Published online May 28, 2008. doi: 10.3748/wjg.14.3249
Revised: February 27, 2008
Accepted: March 5, 2008
Published online: May 28, 2008
AIM: To observe the effect of proteasome inhibitor MG-132 on severe acute pancreatitis (SAP) and associated lung injury of rats.
METHODS: Male adult SD rats were randomly divided into SAP group, sham-operation group, and MG-132 treatment group. A model of SAP was established by injection of 5% sodium taurocholate into the biliary-pancreatic duct of rats. The MG-132 group was pretreated with 10 mg/kg MG-132 intraperitoneally (ip) 30 min before the induction of pancreatitis. The changes in serum amylase, myeloperoxidase (MPO) activity of pancreatic and pulmonary tissue were measured. The TNF-α level in pancreatic cytosolic fractions was assayed with an enzyme-linked immunosorbent assay (ELISA) kit. Meanwhile, the pathological changes in both pancreatic and pulmonary tissues were also observed.
RESULTS: MG-132 significantly decreased serum amylase, pancreatic weight/body ratio, pancreatic TNF-α level, pancreatic and pulmonary MPO activity (P < 0.05). Histopathological examinations revealed that pancreatic and pulmonary samples from rats pretreated with MG-132 demonstrated milder edema, cellular damage, and inflammatory activity (P < 0.05).
CONCLUSION: The proteasome inhibitor MG-132 shows a protective effect on severe acute pancreatitis and associated lung injury of rats.
- Citation: Chen X, Li SL, Wu T, Liu JD. Proteasome inhibitor ameliorates severe acute pancreatitis and associated lung injury of rats. World J Gastroenterol 2008; 14(20): 3249-3253
- URL: https://www.wjgnet.com/1007-9327/full/v14/i20/3249.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.3249
Acute pancreatitis (AP) is a common clinical disease and its incidence has been increasing in recent years. Although most patients experience mild and self-limited AP, some patients have severe acute pancreatitis (SAP)[1–3]. Abnormal activation of digestive enzymes within pancreatic acinar cells is thought to be a critical initiating event[4]. Pancreatic injury leads to a localized and a subsequent systemic inflammatory response which determines the severity of pancreatitis. It may lead to the development of multiple organ dysfunction syndrome (MODS), which is responsible for the mortality rate associated with this disease. The major component of MODS is lung injury, clinically manifested as acute respiratory distress syndrome[5]. So how to ameliorate the injury of pancreatic acinar cells and downstream events is the way to influence the severity of pancreatitis.
MG-132 (Z-Leu-Leu-Leu-aldehyde) is a member of the peptide aldehyde proteasome inhibitors, including calpains, cathepsins and the proteasome[67]. Both calpain I and proteasome can degrade Iκb and enhance the activity of NF-κb[8]. Cathepsins , especially cathepsin B, has been confirmed to be a key agent for the abnormal activation of digest enzymes within pancreatic acinar cells[9]. MG-132 can also inhibit the activation of NF-κb by blocking Iκb degradation and enhance the expression of heat shock proteins (HSP) that suppress the inflammatory response[10]. In the present study, we used a model of severe acute pancreatitis (SAP) established by retrograde injection of 5% sodium taurocholate (1 mL/kg) into the pancreatic duct to observe the effect of the proteasome inhibitor MG-132 on severe acute pancreatitis and associated lung injury of rats.
Male Sprague-Dawley rats, weighing 250-300 g, were obtained from Experimental Animals Center of Medical College of Xi’an Jiaotong University. The animals were fasted overnight with free access to water and standard rat chow diet before the experiment. Five percent sodium taurocholate was purchased from Sigma, USA. MG-132 was purchased from Calbiochem, Germany. TNF-α ELISA kit was obtained from Genzyme, USA. Serum amylase kit and MPO kit were purchased from Nanjing Jiancheng Company, China.
The rats were randomly divided into control group, SAP group and MG-132 treatment group (SAP + MG-132), 10 in each group. The animals were anesthetized with ketamine and subjected to a midline laparotomy. A blunt needle was inserted transduodenally into the common biliary-pancreatic duct as previously described[11]. The hepatic duct was closed at the hilum of the liver with a bulldog clamp to prevent backflow. Sodium taurocholate (5% in saline) was infused using a fine needle inserted into 5 mm of the common biliary-pancreatic duct and each rat received a total volume of 1 mL/kg body weight for 1 min. After 5 min, the needle and clamp were removed, and the laparotomy incision was closed. Animals in the treatment group were injected intraperitoneally (ip) with 10 mg/kg MG-132 dissolved in 0.25 mL dimethyl sulfoxide (DMSO). Thirty minutes later, pancreatitis was induced as above. Animals in the control group underwent a sham operation consisting of laparotomy and puncture of the duodenum, under an identical anesthesia. Six hours after duct infusion or sham operation, the animals were killed by depletion and samples were taken for study. Blood was collected by cardiac puncture using heparinized syringes, centrifuged at 4000 r/min for 10 min, and stored at 4°C for further analysis. The pancreas and lungs were carefully isolated and weighed for subsequent experiments. Tissues for histological examination were isolated, fixed in 10% formalin and embedded in paraffin for sectioning.
Changes in pancreatic weight were assessed for pancreatic interstitial edema. The whole pancreas was removed and weighed. Weight of each pancreatic sample was used to estimate the water content in pancreas as previously described[12], which was presented as a ratio of pancreas/body weight for evaluating the consequence of pancreatic edema.
For quantification of lung edema, the left lung was resected, blotted dry, and weighed (wet weight). Thereafter, the left lung was desiccated for 24 h at 80°C and weighed again (dry weight). Water content in the lung was determined by calculating the wet weight/dry weight ratio as previously described[13].
Serum amylase was assayed with an amylase kit with a kinetic spectrophotometric method according to the manufacturer’s instructions. Briefly, the methodology is based on the enzymatic degradation of ethylidene-p-nitrophenol-G7 by amylase coupled with glucosidase, thus producing p-nitrophenol which exhibits strong absorbance at 405 nm. Enzymatic activity was expressed as units/liter.
Pancreatic and lung myeloperoxidase (MPO) activity, a quantitative indicator of neutrophil infiltration, was assessed according to the instructions from Nanjing Jiancheng Company. Briefly, tissues were thawed, homogenized in a 20 mmol/L phosphate buffer (pH 7.4), centrifuged at 13 000 r/min for 10 min at 4°C. The resulting pellet was re-suspended in 50 mmol/L phosphate buffer (pH 6.0) containing 0.5% hexadecyl trimethylammonium bromide. The suspension was subjected to four cycles of freezing and thawing. The sample was then centrifuged at 10 000 r/min for 5 min at 4°C. The supernatant was used for MPO assay. The absorbance at 450 nm of the resulting mixture was determined. The MPO activity was expressed as U/g tissue.
TNF-α level in pancreatic cytosolic fractions was measured with a commercial ELISA kit according to the instructions of its manufacturer. The absorbance was read on a microplate reader and concentrations were calculated according to the standard curve.
Paraffin-embedded tissues ware sectioned, stained with hematoxylin and eosin, and assessed by two different attending physicians unaware of the experimental protocol, at Department of Pathology of Xi’an Jiaotong University. The pancreas damage evaluation system was used as previously described[14], the sections were examin-ed and scored on a scale of 0-3 with 0 being normal and 3 being severe. Six characteristics were included, namely the presence of edema, acinar necrosis, inflammatory infiltrate, hemorrhage, fat necrosis, and perivascular inflammation. Five parameters were used as criteria for lung injury, manifested as alveolar thickening, vascular congestion, hemorrhage, edema, and leukocyte infiltration. Each observer was required to give a score from 0 to 2 for each slide according to the criteria mentioned above. A score of 0 indicates that there was no histological damage. A score of 1 indicates only mild or intermediate histological damage in the slides, and a score of 2 was given to the tissue sections with severe morphological deterioration in most of the areas observed.
All results are expressed as mean ± SE. Statistical analysis of data was accomplished by analysis of variance (ANOVA). P < 0.05 was considered statistically significant.
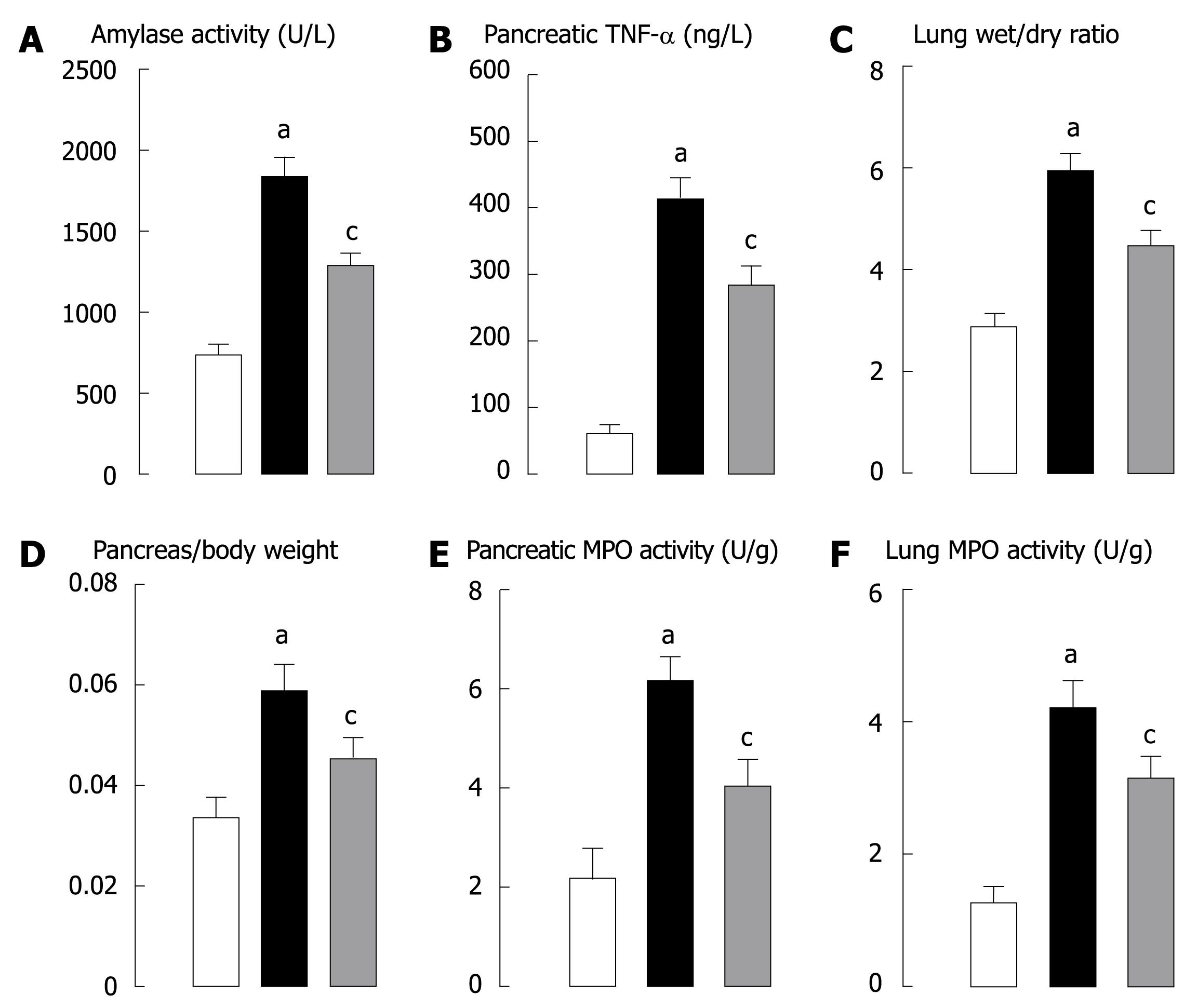
Serum amylase activity, a most common indicator for assessing pancreatitis, was markedly increased in the SAP animals (Figure 1A). The effect of MG-132 on pancreatitis was statistically significant.
In the SAP animals, the concentration of TNF-α was increased (Figure 1B), which could be ameliorated in rats treated with MG-132, showing that TNF-α could improve pancreatitis.
The ratio of lung wet/dry weight, a commonly used indicator for estimating the water content in acute lung injury was significantly increased in the SAP group compared with the sham group (Figure 1C). Treatment with MG-132 could reduce the water content lung.
Pancreatic edema, one of the major criteria for assessing pancreatitis was found in our experiment. Injection of 5% sodium taurocholate into the biliary-pancreatic duct of rats could significantly increase the ratio of pancreas to body weight (Figure 1D). Treatment with MG-132 showed a beneficial effect on pancreatic edema.
SAP is associated with a rise in both pancreatic and lung MPO activity, indicating the presence of sequestered neutrophils[15]. Pre-treatment of the animals with MG-132 significantly reduced the MPO activity both in pancreas and in lung (Figure 1E and F).
To assess the effects of MG-132 on local pancreatic injury, the morphology of pancreas was examined and compared with the treatment group. The results showed that the SAP group exhibited severe edema and a high degree of destruction of histoarchitecture of the acini cells. The architecture and integrity of acini cells were improved in the MG-132 group.
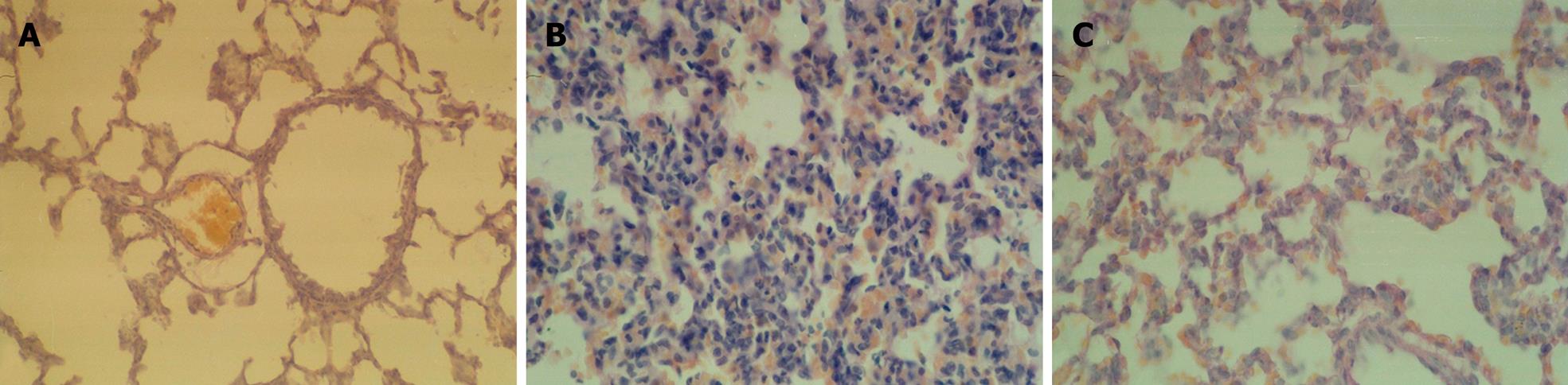
Normal lung tissue morphology (Figure 2A) was observed in the sham group. Histological examination of the sections confirmed lung injury with significant alveolate thickening, vasocongestion and infiltration with leukocytes observed in the SAP group (Figure 2B). In contrast, the lung injury was significantly ameliorated in the animals treated with MG-132 (Figure 2C). The scores of histological evaluation of pancreatitis and lung injury are summarized in Table 1.
Acute pancreatitis is a life-threatening disease with a high mortality rate, especially in the setting of systemic inflammatory response and multiple organ failure when severe infection of necrosis occurs[16]. Under physiological conditions, digestive enzymes are synthesized by and stored in pancreatic acinar cells as inactive proenzymes known as zymogens which are secreted into the duodenum where enterokinase initiates their activation. The pathogenesis of acute pancreatitis remains obscure. However, it is believed that the premature activation of zymogens within acinar cells is a critical initiating event, thus leading to auto-digestion of the gland. Afflicted acinar cells release factors that lead to recruitment of inflammatory cells and generation of multiple mediators, such as reactive oxygen species and cytokines[17]. Two potential key elements involved in this process are cathepsin B and NF-κb.
Cathepsin B is a lysosomal hydrolase, which activates human trypsinogen in vitro and is redistributed in a zymogen-granule enriched subcellular compartment during the early course of experimental pancreatitis[1819]. It was reported that inhibition of lysosomal protease cathepsin B can suppress pancreatic inflammation[2021]. Studies in cathepsin B gene knocked-out mice showed that the premature and intracellular activation of trypsinogen largely depends on the presence of cathepsin B[22].
NF-κb is a member of the transcription factors which, under normal conditions, are coupled to an inhibitor (Iκb) in cytoplasm[2324]. In response to stress, a cascade of phosphorylation events results in Iκb phosphorylation and degradation by proteasome. NF-κb with subsequent up-regulation of the expression of genes coding for a variety inflammatory factors including cytokines and chemokines such as TNF-α, IL-1, IL-6, IL-8. In acute pancreatitis[25], NF-κb activation can be inhibited by blocking the degradation of Iκb, which has been shown to ameliorate the severity of pancreatitis[26].
During acute pancreatitis, lung injury is associated with the accumulation of neutrophils within the interstitial and alveolar spaces. Leukocyte sequestration within an inflamed area is a multistep process that begins with leukocyte activation[27], followed by the rolling of inflammatory cells and the adhesion of circulating activated inflammatory cells to the endothelium via adhesion molecules, such as intercellular adhesion molecule-1 (ICAM-1)[28]. Cytokines, such as IL-1 and TNF-α, can also increase lung injury and spread to other distant organs, indicating that NF-κb plays key role in this proinflammatory process[29–31].
MG-132 is a cell-permeable aldehyde proteasome inhibitor, which has been shown to suppress the inflammatory cascade by decreasing NF-κb activity through blocking Iκb degradation and increasing cellular HSP level[32]. MG-132 can also suppress pancreatic inflammation by inhibiting calpains and cathepsin B.
Since the SAP model established by injecting sodium taurocholate is most similar to the situation in human severe acute pancreatitis[33], we chose it to examine the effect of proteasome inhibitor MG-132 on pancreatitis and lung injury in order to simulate the situation in humans.
In the present study, administration of MG-132 significantly suppressed the elevation of serum amylase, TNF-α and pancreatic MPO activity. Significant improvements were also observed in pancreatic histology after treatment with MG-132. Edema, acinar cell and fat necrosis, perivascular inflammation occurring in almost all inflammatory processes in any organ, were resolved in pancreatic tissues from the animals treated with MG-132.
The group pre-treated with MG-132, as compared with the sham and SAP groups, showed a significant reduction in the lung water content and MPO activity. These results are in close agreement with the histological analysis, suggesting that alveolar thickening, vasocongestion and recruitment of leukocytes in lung tissue can be suppressed with MG-132.
In conclusion, MG-132, a proteasome inhibitor, can ameliorate sodium taurocholate-induced SAP and its associated lung injury.
Severe acute pancreatitis (SAP) is still a fatal disease and its pathogenesis has not been fully understood. Pathological activation of digestive zymogens within pancreatic acinar cells is considered the key initiator of AP. The effect of MG-132 was investigated in experimental severe acute pancreatitis in the present study.
Proteasome inhibitors have a broad inhibitory action on pancreatic enzymes and production of proinflammatory cytokines. Therefore, they are expected to prevent necrotic changes in the pancreas and reduce the mortality rate.
The SAP model established by retrograde injection of 5% sodium taurocholate (1 mL/kg) into the pancreatic duct was used to observe the effect of the proteasome inhibitor MG-132 on severe acute pancreatitis and its associated lung injury of rats. Taurocholate-induced pancreatitis is a reliable model of severe acute pancreatitis rats with significantly greater pancreatic damage and systemic inflammatory response in comparison with cerulein-induced pancreatitis. Pancreatic and lung injury was a distant organ injury which is the key determinant of mortality in AP patients.
MG-132 shows its protective effect on SAP induced by sodium taurocholate and its associated lung injury of rats. Moreover, proteasome inhibitors may promote further studies on the treatment of AP.
This paper describes the effect of MG-132 (carbobenzoxy-L-leucyl-L-leucyl-L-leucinal), a proteosome inhibitor, on SAP and its associated lung injury of rats. The model selected is appropriate and induces SAP and lung injury. Further researches are needed to explore its mechanism.
| 1. | Pandol SJ. Acute pancreatitis. Curr Opin Gastroenterol. 2006;22:481-486. |
| 2. | Yadav D, Lowenfels AB. Trends in the epidemiology of the first attack of acute pancreatitis: a systematic review. Pancreas. 2006;33:323-330. |
| 3. | Bhatia M, Wong FL, Cao Y, Lau HY, Huang J, Puneet P, Chevali L. Pathophysiology of acute pancreatitis. Pancreatology. 2005;5:132-144. |
| 4. | Sherwood MW, Prior IA, Voronina SG, Barrow SL, Woodsmith JD, Gerasimenko OV, Petersen OH, Tepikin AV. Activation of trypsinogen in large endocytic vacuoles of pancreatic acinar cells. Proc Natl Acad Sci USA. 2007;104:5674-5679. |
| 6. | Elliott PJ, Zollner TM, Boehncke WH. Proteasome inhibition: a new anti-inflammatory strategy. J Mol Med. 2003;81:235-245. |
| 7. | Lee DH, Goldberg AL. Proteasome inhibitors: valuable new tools for cell biologists. Trends Cell Biol. 1998;8:397-403. |
| 8. | Christman JW, Lancaster LH, Blackwell TS. Nuclear factor kappa B: a pivotal role in the systemic inflammatory response syndrome and new target for therapy. Intensive Care Med. 1998;24:1131-1138. |
| 9. | Saluja AK, Donovan EA, Yamanaka K, Yamaguchi Y, Hofbauer B, Steer ML. Cerulein-induced in vitro activation of trypsinogen in rat pancreatic acini is mediated by cathepsin B. Gastroenterology. 1997;113:304-310. |
| 10. | Salinthone S, Singer CA, Gerthoffer WT. Inflammatory gene expression by human colonic smooth muscle cells. Am J Physiol Gastrointest Liver Physiol. 2004;287:G627-G637. |
| 11. | Aho HJ, Koskensalo SM, Nevalainen TJ. Experimental pancreatitis in the rat. Sodium taurocholate-induced acute haemorrhagic pancreatitis. Scand J Gastroenterol. 1980;15:411-416. |
| 12. | Rongione AJ, Kusske AM, Kwan K, Ashley SW, Reber HA, McFadden DW. Interleukin 10 reduces the severity of acute pancreatitis in rats. Gastroenterology. 1997;112:960-967. |
| 13. | Tsang SW, Ip SP, Leung PS. Prophylactic and therapeutic treatments with AT 1 and AT 2 receptor antagonists and their effects on changes in the severity of pancreatitis. Int J Biochem Cell Biol. 2004;36:330-339. |
| 14. | Kusske AM, Rongione AJ, Ashley SW, McFadden DW, Reber HA. Interleukin-10 prevents death in lethal necrotizing pancreatitis in mice. Surgery. 1996;120:284-288; discussion 289. |
| 15. | Schmidt J, Lewandrowsi K, Warshaw AL, Compton CC, Rattner DW. Morphometric characteristics and homogeneity of a new model of acute pancreatitis in the rat. Int J Pancreatol. 1992;12:41-51. |
| 16. | Mullane KM, Kraemer R, Smith B. Myeloperoxidase activity as a quantitative assessment of neutrophil infiltration into ischemic myocardium. J Pharmacol Methods. 1985;14:157-167. |
| 17. | Shi C, Zhao X, Wang X, Andersson R. Role of nuclear factor-kappaB, reactive oxygen species and cellular signaling in the early phase of acute pancreatitis. Scand J Gastroenterol. 2005;40:103-108. |
| 18. | Baron TH, Morgan DE. Acute necrotizing pancreatitis. N Engl J Med. 1999;340:1412-1417. |
| 19. | Teich N, Bodeker H, Keim V. Cathepsin B cleavage of the trypsinogen activation peptide. BMC Gastroenterol. 2002;2:16. |
| 20. | Saluja AK, Donovan EA, Yamanaka K, Yamaguchi Y, Hofbauer B, Steer ML. Cerulein-induced in vitro activation of trypsinogen in rat pancreatic acini is mediated by cathepsin B. Gastroenterology. 1997;113:304-310. |
| 21. | Van Acker GJ, Saluja AK, Bhagat L, Singh VP, Song AM, Steer ML. Cathepsin B inhibition prevents trypsinogen activation and reduces pancreatitis severity. Am J Physiol Gastrointest Liver Physiol. 2002;283:G794-G800. |
| 22. | Halangk W, Lerch MM, Brandt-Nedelev B, Roth W, Ruthenbuerger M, Reinheckel T, Domschke W, Lippert H, Peters C, Deussing J. Role of cathepsin B in intracellular trypsinogen activation and the onset of acute pancreatitis. J Clin Invest. 2000;106:773-781. |
| 23. | Hietaranta AJ, Saluja AK, Bhagat L, Singh VP, Song AM, Steer ML. Relationship between NF-kappaB and trypsinogen activation in rat pancreas after supramaximal caerulein stimulation. Biochem Biophys Res Commun. 2001;280:388-395. |
| 24. | Hietaranta A, Mustonen H, Puolakkainen P, Haapiainen R, Kemppainen E. Proinflammatory effects of pancreatic elastase are mediated through TLR4 and NF-kappaB. Biochem Biophys Res Commun. 2004;323:192-196. |
| 25. | Makhija R, Kingsnorth AN. Cytokine storm in acute pancreatitis. J Hepatobiliary Pancreat Surg. 2002;9:401-410. |
| 26. | Altavilla D, Famulari C, Passaniti M, Campo GM, Macri A, Seminara P, Marini H, Calo M, Santamaria LB, Bono D. Lipid peroxidation inhibition reduces NF-kappaB activation and attenuates cerulein-induced pancreatitis. Free Radic Res. 2003;37:425-435. |
| 27. | Lau HY, Wong FL, Bhatia M. A key role of neurokinin 1 receptors in acute pancreatitis and associated lung injury. Biochem Biophys Res Commun. 2005;327:509-515. |
| 28. | Bhatia M, Moochhala S. Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J Pathol. 2004;202:145-156. |
| 29. | O'Reilly DA, Roberts JR, Cartmell MT, Demaine AG, Kingsnorth AN. Heat shock factor-1 and nuclear factor-kappaB are systemically activated in human acute pancreatitis. JOP. 2006;7:174-184. |
| 30. | Shi C, Zhao X, Wang X, Andersson R. Role of nuclear factor-kappaB, reactive oxygen species and cellular signaling in the early phase of acute pancreatitis. Scand J Gastroenterol. 2005;40:103-108. |
| 31. | Long J, Song N, Liu XP, Guo KJ, Guo RX. Nuclear factor-kappaB activation on the reactive oxygen species in acute necrotizing pancreatitic rats. World J Gastroenterol. 2005;11:4277-4280. |
| 32. | Lee DH, Goldberg AL. Proteasome inhibitors cause induction of heat shock proteins and trehalose, which together confer thermotolerance in Saccharomyces cerevisiae. Mol Cell Biol. 1998;18:30-38. |