infiltration which was less severe than in 5FU group. The damage liver in the 5-Fu group was more severe than that in the CTS-Pasp-5Fu group.
| Groups | Tumor number | Score of histological tumor necrosis | |||
| - | + | ++ | +++ | ||
| NS | 8 | 3 | 5 | 0 | 0 |
| CTS-Pasp | 8 | 2 | 6 | 0 | 0 |
| 5-Fu | 6 | 1 | 4 | 1 | 0 |
| CTS-Pasp-5Fu | 8 | 0 | 4 | 3 | 1 |
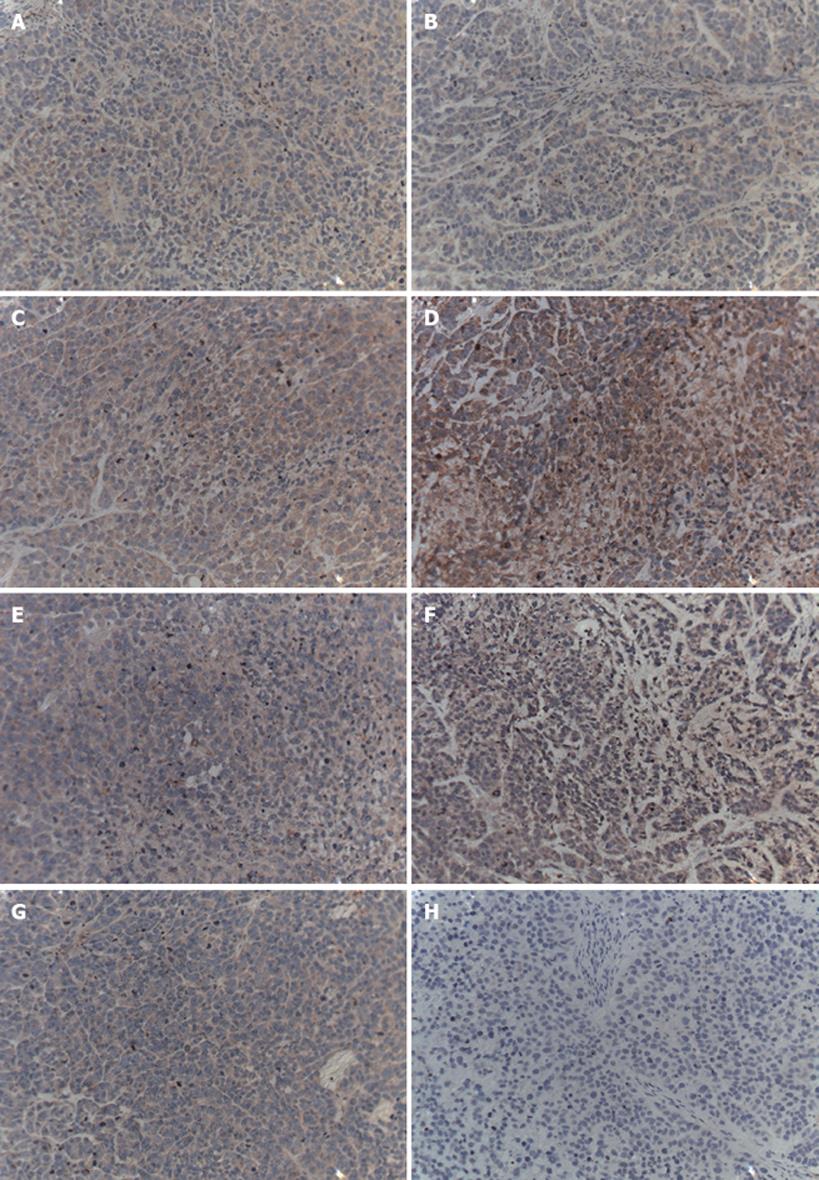
5-Fu and CTS-Pasp-5Fu down-regulate the Bcl-2 expression and up-regulation of the Bax expression were observed in the 5-Fu and CTS-Pasp-5Fu groups, but not in the CTS-Pasp group and NS group. The accommodate effect of CTS-Pasp-5Fu was more obvious than that of 5-Fu (Table 6, Figures 5 and 6).
Figure 6 shows the expression of the apoptotic proteins Bcl-2 and Bax in the four groups. The masculine expression of Bax in the CTS-Pasp-5Fu was more obvious than that in the 5-Fu group. CTS-Pasp-5Fu and 5Fu could up-regulate Bax expression. The masculine expression of Bcl-2 in the CTS-Pasp-5Fu group was obviously lower than that in the other three groups. The difference in the expression of Bax and Bcl-2 between NS group and CTS-Pasp group was not significant (Figure 6).
5-Fu is a thymidylic acid synthetase(TS)inhibitor that undermines the stability of DNA molecular structure[23], thus it is among the top priority of consideration in chemotherapy for gastrointestinal tumors. 5-Fu is also disadvantageous in terms of irregular absorption, treatment dose, toxicity and severe side effects with high doses which result in complications by long-term intravenous administration. Consequently, the research and development of highly effective oral flurouracil preparation or flurouracil analogs with mild side effects has been a major topic for many medical scientists.
Biologically degradable drug-loaded nanoparticles are a novel carrier for targeted drug delivery. Its advantages include greater magnitude of drug load, controlled release, longer biological half-life, less administration time, and fewer side effects. Nanoparticles have all the advantages of liposomes including the size property. Some investigators have also observed that the number of nanoparticles which cross the intestinal epithelium is greater than that of the microspheres (> 1 &mgr;m)[24]. Recently, polymer nanoparticles have been widely studied as a carrier for drug delivery. They are expected to be adsorbed in an intact form in the gastrointestinal tract after oral administration[25]. Chitosan (CTS), a poly [(1→4)-β-linked 2-amino-2-deoxy-D-glucose], is prepared from chitin by N-deacetylation. It is a biologically compatible and degradable material with almost no side effects, and is receiving worldwide interest for its industrial uses as antimicrobials, biomedical materials, cosmetics, food additives, separators, sewage disposal, agricultural materials, etc. Nanoparticles prepared with CTS are characterized by delayed release, controlled release and targeted delivery with a higher bioavailability and fewer side effects. We have developed nanocarriers made of CS which have shown a great capacity in drug controlled release[2627].
Polyaspartic acid is another favorable drug carrier[28]. Polyaspartic acid or its salts are a kind of newly innocuous, biodegradable bio-organic polymer. It has been widely applied in many areas such as agriculture, medicine, commodity, water treatment, petroleum, etc[2930]. The synthesis and application of polyaspartic acid have been studied in many companies over the past years. The medical realm of polyaspartic acid has gradually caught more attention of people. Our previous studies showed that CTS-Pasp-5Fu nanoparticles prepared by ionic gelation in vitro and in vivo can delay release of 5-Fu with longer effective concentration time, and that it may render solution to the 5-Fu problems of a short half-life and more side effects brought by a higher serum concentration soon after administration.
We observed in our experiment that chitosan-polyaspartic acid-5fluorouracil (CTS-Pasp-5Fu) nanoparticles could slowly release 5-Fu compared to 5-Fu. The Cmax of CTS-Pasp-5Fu nanoparticles was decreased, and the AUC was increased. The CTS-Pasp-5Fu nanoparticles were controllablly released.
It is observed in the transplant nude mouse model of human gastric cancer that the inhibition rate of tumor growth (IR) and tumor inhibition rate (TIR) for the CTS-Pasp-5Fu group were significantly higher than those for the 5-Fu group (70.82%, 58.69%, 72.79%, 65.3%), and oncopathology documented prominent degeneration and necrosis of tumor cells in the CTS-Pasp-5Fu group compare with the other three groups. These results demonstrated that the anti-tumor effects of CTS-Pasp-5Fu were better than those of 5-Fu at the same dose, and this preparation method enhanced the pharmacodynamics of the drugs.
Nude mice in the 5-Fu group seemed to be obviously emaciated, in which 2 died at the end of the study. The total serum bilirubin and ALT in the 5-Fu group were higher than those in the other three groups, and CFU-GM values were much lower. However, biochemical examination and CFU-GM values were not significantly different betweening CTS-Pasp-5Fu, CTS-Pasp and NS groups, indicat that single use of 5-Fu brings about liver toxicity and bone marrow suppression. Nevertheless,there was no significant difference in liver or kidney functions and CSF-GM values of the bone marrow between CTS-Pasp-5Fu and NS groups, suggesting that chitosan-Pasp nanoparticles coated with 5-Fu bring about less bone marrow suppressing effect, and can be used as a safe and effective drug carrier.
The Bcl-2 gene family is an important regulatory factor group for apoptosis, including Bcl-2, Bal-xl, etc. The ratio of Bcl-2/Bax is an important index affecting apoptosis[31]. Bcl-2 inhibits cell apoptosis by inhibiting the release of cytochrome c while bax promotes cell apoptosis by promoting the release of cytochrome c and activates the key enzyme caspase in the process of apoptosis. Activated caspase causes tumor apoptosis by its effect on substrate[32]. The previous investigation in vitro showed that 5-Fu can up-regulate bax protein expression in some tumor cells[33]. Our immunochemical detection of the apoptosis-related genes among four tumor groups showed that 5-Fu and CTS-Pasp-5Fu could down-regulate the bcl-2 expression, and up-regulate bax expression. Since the regulation effect of CTS-Pasp-5Fu nanoparticles is more powerful, it can enhance the inhibitory effect compared with 5-Fu alone, and induce the apoptosis of the gastric carcinoma.
In conclusion, in this study we successfully synthesized CTS-PASP nanoparticles coated with 5-Fu, which demonstrated significant anti-tumor effects in the transplanted nude mouse model of human gastric cancer, with less bone marrow suppression. Therefore, it may be used as a safe and effective novel anti-tumor preparation with profound prospects in clinical application.
5-Fluorouracil (5-Fu) is often used as an anti-tumor agent in the treatment of gastrointestinal cancer. However, it often causes inconvenience of patients because of its short plasma half-life and requires continuous infusion (CI) schedules. So it is important to investigate and develop new drugs which can reduce the side efforts of 5-Fu.
Chitosan (CTS) has received more attention recently in the pharmaceutical field for a wide range of drug-delivery applications. Polyaspartic acid is a kind of a new biodegradable, innocuous and friendly environmental bio-organic polymer, recognized as a green material, and widely used in the areas such as agriculture, medicine, commodity, water treatment, etc.
In this study, the authors successfully synthesized chitosan-polyaspartic acid-5 fluorouracil (CTS-Pasp-5Fu) nanoparticles and investigated their anti-carcinoma effect and toxicity to overcome the disadvantage of 5-Fu in gastric carcinoma mouse model. The results show that the tumor inhibition rate of CTS-Pasp-5Fu nanoparticles was much higher than that of 5-Fu alone, and the inhibition on bone marrow was alleviated efficiently. Thus, it may be used as a safe and effective novel anti-tumor preparation with profound prospects in clinical application.
Based on the results of this study, it is supposed that CTS-Pasp-5Fu nano-particles may substitute 5-Fu in the future treatment of gastrointestinal cancer.
This study focused on the preparation of CTS-Pasp-5Fu nanoparticles and investigation of its anti-carcinoma effect and toxicity to overcome the disadvantage of 5-Fu in gastric carcinoma mouse model. The data demonstrate that CTS-Pasp-5Fu appears to offer comparable efficacy while providing a more convenient schedule and showed insight into the novel oral fluoropyrimidine.
| 1. | Grem JL. 5-Fluorouracil: forty-plus and still ticking. A review of its preclinical and clinical development. Invest New Drugs. 2000;18:299-313. |
| 2. | Llorca Ferrandiz C, Esquerdo Galiana G, Cervera Grau JM, Briceo Garcia HC, Calduch Broseta JV, Del Pino Cuadrado J. [5-Fluorouracil-induced small bowel toxicity in a patient with colorectal cancer]. Clin Transl Oncol. 2005;7:356-357. |
| 3. | Saif MW. Capecitabine versus continuous-infusion 5-fluorouracil for colorectal cancer: a retrospective efficacy and safety comparison. Clin Colorectal Cancer. 2005;5:89-100. |
| 4. | Diasio RB, Johnson MR. The role of pharmacogenetics and pharmacogenomics in cancer chemotherapy with 5-fluorouracil. Pharmacology. 2000;61:199-203. |
| 5. | Ogawa K, Yui T, Okuyama K. Three D structures of chitosan. Int J Biol Macromol. 2004;34:1-8. |
| 6. | Prabaharan M, Mano JF. Chitosan-based particles as controlled drug delivery systems. Drug Deliv. 2005;12:41-57. |
| 7. | Aspden TJ, Mason JDT, Jones NS. Chitosan as a nasal delivery system: the effect of chitosan solutions on in vitro and in vivo mucociliary transport rates in human turbinates and volunteers. J Pharm Sci. 1997;86:509-513. |
| 8. | Chornet E, Dumitriu S. Inclusion and release of proteins from polysaccharide-based polyion complexes. Adv Drug Deliv Rev. 1998;31:223-246. |
| 9. | Takeuchi H, Yamamoto H, Niwa T, Hino T, Kawashima Y. Enteral absorption of insulin in rats from mucoadhesive chitosan-coated liposomes. Pharm Res. 1996;13:896-901. |
| 10. | Park SB, You JO, Park HY, Haam SJ, Kim WS. A novel pH-sensitive membrane from chitosan--TEOS IPN; preparation and its drug permeation characteristics. Biomaterials. 2001;22:323-330. |
| 11. | Mi FL, Wu YB, Shyu SS, Schoung JY, Huang YB, Tsai YH, Hao JY. Control of wound infections using a bilayer chitosan wound dressing with sustainable antibiotic delivery. J Biomed Mater Res. 2002;59:438-449. |
| 12. | Shahidi F, Abuzaytoun R. Chitin, chitosan, and co-products: chemistry, production, applications, and health effects. Adv Food Nutr Res. 2005;49:93-135. |
| 13. | Tang ES, Huang M, Lim LY. Ultrasonication of chitosan and chitosan nanoparticles. Int J Pharm. 2003;265:103-114. |
| 14. | Janes KA, Fresneau MP, Marazuela A, Fabra A, Alonso MJ. Chitosan nanoparticles as delivery systems for doxorubicin. J Control Release. 2001;73:255-267. |
| 15. | Wu LL, Zheng YL, Shen XZ, Fu SK, Dong L. Pharmacokinetics of Chitosan-Polyaspartic acid-5Fluorouracil Nanoparticles in vitro. Fudan Univ J Med Sci. 2006;33:757-760. |
| 16. | Nakato T, Kusuno A, Kakuchi T. Synthesis of poly (succinimide) by bulk polycondensation of L-aspartic acid with an acid catalyst. Polym Sci Pol Chem. 2000;38:117-122. |
| 17. | Calvo P, Remunan-Lopez C, Vila-Jato JL, Alonso MJ. Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers. Journal of Applied Polymer Science. 1997;63:125-132. |
| 18. | Chu B, Wang Z, Yu J. Dynamic light scattering study of internal motions of polymer coils in dilute solution. Macromolecules. 1991;24:6832–6838. |
| 19. | Moehler M, Teufel A, Galle PR. New chemotherapeutic strategies in colorectal cancer. Recent Results Cancer Res. 2005;165:250-259. |
| 20. | Okuno S, Harada M, Yano T, Yano S, Kiuchi S, Tsuda N, Sakamura Y, Imai J, Kawaguchi T, Tsujihara K. Complete re-gression of xenografted human carcinomas by camptothecin ana-logue carboxymethyl dextran conjugate (T-0128). Cancer Research. 2000;60:2988-2995. |
| 21. | Kuwata T, Wang IM, Tamura T, Ponnamperuma RM, Levine R, Holmes KL, Morse HC, De Luca LM, Ozato K. Vitamin A deficiency in mice causes a systemic expansion of myeloid cells. Blood. 2000;95:3349-3356. |
| 22. | Jenkins WT, Evans SM, Koch CJ. Hypoxia and necrosis in rat 9L glioma and Morris 7777 hepatoma tumors: comparative measurements using EF5 binding and the Eppendorf needle electrode. Int J Radiat Oncol Biol Phys. 2000;46:1005-1017. |
| 23. | Chu E, Drake JC, Koeller DM, Zinn S, Jamis-Dow CA, Yeh GC, Allegra CJ. Induction of thymidylate synthase associated with multidrug resistance in human breast and colon cancer cell lines. Mol Pharmacol. 1991;39:136-143. |
| 24. | Desai MP, Labhasetwar V, Amidon GL, Levy RJ. Gastrointestinal uptake of biodegradable microparticles: effect of particle size. Pharm Res. 1996;13:1838-1845. |
| 25. | Jung T, Kamm W, Breitenbach A, Kaiserling E, Xiao JX, Kissel T. Biodegradable nanoparticles for oral delivery of peptides: is there a role for polymers to affect mucosal uptake? Eur J Pharm Biopharm. 2000;50:147-160. |
| 26. | Zheng Y, Wu Y, Yang W, Wang C, Fu S, Shen X. Preparation, characterization, and drug release in vitro of chitosan-glycyrrhetic acid nanoparticles. J Pharm Sci. 2006;95:181-191. |
| 27. | Wu Y, Yang WL, Wang CC, Hu JH, Fu SK. Chitosan nanoparticles as a novel delivery system for ammonium glycyrrhizinate. Int J Pharm. 2005;295:235-245. |
| 28. | Tauro JR, Gemeinhart RA. Development of amine-containing polymeric particles. J Biomater Sci Polym Ed. 2005;16:1233-1244. |
| 29. | Ehtezazi T, Govender T, Stolnik S. Hydrogen bonding and electrostatic interaction contributions to the interaction of a cationic drug with polyaspartic acid. Pharm Res. 2000;17:871-878. |
| 30. | Jiang HL, Zhu KJ. Comparison of poly(aspartic acid) hydrogel and poly(aspartic acid)/gelatin complex for entrapment and pH-sensitive release of protein drugs. J Appl Polymer Sci. 2006;9:2320-2329. |
| 31. | Mirjolet JF, Barberi-Heyob M, Didelot C, Peyrat JP, Abecassis J, Millon R, Merlin JL. Bcl-2/Bax protein ratio predicts 5-fluorouracil sensitivity independently of p53 status. Br J Cancer. 2000;83:1380-1386. |
| 32. | Sawa H, Kobayashi T, Mukai K, Zhang W, Shiku H. Bax overexpression enhances cytochrome c release from mitochondria and sensitizes KATOIII gastric cancer cells to chemotherapeutic agent-induced apoptosis. Int J Oncol. 2000;16:745-749. |
| 33. | Kawakami K, Tsukuda M, Mizuno H, Nishimura G, Ishii A, Hamajima K. Alteration of the Bcl-2/Bax status of head and neck cancer cell lines by chemotherapeutic agents. Anticancer Res. 1999;19:3927-3932. |