Published online May 28, 2008. doi: 10.3748/wjg.14.3254
Revised: April 8, 2008
Accepted: April 15, 2008
Published online: May 28, 2008
AIM: To explore the change of intestinal mucosa barrier function in the progress of non-alcoholic steatohepatitis (NASH) in rats.
METHODS: Thirty-two Sprague-Dawley (SD) rats were randomly divided into control group and model group. Rats in the control group were given normal diet, and rats in the model group were given fat-rich diet. Eight rats in each group were killed at end of the 8th and 12th wk, respectively. The levels of endotoxin, D-xylose, TG, TC, ALT and AST, intestinal tissue SOD and MDA as well as intestinal mucus secretory IgA (sIgA) were measured. The pathology of liver was observed by HE staining.
RESULTS: At end of the 8th wk, there was no marked difference in the levels of endotoxin, D-xylose and sIgA between the two groups. At end of the 12th wk, rats in the model group developed steatohepatitis and had a higher serum level of endotoxin (P = 0.01) and D-xylose (P = 0.00) and a lower serum level of sIgA (P = 0.007).
CONCLUSION: Intestinal mucosa barrier malfunction may exist in NASH rats and may be an important promoter of NASH in rats.
- Citation: Li S, Wu WC, He CY, Han Z, Jin DY, Wang L. Change of intestinal mucosa barrier function in the progress of non-alcoholic steatohepatitis in rats. World J Gastroenterol 2008; 14(20): 3254-3258
- URL: https://www.wjgnet.com/1007-9327/full/v14/i20/3254.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.3254
Non-alcoholic steatohepatitis (NASH) is a type of non-alcoholic fatty liver disease (NAFLD), and may progress to hepatic fibrosis and cirrhosis[1–3]. The pathogenesis of NASH remains unclear. Nowadays, lipid metabolism abnormality, insulin resistance and oxidative stress and lipid peroxidation reaction[4–8] are thought to play an important role in the pathogenesis of NASH[910]. It was reported that the change of intestinal environment may play a role in NASH, which may be a cause of enterogenous endotoxemia[1112]. Since the relationship between intestinal mucosa barrier function and NASH is uncertain, we established an animal model of NASH by giving fat-rich diet to explore the change of intestinal mucosa barrier function in the progress of NASH.
Thirty-two healthy female mice, provided by Nanjing Qinglongshan Experimental Animal Center, were used in this study. D-xylose, SOD and MDA kit were purchased from Nanjing Jiancheng Bioengineering Institute (NJBI). Quantitative chromogenic end-point tachypleus amebocyte lysate kit was purchased from Xiamen Houshiji, Ltd. Secretory IgA (sIgA) kit was purchased from Beijing North Institute of Biological Technology (BNIBT).
Establishment of animal mode: 32 female Sprague-Dawley (SD) rats, weighing 130-150 g, after a week of adaptive feeding, were randomly divided into model group and control group (16 in each group). Rats in the control group were given normal diet and rats in the model group (n = 16) were given fat-rich diet containing 88% normal diet, 10% lard and 2% cholesterol. All rats were maintained at controlled room temperature in a 12-h light/dark cycle with free access to laboratory feed and water. Eight rats in each group were killed at wk 8, 12 respectively during the study. All rats had no access to food and water for 12 h, but received intra-gastric 5% D-xylose (0.5 mL/100 g, BW) and 0.3% pentobarbital (0.15-0.2 mL/kg) via abdominal cavity, 25 min and a short wile, respectively, before they were killed.
Histological evaluation: Liver specimen was obtained from the central part of liver, observed by HE staining, and evaluated according to the guidelines for diagnosis and treatment of nonalcoholic fatty liver diseases revised by Fatty Liver and Alcoholic Liver Study Group of the Chinese Liver Disease Association[13].
Measurement of ALT, AST, TG and TC: Two milliliters blood was taken from abdominal aorta and serum was taken after centrifugation at 4000 r/min for 10 min. The levels of ALT, AST, TG and TC were measured with an automatic biochemical analyzer.
Measurement of D-xylose: Two milliliters blood was taken from abdominal aorta, and collected into a tube (containing heparin) immediately and plasma was taken after the blood was centrifuged at 4000 r/min for 10 min. The Level of D-xylose in plasma was measured with a D-xylose kit.
Measurement of endotoxin: One milliliter blood was taken from portal vein and collected into an apyrogenic tube (containing heparin) immediately. Plasma was taken after the blood was centrifuged at 3000 r/min for 10 min (environmental temperature: 0°C). The levels of endotoxin were measured by limulus amebocyte lysate test.
Detection of sIgA: sIgA was detected as previously described[14]. A 10 cm long tissue was obtained from the small intestine, dissected and washed with normal saline carefully. Intestine mucus was collected into an Eppendorf tube, and centrifuged at 3000 r/min for 10 min (environmental temperature: 0°C) after 1 mL 0.01 mol/L PBS was added. The supernatant was harvested. The level of sIgA was measured by double antibody sandwich immunoradiometric assay. The total protein of intestine mucus was assayed by Bradford brilliant blue method simultaneously. The sIgA content in total protein of one milligram small intestine mucus was detected.
Detection of SOD and MDA in small intestine tissue: The small intestine tissue was weighed to prepare 10% tissue homogenate by adding normal saline according to weighing ratio The homogenate was centrifuged at 3000 r/min for 10 min (environmental temperature: 0°C). The supernatant was harvested to make 1% tissue homogenate by adding normal saline. The levels of SOD and MDA in tissue homogenate were measured.
All statistical analyses were performed using SPSS 11.5 software package. All data were expressed as mean ± SD. Group comparison was done by one-factor analysis of variance. P < 0.05 was considered statistically significant.
The structure of hepatic lobules and the morphology of liver cells were normal in the control group. Lipid droplets were observed in 50%-75% of hepatic cells in the model group after 8 wk of fat-rich diet, predominantly bullules, consistent with the diagnostic criteria for simple fatty liver disease. Fatty degeneration in hepatic cells exceeded 75% and patch necrosis, mild to moderate chronic inflammation could be seen after 12 wk of fat-rich diet, consistent with the diagnostic criteria for steatohepatitis.
Serum TC, ALT and AST levels were higher in the model group than in the control group in the 8th, and 12th wk. There was a statistical difference between the two groups (P < 0.05). The serum TG level was slightly higher in the model group than in the control group, but there was no statistical difference between the two groups (Table 1).
At end of the 8th wk, there was no significant difference in the levels of endotoxin, D-xylose and sIgA between the two groups. At end of the 12th wk, rats in the model group developed steatohepatitis and had a higher serum level of endotoxin and D-xylose (P < 0.05), but a lower level of sIgA (P < 0.05) (Table 2).
The level of SOD in small intestine tissue was lower in the model group than in the control group in the 8th and 12th wk. There was a statistical difference between the two groups (P < 0.05). The level of MDA in small intestine tissue was higher in the model group than in control group in the 8th and 12th wk. There was a statistical difference between the two groups (P < 0.05) (Table 3).
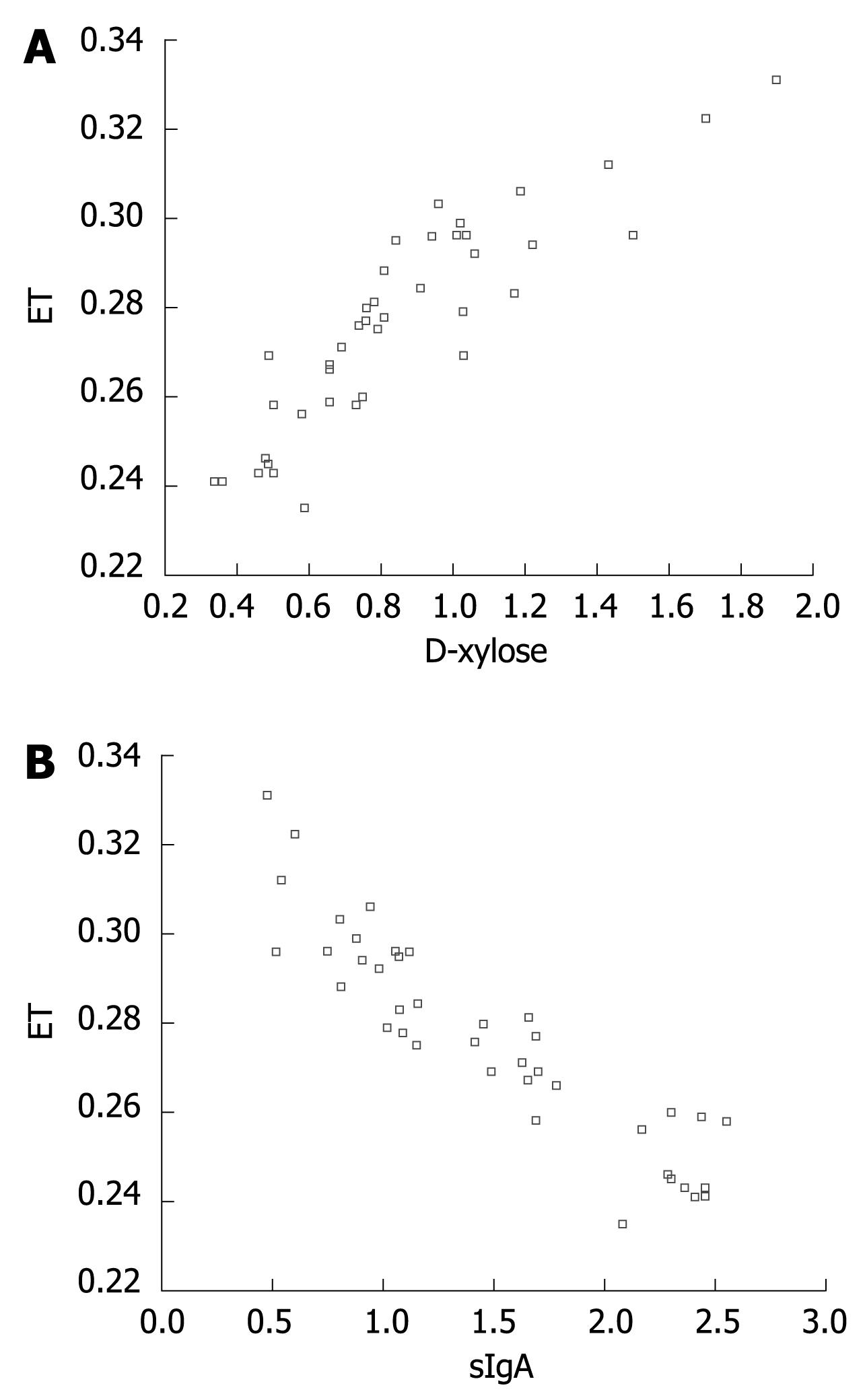
A line tendency could be observed in scatter plots bellow. The serum level of endotoxin in portal vein was positively correlated with that of D-xylose in abdominal aorta (r = 0.846, n = 8, P < 0.01) and negatively correlated that of sIgA in intestine mucus (r = -0.873, n = 8, P < 0.01) (Figure 1A and B).
At present, the specific pathogenesis and progress of NASH remain unclear. It was reported that there is enterogenous endotoxemia in NASH, suggesting that NASH is closely related to endotoxin[15–17]. Wigg et al[11] reported that small intestinal bacterial overgrowth is present in 50% of patients with non-alcoholic steatosis not accompanying increased intestinal permeability or elevated endotoxin levels. Brun P et al[12] showed that obese mice with NASH have higher intestinal mucosa permeability and circulating level of endotoxemia in portal vein than the control mice, suggesting that genetically obese mice display an enhanced intestinal permeability, leading to severe endotoxemia in portal vein. Therefore, whether there is an increased intestinal permeability and a change in intestinal mucosa barrier function in NASH patients needs to be further explored.
Intestinal mucosa barriers include mechanical barrier, chemical barrier, immunologic barrier and biology barrier[18–21], any damage of these barriers will damage intestinal mucosa barrier function. In this study, we used plasma D-xylose, endotoxin and intestine mucus sIgA to evaluate the intestinal mucosa barrier function in rats with NASH and to observe its change in NASH rats. Intestine mucus sIgA is a major ingredient of intestinal immunologic barrier, mostly secreted by plasmocytes of intestinal mucosa, and may restrain intestinal bacteria to adhere to intestinal mucosa surface, to counteract toxin, enzyme and virus in the intestinal tract, thus playing an important role in intestinal immunity[22–24]. SOD is the most important anti-oxidation enzyme in anti-oxidation defense system and MDA is the end product of lipid peroxidation, which can cause tissue injury. SOD activity and MDA level can reflect the degree of lipid peroxidation and oxidative stress. It was reported that NASH is closely related to lipid peroxidation and oxidative stress[25–27]. In the present study, we successfully established the NASH model by giving fat-rich diet, and observed the change of intestinal mucosa barrier function in simple fatty liver disease and NASH. The results showed that there was no statistical difference in serum D-xylose, endotoxin and intestine mucus sIgA between the two groups at the 8th wk, suggesting that there might be no damage to the intestinal mucosa barrier at the stage of simple fatty liver disease. However, the SOD activity was decreased in intestinal tissue, while the level of MDA was increased, suggesting that lipid peroxidation and anti-oxidation are imbalanced. There was a significant difference in serum D-xylose, endotoxin and intestine mucus sIgA between the two groups (P < 0.05) at the 12th wk. Serum D-xylose and endotoxin levels were higher in the model group than in the control group, while the intestine mucus sIgA levels were lower in the model group, suggesting that the intestinal mucosa barrier is damaged at the stage of NASH and that the SOD activity is further decreased in the intestinal tissue while MDA level is further elevated and lipid peroxidation reaction is further aggravated. The fact that serum endotoxin level in portal vein was positively correlated with that of serum D-xylose in abdominal aorta, but negatively correlated with that of sIgA in intestine mucus, suggesting that the damage to intestinal mucosa barrier may cause enterogenous endotoxemia.
In our study, no damage to intestinal mucosa barrier occurred at the early stage of nonalcoholic fatty liver disease. With the progress from simple fatty liver disease to NASH, severe damage to intestinal mucosa barrier occurred. The pathogenesis of intestinal mucosa barrier damage is unclear. It may be due to the increased lipid peroxidation reaction in intestinal tissue and intestinal mucosa damage caused by endotoxin[28]. It needs to be further studied. Increased sIgA levels in intestine mucus would lead to the ability of intestinal bacterium to inhibit adherence to intestinal mucosa surface and decrease counteracting toxin, so bacteria and endotoxin are increased in the intestinal tract. Wigg et al[11] reported that bacteria grow in small intestine of patients with non-alcoholic steatosis, suggesting that and decreased sIgA promotes overgrowth of bacteria in small intestine. We suppose that small intestinal bacterial overgrowth in small intestine can produce more endotoxin in enteric cavity, thus damaging intestinal mucosa barrier and absorbing more endotoxin, finally leading to enterogenous endotoxemia. It was reported that endotoxin can not only injure hepatic cells but also activate Kupffer cells by combing receptor CD14 and signal receptor TLR4. The activated Kupffer cells release a series of bioactive substances, such as TNF-α, causing hepatic injury, thus aggravating the effect of endotoxin and promoting development of NASH[29–31].
The pathogenesis of non-alcoholic steatohepatitis (NASH) remains unclear. Insulin resistance, obesity-related inflammation, oxidative stress, microcirculation disturbance, and malnutrition are thought to play a key role in the pathogenesis of NASH. Studies have also demonstrated that change in intestinal environment may also play a role in the pathogenesis of NASH, and may be a cause of enterogenous endotoxemia. It has been found that a higher intestinal permeability may also play a role in the process of NASH. However, it is not accepted that there exists enterogenous endotoxemia in NASH.
Great effort has been made to clarify the pathogenesis of NASH. The source and pathogenesis of endotoxin in the process are two hot spots.
In this study, the relationship between intestinal mucosa barrier function and NASH was studied.
The intestinal mucosa barrier malfunction may lead to NASH. There might be a vicious circle between intestinal mucosa barrier malfunction and NASH.
NASH is a kind of liver disease which resembles alcoholic liver disease accompanying steatosis, inflammation, necrosis, and fibrosis. Intestinal mucosa barriers include mechanical barrier, chemical barrier, immunologic barrier and biology barrier.
This paper explores the change of intestinal mucosa barrier function in the progress of NASH in rats. The well designed study demonstrated that the intestinal mucosa barrier malfunction may exist in NASH rats, and may be an important promoter of NASH in rats.
| 1. | Day CP. Non-alcoholic steatohepatitis (NASH): where are we now and where are we going? Gut. 2002;50:585-588. |
| 2. | Powell EE, Cooksley WG, Hanson R, Searle J, Halliday JW, Powell LW. The natural history of nonalcoholic steatohepatitis: a follow-up study of forty-two patients for up to 21 years. Hepatology. 1990;11:74-80. |
| 3. | Harrison SA, Torgerson S, Hayashi PH. The natural history of nonalcoholic fatty liver disease: a clinical histopathological study. Am J Gastroenterol. 2003;98:2042-2047. |
| 4. | Koruk M, Savas MC, Yilmaz O, Taysi S, Karakok M, Gundogdu C, Yilmaz A. Serum lipids, lipoproteins and apolipoproteins levels in patients with nonalcoholic steatohepatitis. J Clin Gastroenterol. 2003;37:177-182. |
| 5. | Baskol G, Baskol M, Kocer D. Oxidative stress and antioxidant defenses in serum of patients with non-alcoholic steatohepatitis. Clin Biochem. 2007;40:776-780. |
| 6. | Kojima H, Sakurai S, Uemura M, Fukui H, Morimoto H, Tamagawa Y. Mitochondrial abnormality and oxidative stress in nonalcoholic steatohepatitis. Alcohol Clin Exp Res. 2007;31:S61-S66. |
| 7. | Mitsuyoshi H, Itoh Y, Okanoue T. [Role of oxidative stress in non-alcoholic steatohepatitis]. Nippon Rinsho. 2006;64:1077-1082. |
| 8. | Leclercq IA. Pathogenesis of steatohepatitis: insights from the study of animal models. Acta Gastroenterol Belg. 2007;70:25-31. |
| 9. | Angulo P, Lindor KD. Non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2002;17 Suppl:S186-S190. |
| 10. | Choudhury J, Sanyal AJ. Insulin resistance in NASH. Front Biosci. 2005;10:1520-1533. |
| 11. | Wigg AJ, Roberts-Thomson IC, Dymock RB, McCarthy PJ, Grose RH, Cummins AG. The role of small intestinal bacterial overgrowth, intestinal permeability, endotoxaemia, and tumour necrosis factor alpha in the pathogenesis of non-alcoholic steatohepatitis. Gut. 2001;48:206-211. |
| 12. | Brun P, Castagliuolo I, Di Leo V, Buda A, Pinzani M, Palu G, Martines D. Increased intestinal permeability in obese mice: new evidence in the pathogenesis of nonalcoholic steatohepatitis. Am J Physiol Gastrointest Liver Physiol. 2007;292:G518-G525. |
| 13. | Fatty Liver and Alcoholic Liver Disease Study Group of the Chinese Liver Disease Association. [Guidelines for diagnosis and treatment of nonalcoholic fatty liver diseases]. Zhonghua Ganzangbing Zazhi. 2006;14:161-163. |
| 14. | Zhang YP, Shi ZR. Effects of probiotics on the bacterial groups of intestinal and sIgA in severely burned rats. Zhongguo Weishengwu Zazhi. 2004;5:257-259. |
| 15. | Kirsch R, Clarkson V, Verdonk RC, Marais AD, Shephard EG, Ryffel B, de la M Hall P. Rodent nutritional model of steatohepatitis: effects of endotoxin (lipopolysaccharide) and tumor necrosis factor alpha deficiency. J Gastroenterol Hepatol. 2006;21:174-182. |
| 16. | Li X, Han de W, Zhao LF, Yin L. [Effect of Endotoxin on the expression of peroxisome proliferator-activated receptor alpha in the development of nonalcoholic steatohepatitis in rats]. Zhonghua Ganzangbing Zazhi. 2005;13:89-91. |
| 17. | Fan JG, Xu ZJ, Wang GL, Ding XD, Tian LY, Zheng XY. [Change of serum endotoxin level in the progress of nonalcoholic steatohepatitis in rats]. Zhonghua Ganzangbing Zazhi. 2003;11:73-76. |
| 18. | Baumgart DC, Dignass AU. Intestinal barrier function. Curr Opin Clin Nutr Metab Care. 2002;5:685-694. |
| 19. | Sakaguchi T, Brand S, Reinecker HC. Mucosal barrier and immune mediators. Curr Opin Gastroenterol. 2001;17:573-577. |
| 20. | Tlaskalova-Hogenova H, Farre-Castany MA, Stepankova R, Kozakova H, Tuckova L, Funda DP, Barot R, Cukrowska B, Sinkora J, Mandel L. The gut as a lymphoepithelial organ: the role of intestinal epithelial cells in mucosal immunity. Folia Microbiol (Praha). 1995;40:385-391. |
| 21. | Sydora BC, Martin SM, Lupicki M, Dieleman LA, Doyle J, Walker JW, Fedorak RN. Bacterial antigens alone can influence intestinal barrier integrity, but live bacteria are required for initiation of intestinal inflammation and injury. Inflamm Bowel Dis. 2006;12:429-436. |
| 22. | Mazanec MB, Nedrud JG, Kaetzel CS, Lamm ME. A three-tiered view of the role of IgA in mucosal defense. Immunol Today. 1993;14:430-435. |
| 23. | Takahashi I, Kiyono H. Gut as the largest immunologic tissue. JPEN J Parenter Enteral Nutr. 1999;23:S7-S12. |
| 24. | Lamm ME. Interaction of antigens and antibodies at mucosal surfaces. Annu Rev Microbiol. 1997;51:311-340. |
| 25. | Day CP, James OF. Steatohepatitis: a tale of two "hits"? Gastroenterology. 1998;114:842-845. |
| 26. | Koruk M, Taysi S, Savas MC, Yilmaz O, Akcay F, Karakok M. Oxidative stress and enzymatic antioxidant status in patients with nonalcoholic steatohepatitis. Ann Clin Lab Sci. 2004;34:57-62. |
| 27. | Baskol G, Baskol M, Kocer D. Oxidative stress and antioxidant defenses in serum of patients with non-alcoholic steatohepatitis. Clin Biochem. 2007;40:776-780. |
| 28. | Mercer DW, Smith GS, Cross JM, Russell DH, Chang L, Cacioppo J. Effects of lipopolysaccharide on intestinal injury; potential role of nitric oxide and lipid peroxidation. J Surg Res. 1996;63:185-192. |
| 29. | Rivera CA, Adegboyega P, van Rooijen N, Tagalicud A, Allman M, Wallace M. Toll-like receptor-4 signaling and Kupffer cells play pivotal roles in the pathogenesis of non-alcoholic steatohepatitis. J Hepatol. 2007;47:571-579. |