Published online Jun 14, 2008. doi: 10.3748/wjg.14.3504
Revised: May 9, 2008
Accepted: May 16, 2008
Published online: June 14, 2008
AIM: To compare the diagnostic accuracy of pelvic phased-array magnetic resonance imaging (MRI) and endorectal ultrasonography (ERUS) in the preoperative staging of rectal carcinoma.
METHODS: Thirty-four patients (15 males, 19 females) with ages ranging between 29 and 75 who have biopsy proven rectal tumor underwent both MRI and ERUS examinations before surgery. All patients were evaluated to determine the diagnostic accuracy of depth of transmural tumor invasion and lymph node metastases. Imaging results were correlated with histopathological findings regarded as the gold standard and both modalities were compared in terms of predicting preoperative local staging of rectal carcinoma.
RESULTS: The pathological T stage of the tumors was: pT1 in 1 patient, pT2 in 9 patients, pT3 in 21 patients and pT4 in 3 patients. The pathological N stage of the tumors was: pN0 in 19 patients, pN1 in 9 patients and pN2 in 6 patients. The accuracy of T staging for MRI was 89.70% (27 out of 34). The sensitivity was 79.41% and the specificity was 93.14%. The accuracy of T staging for ERUS was 85.29% (24 out of 34). The sensitivity was 70.59% and the specificity was 90.20%. Detection of lymph node metastases using phased-array MRI gave an accuracy of 74.50% (21 out of 34). The sensitivity and specificity was found to be 61.76% and 80.88%, respectively. By using ERUS in the detection of lymph node metastases, an accuracy of 76.47% (18 out of 34) was obtained. The sensitivity and specificity were found to be 52.94% and 84.31%, respectively.
CONCLUSION: ERUS and phased-array MRI are complementary methods in the accurate preoperative staging of rectal cancer. In conclusion, we can state that phased-array MRI was observed to be slightly superior in determining the depth of transmural invasion (T stage) and has same value in detecting lymph node metastases (N stage) as compared to ERUS.
-
Citation: Halefoglu AM, Yildirim S, Avlanmis O, Sakiz D, Baykan A. Endorectal ultrasonography
versus phased-array magnetic resonance imaging for preoperative staging of rectal cancer. World J Gastroenterol 2008; 14(22): 3504-3510 - URL: https://www.wjgnet.com/1007-9327/full/v14/i22/3504.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.3504
In recent years, the treatment of rectal carcinoma has been improved by the introduction of new surgical techniques and neoadjuvant therapies. Surgery is still the method of choice for the treatment of rectal carcinoma. The depth of tumor infiltration into the rectal wall and involvement of the regional lymph nodes are the major factors in determining prognosis[12]. Therefore, assessment of the invasion depth (T stage) and lymph node involvement (N stage) are vital components of preoperative staging. The three main techniques currently used are computed tomography (CT), endorectal ultrasonography (ERUS), and magnetic resonance imaging (MRI) using various coils. Positron emission tomography (PET) is useful adjunct to systemic and regional staging, especially of a recurrent rectal cancer, but is rarely used as part of loco-regional staging of rectal cancer preoperatively[34]. The utility of ERUS and MRI for preoperative local staging of rectal carcinoma has been widely demonstrated[5–7]. In our study, we compared the ability of ERUS and pelvic phased-array MRI for preoperative local staging of rectal carcinoma. The imaging results obtained by both examinations were correlated with the histopathological gold standard evaluations of the surgical specimens.
Between June 2005-July 2007, 34 consecutive patients (15 male and 19 female), with a mean age of 58.7 (ranging from 29 to 75 years) who had biopsy proven rectal carcinoma were included in this study. All of the patients had underwent colonoscopic examination in which a biopsy procedure was also performed preoperatively. Following histopathological analysis of the endoscopic biopsy specimens, patients were diagnosed as having rectal adenocarcinoma. Patients who previously underwent chemotherapy or radiotherapy were excluded from the study. Regarding the location of the rectal tumors, the rectum is considered starting from the anal verge and extending to the rectosigmoid junction. Five cancers (14.71%) were in the upper third of the rectum, 17 cancers (50%) were in the middle third of the rectum and 12 cancers (35.29%) were in the lower third of the rectum.
Fifty patients (44.12%) underwent an abdomino-perineal resection and 19 patients (55.88%) a low-anterior resection. Following surgery, operative specimens were analysed by a pathologist (D.S.) who was unaware of ERUS and MRI results. The sections were evaluated microscopically in terms of determining the depth of transmural tumor invasion and lymph node metastases according to TNM criteria[8].
ERUS examinations were performed by an experienced colorectal endoscopist (A.B.) on this area using a B-K Falcon 2101 ultrasound machine with a 7 and 10 MHz rotating superficial endoprobes. All patients were given enema the day before the examination. Informed consents were obtained from all of the patients prior to the examination.
Endorectal ultrasound was carried out with the patients in the left lateral decubitus position without needing any sedation. The tip of the transducer was covered with a latex balloon filled with degassed water.
The bowel wall is represented in five sonographic layers as a result of differences in acoustic impedance[9]. Beginning with the lumen, the five layers are: (1) hypere-choic layer from the interface between mucosa and ultrasound probe; (2) hypoechoic layer produced from the mucosa and muscularis mucosae; (3) hyperechoic layer corresponding to the submucosa; (4) hypoechoic layer corresponding to the muscularis propria; and (5) hyperechoic layer being the interface between the muscularis propria and perirectal fat/serosa[910].
Ultrasonographic staging of tumor depth is denoted by the prefix “u”. The ultrasonographic staging corresponds to the TNM classification: (1) uT1, tumor confined to mucosa and submucosa; (2) uT2, tumor infiltrating muscularis propria; (3) uT3, tumor invading perirectal fat; and (4) uT4, tumor infiltrating surrounding organs[11].
The sonographic criteria for identifying involved lymph nodes consist of size greater than 5 mm, mixed signal intensity, irregular margins and spherical rather than ovoid or flat shape.
MRI examinations of the same patient group were performed by means of a 1.5 tesla superconducting magnet (GE, Signa, Milwaukee,Wisconsin, USA). All patients gave informed consent for the examination.
During the examination a pelvic coil was used which is a wrap-around surface coil around the pelvis. Patients did not undergo rectal air insufflation, nor did they receive bowel preparation or intravenous contrast. The patiens were placed in head - first supine position in the magnet. We did not perform T1 weighted images. The imaging protocol included T2 weighted images obtained by acquiring a non-breath hold FSE sequence by using the following parameters: TR: 3700 ms; TE: 105 ms; Echo train length: 16; Matrix size: 512 × 256; Section thickness: 4 mm; Field of view: 26 cm × 26 cm. T2 weighted images were obtained in axial, sagittal and coronal planes.
A single specialized radiologist (A.M.H.) who was blinded to the ERUS examination results evaluated these images. The layers as showed by MRI are defined as follows: (1) mucosa; thin, low-signal intensity line; (2) submucosa; thicker, higher-signal intensity; (3) muscularis propria; low signal; (4) perirectal fat; high signal layer; and (5) mesorectal fascia; fine, low-signal intensity layer enveloping the perirectal fat and rectum[10].
This fascia represents the plane of dissection during total mesorectal excision and the tumor proximity to within 1 mm of this fascia was taken as a marker of tumor involvement of the circumferential resection margin.
T2 weighted images are more useful for evaluating the rectal wall layers and revealing involvement of other pelvic structures. On these images both the depth of transmural tumor invasion (T staging) and lymph node involvement (N staging) were assessed.
The depth of tumor invasion (T stage) and lymph node involvement (N stage) were classified according to the TNM classification[8]. In this staging system: (1) T1 tumors are confined to mucosa and submucosa; (2) T2 tumors invade muscularis propria; (3) T3 tumors extend to mesorectal fat; (4) T4 tumors show adjacent organ invasion. N0: No nodal involvement; N1: One to three regional nodes positive for tumor; N2: Four or more regional nodes positive for tumor.
Spiculation from the tumor margin was considered to indicate malignant tumoral infiltration and, therefore, as in the Maastricht study[12], spiculated lesions (i.e. showing perirectal strandings) were classified as T3 disease.
Any discrete hypointense lesion detected in the mesorectal fat was interpreted as a lymph node. Lymph nodes of 5 mm diameter or greater were reported as nodal metastases while those lesser than 5 mm diameter were considered to be uninvolved[13].
The overall accuracy, sensitivity, specificity, positive predictive value and negative predictive value were calculated for ERUS and MRI to predict transmural tumor invasion and lymph node involvement using the histopathological findings as the gold standard.
All tumors could be detected by both ERUS and MRI. The histopathological evaluation of resected tumors revealed adenocarcinoma for all of the patients. Mean histological tumor size was 3.7 cm (range 1.5-6.8 cm). The pathological T stage of these adenocarcinomas was: pT1 in 1 patient, pT2 in 9 patients, pT3 in 21 patients and pT4 in 3 patients. The pathological N staging of these tumors was: pN0 in 19 patients, pN1 in 9 patients and pN2 in 6 patients.
Comparison of T staging results obtained with phased-array MRI and ERUS with the pathology is summarized in Tables 1 and 2.
| p-T1 | p-T2 | p-T3 | p-T4 | |
| MR-T1 | 1 | 0 | 0 | 0 |
| MR-T2 | 0 | 5 | 1 | 0 |
| MR-T3 | 0 | 4 | 18 | 0 |
| MR-T4 | 0 | 0 | 2 | 3 |
| No | 1 | 9 | 21 | 3 |
| p-T1 | p-T2 | p-T3 | p-T4 | |
| ERUS-T1 | 0 | 0 | 0 | 0 |
| ERUS-T2 | 1 | 4 | 3 | 0 |
| ERUS-T3 | 0 | 5 | 18 | 1 |
| ERUS-T4 | 0 | 0 | 0 | 2 |
| No | 1 | 9 | 21 | 3 |
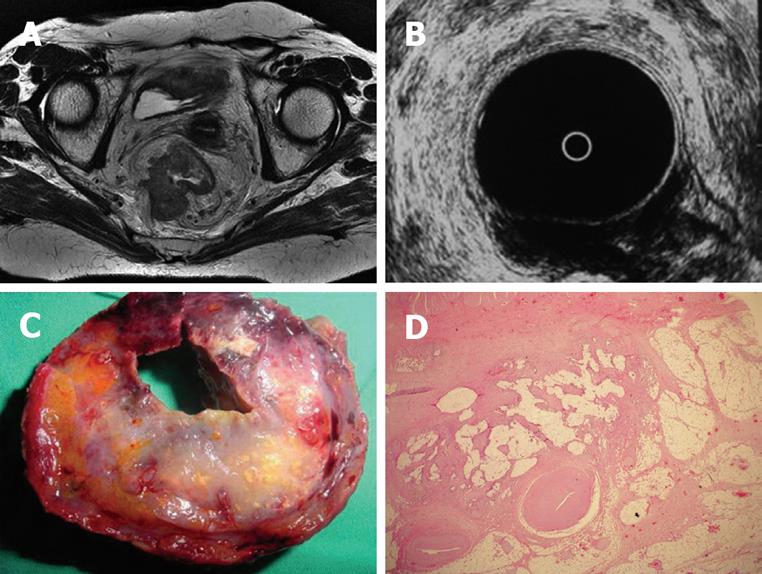
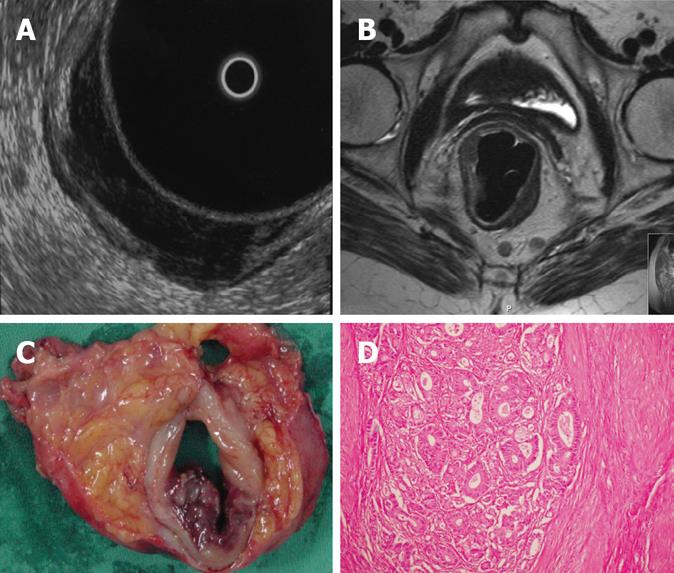
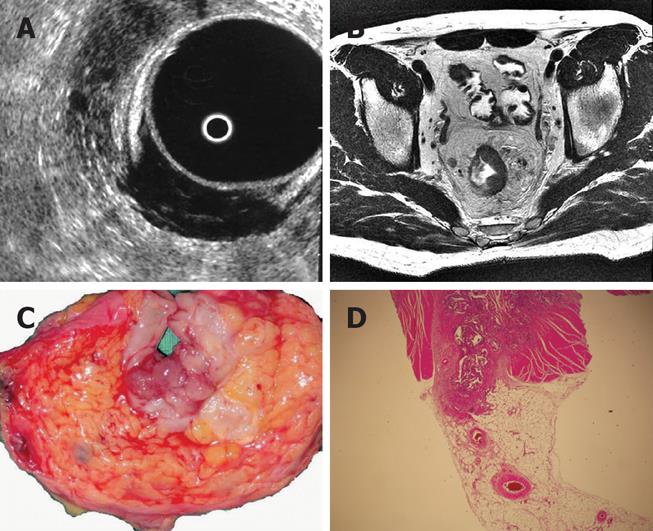
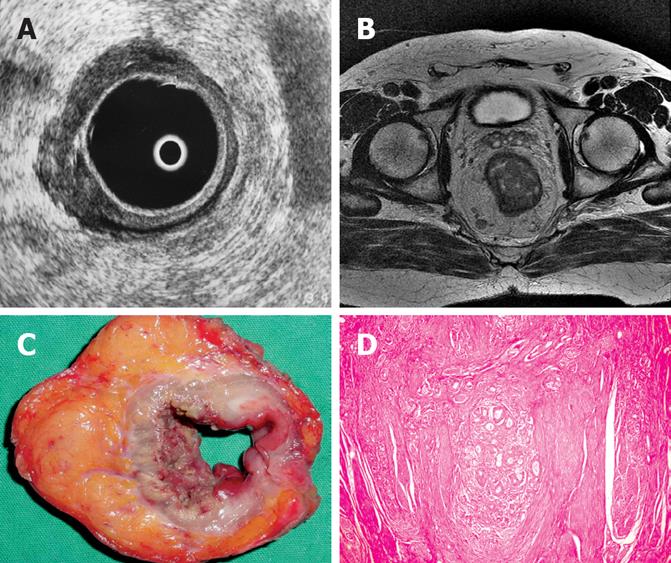
Regarding MRI, one patient with pT3 tumor was understaged as T2 tumor. In 4 patients with pT2 tumors, MRI overestimated as T3 tumors and in 2 patients with pT3 tumors, MRI overestimated as T4 (Figure 1A, C and D). In the remaining cases, with a pT1 tumor in 1 patient, pT2 tumor in 5 patients (Figure 2B, C and D), pT3 tumor in 18 patients (Figure 3B, C and D) and pT4 tumor in 3 patients, MRI correctly assessed the stage of transmural tumor invasion. The accuracy of T staging was 89.70% (27 out of 34). The sensitivity was 79.41% and the specificity was 93.14%. MRI correctly predicted invasion in 23 patients and no invasion in 6 patients, thus the overall accuracy in terms of discriminating between pT1-pT2 and pT3-pT4 tumors was found to be 85.29% with a 95.8% sensitivity and 60% specificity. The positive and negative predictive values were calculated as 85.19% and 85.7%, respectively. With ERUS, 4 patients were underestimated. In 3 patients with pT3 tumors, ERUS staged as T2 and in 1 patient with pT4 tumor, ERUS staged as T3. In 6 patients, overestimation did occur where 5 pT2 tumors were overestimated as T3 (Figure 4A, C and D) and 1 pT1 tumor was overesti-mated as T2.
In the remaining 4 cases with pT2 (Figure 2A, C and D), 18 with pT3 (Figure 3A, C and D) and 2 with pT4 tumors, ERUS correctly predicted the T staging.
The accuracy of T staging was 85.29% (24 out of 34). The sensitivity was 70.59% and the specificity was 90.20%. ERUS was correctly predicted invasion in 21 patients and no invasion in 5 patients, thus the overall accuracy of ERUS in terms of discriminating between pT1-pT2 and pT3-pT4 tumors was found to be 76.47% with a 87.5% sensitivity and 50% specificity. The positive and negative predictive values were calculated as 80.77% and 62.50%, respectively.
Comparison of N staging results obtained with phased-array MRI and ERUS with the pathology is summarized in Table 3. Detection of lymph node metastases using phased-array MRI gave an accuracy of 74.50% (21 out of 34). The sensitivity and specificity were found to be 61.6% and 80.88%, respectively. By using ERUS in the detection of lymph node metastases, an accuracy of 76.47% (18 out of 34) was obtained. The sensitivity and specificity were found to be 52.94% and 84.31%, respectively. Overstaging and understaging of phased-array MRI and ERUS in terms of predicting T and N stage are summarized in Table 4.
| Pathology | |||
| N0 | N1 | N2 | |
| Phased-array MRI | |||
| N0 | 8 | 1 | 1 |
| N1 | 11 | 8 | 0 |
| N2 | 0 | 0 | 5 |
| ERUS | |||
| N0 | 7 | 2 | 2 |
| N1 | 12 | 7 | 0 |
| N2 | 0 | 0 | 4 |
| T staged | N staged | |||
| Overstaged | Understaged | Overstaged | Understaged | |
| MRI | 6 | 1 | 11 | 2 |
| ERUS | 6 | 4 | 12 | 4 |
Treatment options for rectal cancer depend on the stage at presentation[1]. Since staging of rectal cancer with digital rectal examination is unreliable, preoperative staging is mostly based on imaging[14]. Accurate staging is particularly important because stage 1 tumors are best treated with surgery alone, whereas stage 2 and 3 tumors require preoperative chemoradiotherapy[15].
CT, ERUS and MRI are the imaging modalities predominantly utilized in the preoperative staging of rectal cancer. CT is unable to differentiate the different layers of the rectal wall and has lower overall predictive accuracy than ERUS and MRI. Initally, preoperative local staging of rectal carcinoma using body coil MRI was only 60% accurate in predicting the transmural tumor invasion[16].
This poor result can be attributed to the use of body coil which suffers from low spatial resolution. But in the recent years, the advent of endorectal MRI has made it possible to generate images with high signal-to-noise ratio (SNR) near the coil with better identification of the rectal wall. The reported accuracy ranging between 81% and 89%[1718] compares favorably with that of ERUS. However, endorectal MRI does have the same limitations as ERUS. Major pitfalls include poor resolution of pelvic structures surrounding the rectum due to the small field of view, and failure to insert the coil in patients with stenosing tumors.
MRI with a pelvic phased-array coil provides slightly lower resolution of the rectal wall compared to the endorectal coil, but allows the entire pelvis to be imaged, and thus, more distant spread can be assessed. In addition, this coil is noninvasive and useful for all rectal tumors regardless of site and size.
ERUS can distinguish the different anatomic layers of the bowel and thus, it appears to have advantages over both CT or MRI in assessing mural penetration and is invaluable in assessing patients considered for local resection. However, it is highly operator dependent, has poor patient acceptability, has limited depth penetration and can not be performed in stenotic tumors or tumors in the upper rectum[1920]. The assessment of the mesorectal fascia is also hampered by its limited field of view.
Kwok et al[21] concluded that ERUS was the most accurate technique for assessing wall penetration. However, in studies that compared MRI with an endorectal coil with ERUS, the former was found to be as effective as ERUS for assessing T stage and was more effective in assessing nodal involvement. They concluded that MRI using an endorectal coil was the most accurate technique for predicting the pathological stage of rectal cancer.
The meta-analysis of Bipat et al[22], also found that ERUS was the best technique for assessing local invasion, but stressed its limitations: operator depen-dency, no assessment of stenotic tumor, inability to visualize with a rigid probe, tumors located in the upper rectum, inability to detect lymph nodes outside the range of the transducer, and inability to visualize mesorectal fascia. The authors emphasized that none of the techniques were able to identify involved lymph nodes with satisfactory accuracy.
Overall accuracy in the ERUS assessment of tumor depth ranges from 63%-96% with an average of 81.8% in 2718 patients[91120]. In a review of cross-sectional studies investigating tumor depth in 873 patients, the overall accuracy was 85%, with sensitivity ranging from 84% in T1 to 76% in T4[23].
Overstaging of tumor depth frequently occurs as a result of perineoplastic inflammation as ultrasound can not clearly differentiate between inflammatory and neoplastic tissue[1124]. Similarly, preoperative biopsy causes hemorrhage and obliteration of sonographic layers[11].
Phased-array coils or pelvic coils have improved spatial resolution with improved signal-to-noise ratio[7], without the techniqual limitations of endorectal MRI[25]. They have the advantage of having a larger field of view of the mesorectal fascia. The role of circumferential resection margin as an important prognostic indicator of local recurrence is evident and several MRI studies have shown a high accuracy in this regard[122526]. Beets-Tan et al[12] used contrast-enhanced thin section MRI on a 1.5 tesla scanner with a quadrature phased-array spine coil and reported that the depth of transmural tumor invasion and mesorectal fascia involvement were predicted correctly in 83% and 100% of their patients, respectively. Although contrast enhancement may be helpful for differentiating reactive changes from true tumor invasion, they reported that MRI could not be used to distinguish reliably between fibrosis with and fibrosis without tumor cells.
On the other hand, Tatli et al[27] in their study using gadolinium-enhanced combined pelvic- phased array and endo-rectal coil MRI, surgical treatment groups (stage 1 vs stage 2/3) were accurately predicted in 33 out of 39 patients (85%). Overall, a 93% sensitivity, 86% specificity, and 88% accuracy were achieved in the identification of mesorectal fat invasion.
Brown et al[28] conducted a prospective study that found correct invasion depth assessment attained in 100% of their cases. Judging from the excellent results reported by this group who were able to differentiate between desmoplastic spiculation and true invasion, the best technique may be the one described by these authors and involves more precise image acquisition and administration of effective contrast material. Thus, thin section MRI performed on a 1.5 tesla scanner with a phased-array coil in general can be considered to provide moderate to good accuracy in the prediction of invasion depth and good accuracy in the prediction of mesorectal fascia involvement. These data are comparable to accuracy rates of 82%-88%[2329] obtained with ERUS for the prediction of invasion depth.
Although overall T stage accuracies by MRI are similar to that of ERUS, MRI has higher accuracies when assessing T3 and T4 tumors as compared to early T stages (T1 and T2)[2526]. When directly compared to ERUS for a T3 tumor, Blomqvist et al[30] had 11 false positives with ERUS compared to 8 for MRI. On the other hand, Akasu et al[31] in their series found that, two third of staging errors in invasion depth resulted from overstaging and were most common with pT2 tumors.
This data is consistent with our study in which most staging errors arised from overstaging with both modalities and these were mostly pT2 tumors (4 pT2 cases were overestimated by MRI and 5 pT2 cases were overestimated by ERUS).
Recent studies confirmed that ERUS can accurately stage the depth of tumor invasion particularly in T1 and T2 tumors[20223233], whereas MRI seems superior in more locally advanced disease[2526].
Although our patient population is too small to make a final statement, we found that phased-array MRI had slightly better accuracy (89.70%), sensitivity (79.41%) and specificity (93.14%) as compared to ERUS (85.29%, 70.59% and 90.20%, respectively) for detecting the depth of transmural tumor invasion.
Also we obtained better results by phased-array MRI in terms of predicting early T stages (6 out of 10) as compared to ERUS (4 out of 10).
The preoperative assessment of regional lymph node status forms part of the overall staging of any rectal tumor. The overall accuracy of assessing lymph node involvement ranges between 59% and 95%[17]. Nearly all published MR imaging studies of rectal cancer have used size as a criterion for predicting nodal involvement[1734], although there is no particular size cut-off that can be used to discriminate between benign and malignant lymph nodes.
Brown et al[35] confirmed that in mesorectal lymph nodes greater than 3 mm, morphological criteria such as an irregular border and mixed signal intensity is definitely a better predictor of lymph node status than size alone. More recent studies suggest that multiple criteria should be used to improve accuracy[18].
Regarding the detection of lymph node metastases, we obtained an accuracy of 74.50% with phased-array MRI and 76.47% with ERUS. Sensitivity was slightly better with MRI than ERUS (61.76% and 52.94% respectively).
ERUS and phased-array MRI are complementary methods for accurate preoperative staging of rectal cancer. Neither ERUS nor MRI can accurately stage tumoral invasion for all T stages. Nodal staging, although better defined with phased-array MRI, is limited for both of the methods[10].
In conclusion, in this study comparing those two modalities we can state that phased-array MRI is slightly superior in determining the depth of transmural tumor invasion (T stage) and has same value in detecting lymph node metastases (N stage) as compared to ERUS.
The preoperative staging of rectal cancer is very important in terms of planning appropriate therapy and determining prognosis. Therefore, the assessment of transmural tumor invasion depth and detection of lymph node metastases are of major importance for which purpose authors have investigated the accuracy of most recently used imaging techniques, namely endorectal ultrasonography (ERUS) and phased-array magnetic resonance imaging (MRI).
ERUS and MRI using various coils are currently used imaging modalities for the preoperative staging of rectal carcinoma.The utility of these techniques for staging of rectal carcinoma has been demonstrated well in the literature and both are regarded very useful. In this prospective study, authors’ aim was to determine the diagnostic accuracy of each technique in the same cohort of patient population and then to postsurgically compare the obtained results to find out which modality was more effective in the preoperative staging of rectal cancer.
The results suggested that phased-array MRI is slightly superior to ERUS in determining the transmural tumor invasion depth whereas both techniques seem to yield similar values in detecting lymph node metastases.
Based on this study, authors can state that ERUS and phased-array MRI can be used for the preoperative staging of rectal carcinoma and both techniques are accurate determinants of the T and N stages of tumors in most cases. They can also be applied as complementary methods for accurate preoperative staging of rectal cancer.
Pelvic phased-array MR technique provides a full evaluation of rectal wall layers with a large field of view compared with the standard MR techniques. It uses the pelvic phased-array coil which is a wrap-around surface coil around the pelvis. This coil has the advantages of the surface coil by obtaining higher signal but with greater coverage than a single surface coil and improved homogeneity resulting in higher spatial resolution images.
This is a very clear text, well presented and well written, the compared effectiveness of MRI and endoscopic ultrasound is compared in 34 patients having a colorectal cancer, with have a pathology control. The results are very clear and important.
| 1. | Lindmark G, Gerdin B, Pahlman L, Bergstrom R, Glimelius B. Prognostic predictors in colorectal cancer. Dis Colon Rectum. 1994;37:1219-1227. |
| 2. | Moriya Y, Sugihara K, Akasu T, Fujita S. Patterns of recurrence after nerve-sparing surgery for rectal adenocarcinoma with special reference to loco-regional recurrence. Dis Colon Rectum. 1995;38:1162-1168. |
| 3. | Heriot AG, Hicks RJ, Drummond EG, Keck J, Mackay J, Chen F, Kalff V. Does positron emission tomography change management in primary rectal cancer? A prospective assessment. Dis Colon Rectum. 2004;47:451-458. |
| 4. | Dobos N, Rubesin SE. Radiologic imaging modalities in the diagnosis and management of colorectal cancer. Hematol Oncol Clin North Am. 2002;16:875-895. |
| 5. | Ahmad NA, Kochman ML, Ginsberg GG. Endoscopic ultrasound and endoscopic mucosal resection for rectal cancers and villous adenomas. Hematol Oncol Clin North Am. 2002;16:897-906. |
| 6. | Brown G, Davies S, Williams GT, Bourne MW, Newcombe RG, Radcliffe AG, Blethyn J, Dallimore NS, Rees BI, Phillips CJ. Effectiveness of preoperative staging in rectal cancer: digital rectal examination, endoluminal ultrasound or magnetic resonance imaging? Br J Cancer. 2004;91:23-29. |
| 7. | Gagliardi G, Bayar S, Smith R, Salem RR. Preoperative staging of rectal cancer using magnetic resonance imaging with external phase-arrayed coils. Arch Surg. 2002;137:447-451. |
| 8. | Sobin LH, Wittekind C, editors . International union against cancer (UICC). TNM classification of malignant tumors. 6th ed. Baltimore, New York: Wiley-Liss 2002; 199-202. |
| 9. | Kumar A, Scholefield JH. Endosonography of the anal canal and rectum. World J Surg. 2000;24:208-215. |
| 10. | Bartram C, Brown G. Endorectal ultrasound and magnetic resonance imaging in rectal cancer staging. Gastroenterol Clin North Am. 2002;31:827-839. |
| 11. | Massari M, De Simone M, Cioffi U, Rosso L, Chiarelli M, Gabrielli F. Value and limits of endorectal ultrasonography for preoperative staging of rectal carcinoma. Surg Laparosc Endosc. 1998;8:438-444. |
| 12. | Beets-Tan RG, Beets GL, Vliegen RF, Kessels AG, Van Boven H, De Bruine A, von Meyenfeldt MF, Baeten CG, van Engelshoven JM. Accuracy of magnetic resonance imaging in prediction of tumour-free resection margin in rectal cancer surgery. Lancet. 2001;357:497-504. |
| 13. | McNicholas MM, Joyce WP, Dolan J, Gibney RG, MacErlaine DP, Hyland J. Magnetic resonance imaging of rectal carcinoma: a prospective study. Br J Surg. 1994;81:911-914. |
| 14. | Nicholls RJ, Mason AY, Morson BC, Dixon AK, Fry IK. The clinical staging of rectal cancer. Br J Surg. 1982;69:404-409. |
| 15. | Minsky BD. Adjuvant radiation therapy for rectal cancer: is there finally an answer? Lancet. 2001;358:1285-1286. |
| 16. | Hodgman CG, MacCarty RL, Wolff BG, May GR, Berquist TH, Sheedy PF 2nd, Beart RW Jr, Spencer RJ. Preoperative staging of rectal carcinoma by computed tomography and 0.15T magnetic resonance imaging. Preliminary report. Dis Colon Rectum. 1986;29:446-450. |
| 17. | Vogl TJ, Pegios W, Mack MG, Hunerbein M, Hintze R, Adler A, Lobbeck H, Hammerstingl R, Wust P, Schlag P. Accuracy of staging rectal tumors with contrast-enhanced transrectal MR imaging. AJR Am J Roentgenol. 1997;168:1427-1434. |
| 18. | Kim NK, Kim MJ, Yun SH, Sohn SK, Min JS. Comparative study of transrectal ultrasonography, pelvic computerized tomography, and magnetic resonance imaging in preoperative staging of rectal cancer. Dis Colon Rectum. 1999;42:770-775. |
| 19. | Adams DR, Blatchford GJ, Lin KM, Ternent CA, Thorson AG, Christensen MA. Use of preoperative ultrasound staging for treatment of rectal cancer. Dis Colon Rectum. 1999;42:159-166. |
| 20. | Garcia-Aguilar J, Pollack J, Lee SH, Hernandez de Anda E, Mellgren A, Wong WD, Finne CO, Rothenberger DA, Madoff RD. Accuracy of endorectal ultrasonography in preoperative staging of rectal tumors. Dis Colon Rectum. 2002;45:10-15. |
| 21. | Kwok H, Bissett IP, Hill GL. Preoperative staging of rectal cancer. Int J Colorectal Dis. 2000;15:9-20. |
| 22. | Bipat S, Glas AS, Slors FJ, Zwinderman AH, Bossuyt PM, Stoker J. Rectal cancer: local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging--a meta-analysis. Radiology. 2004;232:773-783. |
| 23. | Solomon MJ, McLeod RS. Endoluminal transrectal ultrasonography: accuracy, reliability, and validity. Dis Colon Rectum. 1993;36:200-205. |
| 24. | Starck M, Bohe M, Simanaitis M, Valentin L. Rectal endosonography can distinguish benign rectal lesions from invasive early rectal cancers. Colorectal Dis. 2003;5:246-250. |
| 25. | Beets-Tan RG. MRI in rectal cancer: the T stage and circumferential resection margin. Colorectal Dis. 2003;5:392-395. |
| 26. | Mathur P, Smith JJ, Ramsey C, Owen M, Thorpe A, Karim S, Burke C, Ramesh S, Dawson PM. Comparison of CT and MRI in the pre-operative staging of rectal adenocarcinoma and prediction of circumferential resection margin involvement by MRI. Colorectal Dis. 2003;5:396-401. |
| 27. | Tatli S, Mortele KJ, Breen EL, Bleday R, Silverman SG. Local staging of rectal cancer using combined pelvic phased-array and endorectal coil MRI. J Magn Reson Imaging. 2006;23:534-540. |
| 28. | Brown G, Richards CJ, Newcombe RG, Dallimore NS, Radcliffe AG, Carey DP, Bourne MW, Williams GT. Rectal carcinoma: thin-section MR imaging for staging in 28 patients. Radiology. 1999;211:215-222. |
| 29. | Akasu T, Sugihara K, Moriya Y, Fujita S. Limitations and pitfalls of transrectal ultrasonography for staging of rectal cancer. Dis Colon Rectum. 1997;40:S10-S15. |
| 30. | Blomqvist L, Machado M, Rubio C, Gabrielsson N, Granqvist S, Goldman S, Holm T. Rectal tumour staging: MR imaging using pelvic phased-array and endorectal coils vs endoscopic ultrasonography. Eur Radiol. 2000;10:653-660. |
| 31. | Akasu T, Iinuma G, Fujita T, Muramatsu Y, Tateishi U, Miyakawa K, Murakami T, Moriyama N. Thin-section MRI with a phased-array coil for preoperative evaluation of pelvic anatomy and tumor extent in patients with rectal cancer. AJR Am J Roentgenol. 2005;184:531-538. |
| 32. | Nesbakken A, Lovig T, Lunde OC, Nygaard K. Staging of rectal carcinoma with transrectal ultrasonography. Scand J Surg. 2003;92:125-129. |
| 33. | Marusch F, Koch A, Schmidt U, Zippel R, Kuhn R, Wolff S, Pross M, Wierth A, Gastinger I, Lippert H. Routine use of transrectal ultrasound in rectal carcinoma: results of a prospective multicenter study. Endoscopy. 2002;34:385-390. |