Published online May 28, 2008. doi: 10.3748/wjg.14.3269
Revised: April 4, 2008
Accepted: April 11, 2008
Published online: May 28, 2008
We described a patient with adenocarcinoma of the stomach combined with choriocarcinoma and neuroendocrine cell carcinoma. An 85-year-old man visited our hospital because of appetite loss. Gastric fiberscopy revealed a large tumor occupying the cardial region and anterior wall of the gastric body. The patient underwent total gastrectomy with lymphnode dissection and partial resection of the liver. Choriocarcinoma, small cell carcinoma and tubular adenocarcinoma existed in the gastric tumor. The choriocarcinomatous foci contained cells positive for beta-subunit of human chorionic gonadotropin (B-hCG) and human placental lactogen mainly in syncytiotrophoblastic cells. The small cell carcinomatous foci contained cells positive for synaptophysin, neuron-specific enolase (NSE), and chromogranin A. The prognosis for gastric adenocarcinoma with choriocarcinoma and neuroendocrine cell carcinoma is exceedingly poor. This patient died about 2 mo after the first complaint from hepatic failure. This is the first reported case of gastric cancer with these three pathological features.
- Citation: Hirano Y, Hara T, Nozawa H, Oyama K, Ohta N, Omura K, Watanabe G, Niwa H. Combined choriocarcinoma, neuroendocrine cell carcinoma and tubular adenocarcinoma in the stomach. World J Gastroenterol 2008; 14(20): 3269-3272
- URL: https://www.wjgnet.com/1007-9327/full/v14/i20/3269.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.3269
Primary carcinoma of the stomach is almost always adenocarcinoma or signet ring cell carcinoma and there have been few reports of choriocarcinoma[1–5] or neuroendocrine cell carcinoma[6–9]. We report a patient with adenocarcinoma of the stomach combined with choriocarcinoma and neuroendocrine cell carcinoma. This is the first reported case of gastric cancer with these three pathological features.
An 85-year-old man was admitted to Kouseiren Takaoka Hospital because of appetite loss in March 2004. He had been treated for hypertension and gout in another hospital. His family history was negative for family and hereditary disease. On examination, the patient was pale because of severe anemia, and had an ill-defined mobile left hypochondrial mass, approximately 10 cm in size. Findings for the chest and heart were normal. Lymphadenopathy, hepatomegaly, and splenomegaly were not observed and the testes and breasts were normal. Blood examination showed severe anemia, leukocytosis, and platelet count was increased. The level of serum carcinoembryonic antigen (CEA) was slightly elevated (Table 1). Radiographic examination of the upper gastrointestinal tract demonstrated a Borrmann type 1 tumor in the cardia. Gastric fiberscopy revealed a large tumor occupying the cardial region and anterior wall of the gastric body accompanied by areas of hemorrhage. Tumor invasion to the esophagus was highly suspected. Biopsy specimens were interpreted as showing adenocarcinoma without features of choriocarcinoma or neuroendocrine cell carcinoma. Contrast-enhanced computed tomography (CT) of the abdomen showed a 7-cm low-density tumor suspected to be regional lymph node metastasis. Liver and lung metastases and abnormality with his testes and breast were not detected radiologically. The patient underwent total gastrectomy on March 15, 2004, with the preoperative diagnosis of primary gastric carcinoma. Liver metastasis, peritoneal dissemination, and ascites were not investigated, and distant metastasis to other organs was not present. There was an invasive tumor encircling the gastric body and cardia and this tumor was invading the liver. Total gastrectomy with lymphnode dissection and partial resection of the liver were performed. The Roux-en-Y method of reconstruction was performed after resection.
| Data | |
| CBC | |
| WBC | 12 100/&mgr;L |
| RBC | 234 × 104/&mgr;L |
| Hb | 6.3 g/dL |
| Ht | 20.8% |
| Plts | 51.9 × 104/&mgr;L |
| Blood chemistry | |
| T-Bil | 0.4 mg/dL |
| D-Bil | 0.4 mg/dL |
| AST | 22 IU/L |
| ALT | 13 IU/L |
| LDH | 260 IU/L |
| ALP | 365 IU/L |
| ZTT | 10.9 K-U |
| TTT | 3.6 M-U |
| Ch-E | 61 IU/L |
| γ-GTP | 27 IU/L |
| T-AMY | 189 IU/L |
| CPK | 41 IU/L |
| Na | 135 mEq/L |
| K | 4.2 mEq/L |
| Cl | 102 mEq/L |
| Ca | 8.4 mg/dL |
| Fe | 16 &mgr;g/dL |
| BUN | 20.3 mg/dL |
| Cr | 1.3 mg/dL |
| UA | 4.2 mg/dL |
| Tch | 141 mg/dL |
| TG | 92 mg/dL |
| FBS | 123 mg/dL |
| TP | 6.2 g/dL |
| Alb | 3.1 g/dL |
| Tumor marker | |
| AFP | 7.5 ng/mL |
| CEA | 5.4 ng/mL |
| CA19-9 | < 2.0 ng/mL |
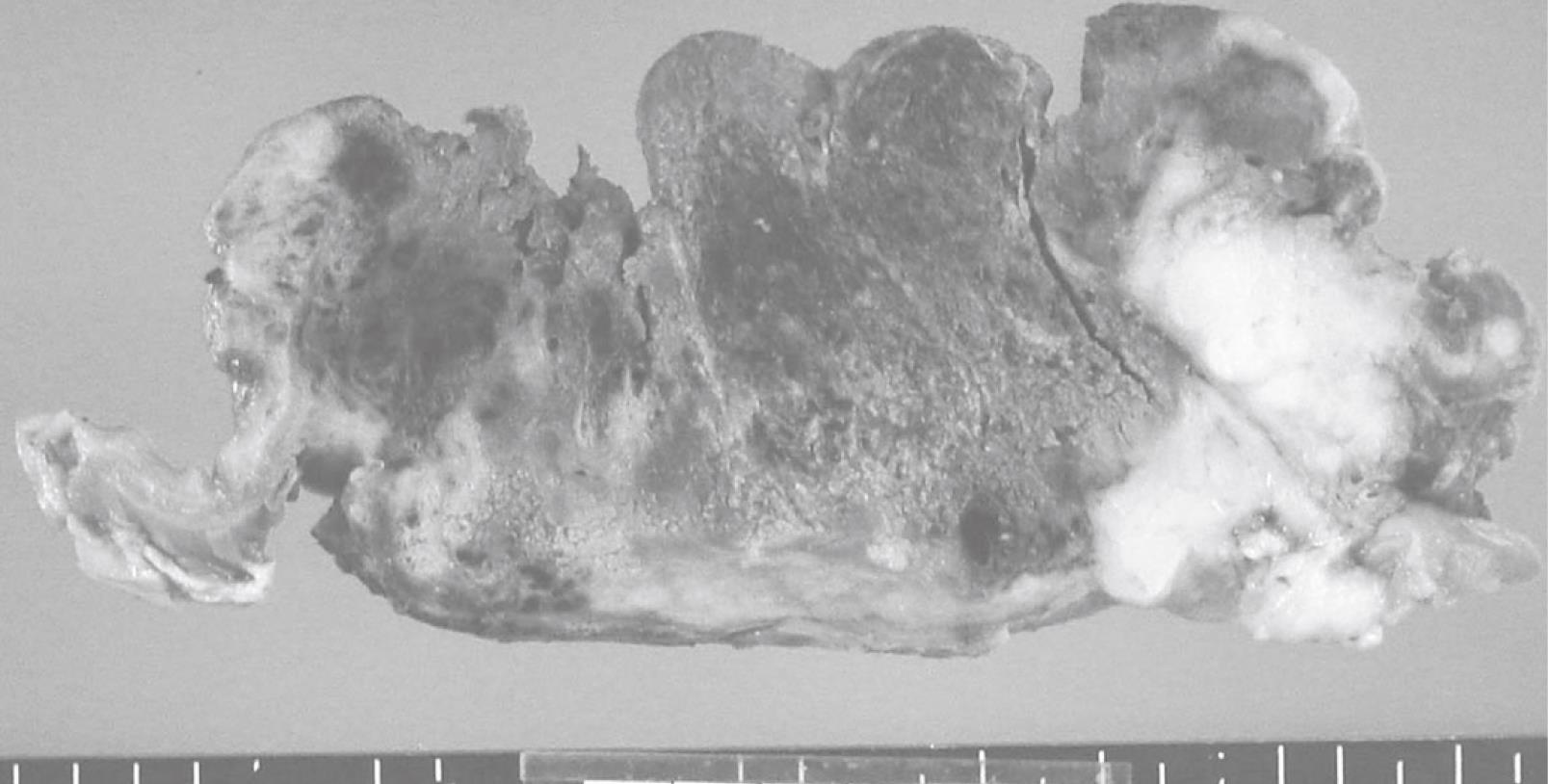
The resected specimen included an elevated tumor with ulcer measuring 12.0 cm × 11.5 cm in the cardia, the body of the stomach, and abdominal esophagus, and the tumor had invaded the liver. Cut surfaces of the tumor showed two different features, a hemorrhagic brown area and a whitish-yellow area. Most of the tumor was composed of the hemorrhagic brown area (Figure 1).
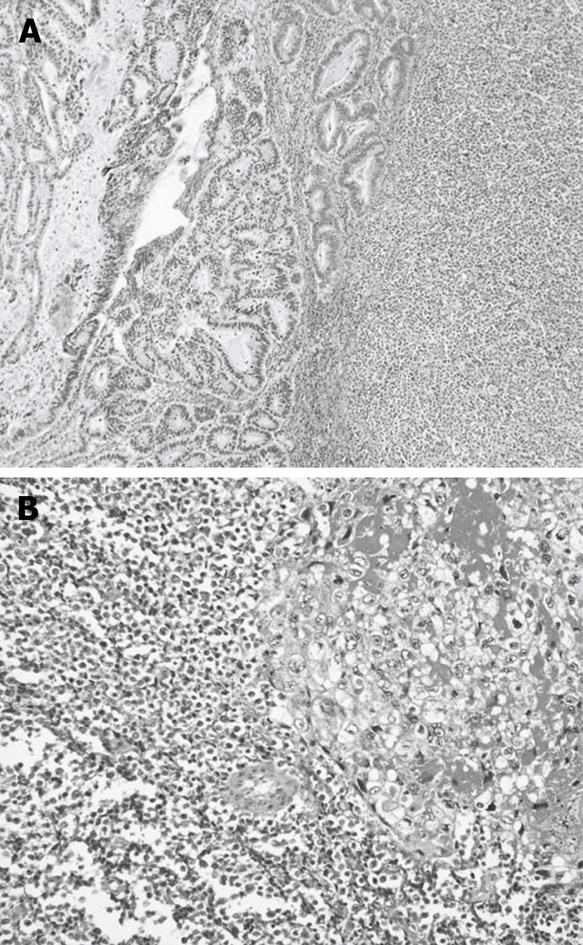
The hemorrhagic brown area was composed of choriocarcinoma, and consisted mostly of clusters of cytotrophoblastic cells separated by steaming masses of syncytiotrophoblasts (Figure 2A). Cytotrophoblastic cells were small cells with large, oval nuclei, and syncytiotrophoblasts were large cells with bizzare nuclei. The whitish-yellow area contained small cell carcinoma, consisting of small amounts of cytoplasm with large nuclei (Figure 2B) and tubular adenocarcinoma.
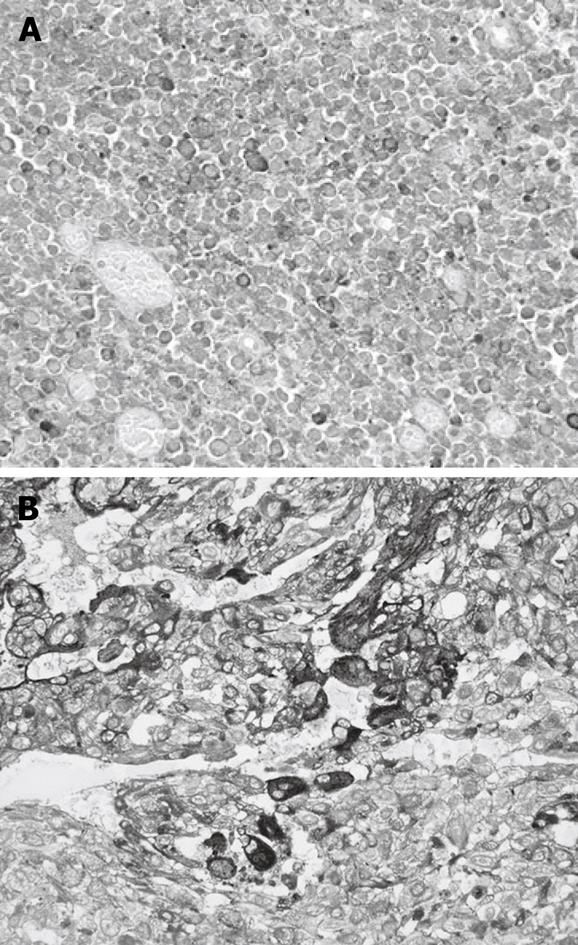
The choriocarcinomatous foci contained cells positive for beta-subunit of human chorionic gonadotropin (B-hCG) and human placental lactogen mainly in syncytiotrophoblastic cells (Figure 3A). These findings enabled us to diagnose these cells as choriocarcinoma. The small cell carcinomatous foci contained cells positive for synaptophysin, neuron-specific enolase (NSE), and chromogranin A (Figure 3B). From these results, we diagnosed them as neuroendocrine cell carcinoma.
The patient was discharged uneventfully 3 wk after surgery. He presented to our hospital with general malaise 2 wk after discharge. CT revealed multiple liver tumors, and his serum hCG level was 67 000 IU/mL. The liver tumor progressed, the patient died eventually from hepatic failure 6 wk after operation.
Choriocarcinoma can be gonadal or extragonadal in origin, and most often arises in the uterus in association with pregnancy[10]. The most common sites for extragonadal tumors are the mediastinum, ovary and testis[11]. There are many reported cases with metastatic choriocarcinoma to the stomach[12], but primary choriocarcinomas of the stomach are extremely rare. Primary neuroendocrine carcinomas are known to arise in the stomach, although they are also rare. Motoyama et al[13] reported a case of combined choriocarcinoma, hepatoid carcinoma, small cell carcinoma, and tubular adenocarcinoma in the esophagus in 1995, but there has been no reported case of combined choriocarcinoma, neuroendocrine carcinoma and tubular adenocarcinoma in the stomach in the English-language literature.
There are several theories of the histopathogenesis of primary choriocarcinoma of the stomach. These hypotheses include origin from a gonadal angle displaced in the abdomen[14], histological resemblance to choriocarcinoma[10], origin from an underlying gastric teratoma[15], and the retrodifferentiation or opisthoplatia of carcinoma cells to the level of the embryonal ectoderm with the ability to form trophoblasts[16]. The finding that gastric choriocarcinomas are frequently accompanied by adenocarcinoma is supported by this retrodifferentiation theory. In the present case, choriocarcinomas, neuroendocrine carcinomas and tubular adenocarcinoma existed in the same tumor of the stomach, and this finding suggests that choriocarcinoma and neuroendocrine carcinoma represent aberrant differentiation in common adenocarcinoma.
In the present case, we failed to diagnose adeno-carcinoma combined with choriocarcinoma and neuroendocrine carcinoma before operation based on pathological examination of biopsy specimens. Therefore, larger biopsy specimens from the whole tumor should be taken when encountering large and hemorrhagic tumors so that pathologic components are not missed.
It is well known that choriocarcinomas and neuroendocrine carcinomas readily metastasize to distant organs and carry a poor prognosis because effective regimens have not been established. Further studies to establish new regimens are required.
| 1. | Imai Y, Kawabe T, Takahashi M, Matsumura M, Komatsu Y, Hamada E, Niwa Y, Kurita M, Shiina S, Shimada T. A case of primary gastric choriocarcinoma and a review of the Japanese literature. J Gastroenterol. 1994;29:642-646. |
| 2. | Liu Z, Mira JL, Cruz-Caudillo JC. Primary gastric choriocarcinoma: a case report and review of the literature. Arch Pathol Lab Med. 2001;125:1601-1604. |
| 3. | Kobayashi A, Hasebe T, Endo Y, Sasaki S, Konishi M, Sugito M, Kinoshita T, Saito N, Ochiai A. Primary gastric choriocarcinoma: two case reports and a pooled analysis of 53 cases. Gastric Cancer. 2005;8:178-185. |
| 4. | Dye DW, Broadwater R, Lamps LW. Uncommon malignancies: case 2. Gastric choriocarcinoma. J Clin Oncol. 2005;23:6251-6253. |
| 5. | Liu Z, Mira JL, Cruz-Caudillo JC. Primary gastric choriocarcinoma: a case report and review of the literature. Arch Pathol Lab Med. 2001;125:1601-1604. |
| 6. | Kuroda N, Oonishi K, Iwamura S, Ohara M, Hirouchi T, Mizumo K, Miyazaki E, Enzan H. Gastric carcinosarcoma with neuroendocrine differentiation as the carcinoma component and leiomyosarcomatous and myofibroblastic differentiation as the sarcomatous component. APMIS. 2006;114:234-238. |
| 7. | Shpaner A, Yusuf TE. Primary gastric small-cell neuroendocrine carcinoma. Endoscopy. 2007;39 Suppl 1:E310-E311. |
| 8. | Fujiyoshi Y, Eimoto T. Chromogranin A expression correlates with tumour cell type and prognosis in signet ring cell carcinoma of the stomach. Histopathology. 2008;52:305-313. |
| 9. | Anjaneyulu , Rao SC, Rao RV. Primary choriocarcinoma of stomach. Indian J Pathol Microbiol. 2000;43:471-474. |
| 10. | Noguchi T, Takeno S, Sato T, Takahashi Y, Uchida Y, Yokoyama S. A patient with primary gastric choriocarcinoma who received a correct preoperative diagnosis and achieved prolonged survival. Gastric Cancer. 2002;5:112-117. |
| 11. | Moran CA, Suster S. Primary mediastinal choriocarcinomas: a clinicopathologic and immunohistochemical study of eight cases. Am J Surg Pathol. 1997;21:1007-1012. |
| 12. | Lombard F, Burtin P, Ketani S, Delaby J, Cales P, Boyer J. Mediastinal posterior choriocarcinoma with hemorrhagic gastric metastasis: endosonographic features. Gastrointest Endosc. 1992;38:187-190. |
| 13. | Motoyama T, Higuchi M, Taguchi J. Combined choriocarcinoma, hepatoid adenocarcinoma, small cell carcinoma and tubular adenocarcinoma in the oesophagus. Virchows Arch. 1995;427:451-454. |
| 14. | Nakao A, Sakagami K, Uda M, Mitsuoka S, Yamashita N, Ito H. Gastric carcinoma with predominant choriocarcinomatous component. Int J Clin Oncol. 1998;3:403-405. |