Published online May 28, 2008. doi: 10.3748/wjg.14.3242
Revised: April 1, 2008
Accepted: April 8, 2008
Published online: May 28, 2008
AIM: To investigate the effect of quercetin (3,3’,4’,5,7-pentahydroxy flavone), a major flavonoid in human diet, on hyper-proliferation of gastric mucosal cells in rats treated with chronic oral ethanol.
METHODS: Forty male Sprague-Dawley rats, weighing 200-250 g, were randomly divided into control group (tap water ad libitum), ethanol treatment group (6 mL/L ethanol), quercetin treatment group (intragastric gavage with 100 mg/kg of quercetin per day), and ethanol plus quercetin treatment group (quercetin and 6 mL/L ethanol). Expression levels of proliferating cell nuclear antigen (PCNA) and Cyclin D1 were detected by Western blot to assay gastric mucosal cell proliferation in rats. To demonstrate the influence of quercetin on the production of extra-cellular reactive oxygen species/nitrogen species (ROS/RNS) in rats, changes in levels of thiobarbituric acid reactive substance (TBARS), protein carbonyl, nitrite and nitrate (NOx) and nitrotyrosine (NT) were determined. The activity of inducible nitric oxide synthase (NOS) including iNOS and nNOS was also detected by Western blot.
RESULTS: Compared to control animals, cell proliferation in the gastric mucosa of animals subjected to ethanol treatment for 7 days was significant increased (increased to 290% for PCNA density P < 0.05, increased to 150 for Cyclin D1 density P < 0.05 and 21.6 ± 0.8 vs 42.3 ± 0.7 for PCNA positive cells per view field), accompanied by an increase in ROS generation (1.298 ± 0.135 &mgr;mol vs 1.772 ± 0.078 &mgr;mol for TBARS P < 0.05; 4.36 ± 0.39 mmol vs 7.48 ± 0.40 mmol for carbonyl contents P < 0.05) and decrease in NO generation (11.334 ± 0.467 &mgr;mol vs 7.978 ± 0.334 &mgr;mol P < 0.01 for NOx; 8.986 ± 1.351 &mgr;mol vs 6.854 ± 0.460 &mgr;mol for nitrotyrosine P < 0.01) and nNOS activity (decreased to 43% P < 0.05). This function was abolished by the co-administration of quercetin.
CONCLUSION: The antioxidant action of quercetin relies, in part, on its ability to stimulate nNOS and enhance production of NO that would interact with endogenously produced reactive oxygen to inhibit hyper-proliferation of gastric mucosal cells in rats treated with chronic oral ethanol.
- Citation: Liu JL, Du J, Fan LL, Liu XY, Gu L, Ge YB. Effects of quercetin on hyper-proliferation of gastric mucosal cells in rats treated with chronic oral ethanol through the reactive oxygen species-nitric oxide pathway. World J Gastroenterol 2008; 14(20): 3242-3248
- URL: https://www.wjgnet.com/1007-9327/full/v14/i20/3242.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.3242
Chronic ethanol consumption is a major risk factor for oropharyngeal, esophageal, and rectal cancer[1]. Chronic ethanol consumption resulting in gastric mucosal lesions might thus be expected to influence the kinetic balance between cell proliferation and cell death. Because hyper-regenerative gastrointestinal mucosa has an increased susceptibility to chemical carcinogens and thus influences carcinogenesis. Various studies have been performed to evaluate the effect of chronic ethanol consumption on gastric mucosal cell turnover[23]. However, the role of ethanol in the altered cell proliferation in rat stomach remains poorly understood. There is evidence that alcohol is involved in gastric mucosa oxidant injury as studies showed that ethanol-induced damage can be prevented if antioxidant treatment or therapy is given concurrently or prior to alcohol exposure[4–6]. Previous studies in our laboratory found that cell proliferation is enhanced in gastric mucosa of rats treated with ethanol in a dose- and time-dependent manner[7]. These findings indicate that ethanol-associated gastric cell proliferation may involve oxidative stress[8].
Oxidative stress occurs when there is a significant imbalance between generation of reactive oxygen species (ROS) and nitrogen species (RNS) and its clearance by antioxidant defenses[9]. 3, 3’, 4’, 5, 7-pentahydroxy flavone (quercetin) is a potent bioflavonoid widely distributed throughout vegetables and fruits. It was reported that quercetin has many beneficial effects on human health, including cardiovascular protection, anticancer activity, anti-ulcer effects, anti-allergy activity, cataract prevention, antiviral activity and anti-inflammatory effects[1011]. These effects of quercetin due to its antioxidant properties of potent anti-oxidant, scavenge free radicals directly[12], inhibit xanthine oxidase and lipid peroxidation[1314], and alter the anti-oxidant defense pathway in vivo and in vitro[15]. It was recently reported that quercetin inhibits oxidative damage in ethanol-induced gastric lesions of rats[16].
In light of these findings, we hypothesized that quercetin has an effect on gastric mucosa cell proliferation in rats that chronically administer ethanol involving inhibition of the ROS-nitric oxide (ROS-NO) pathway. To establish the potential antiproliferative mechanism of quercetin, we detected the expression levels of proliferating cell nuclear antigen (PCNA) and Cyclin D1, which are significantly associated with gastric mucosal cell proliferation in rats. To demonstrate the influence of quercetin on the production of extra-cellular ROS/RNS, changes in thiobarbituric acid reactive substance levels (TBARS) as an index of lipid peroxidation, protein carbonyl content as a marker of free radical-mediated modification of proteins, nitrite and nitrate (NOx) and nitrotyrosine (NT) levels as the marker of NO production, were also determined.
Male Sprague-Dawley rats, weighing 200-250 g, were used in this study. Twenty-four rats were housed in plastic cages in an air-conditioned and light controlled room at 24 ± 2°C and 60% ± 5% humidity. The study protocol was approved by the Nanjing Medical University Animal Care and Use Committee. After a 3 d adaptation period, the rats were randomly divided into four groups (6 in each group). Group 1 had free access to tap water, group 2 had drinking water containing 6 mL/L ethanol as previously described[7], group 3 was given 50 mg/kg quercetin (Sigma, St Louis, MO, USA) by intragastric gavage twice a day, group 4 was given 50 mg/kg quercetin by intragastric gavage twice a day and 6 mL/L ethanol. Quercetin was dissolved using DMSO as the vehicle and diluted in PBS to 2 mL, with the maximum concentration of DMSO being 0.1%. As controls, animals in groups 1 and 2 were also treated with 2 mL 0.1% DMSO, twice a day. The time and doses of ethanol and quercetin treatment were determined on the basis of results from our preliminary experiment. The mean ethanol consumption was 6.52 g/kg body weight per day, the mean plasma ethanol concentration at the time of stomach excision was 18.47 mmol/L in animals of groups 3 and 4. The rats were anesthetized with urethane and sacrificed after 7 d. Their stomachs were dissected and used for this study.
Nuclear extracts from gastric mucosa were prepared using a nuclear extract kit (Active Motif Japan, Japan) following the instructions of its manufacturer. PCNA and Cyclin D1 detected by Western blot were applied to determination of gastric mucosal cell proliferation in rats.
To evaluate the extent of lipid peroxidation, the amount of thiobarbituric acid reactive substances (TBARS) in gastric tissue, a measurement of the extent of lipid peroxidation, was detected with the modified thiobarbituric acid (TBA) method[1718]. Each sample was homogenized in a 1.15% KCl solution containing 10 mmol/L deferoxamine, 0.04% butylated hydroxytoluene (BHT), and 2% ethanol. Each homogenate was incubated for 60 min at 95°C in an oil bath with a stock TCA-TBA-HCl reagent consisting of 15% (w/v) trichloroacetic acid, 0.375% (w/v) thiobarbituric acid, 0.25 mol/L hydrochloric acid and 2% BHT. After cooling, the precipitate was removed by centrifugation, and the extinction coefficient of the supernatant at 535 nm was determined spectrophotometrically and compared with a known TBARS standard.
Protein carbonyls in gastric tissues were determined by spectrometric DNPH assay according to Fagan et al with minor modifications[19]. Briefly, gastric tissues were homogenized by sonication in a lysis buffer containing PBS (pH 7.2), 1% Triton X-100, 1 mmol/L EDTA and 1X protease inhibitor cocktail and removal of insoluble cellular debris was performed by centrifugation. Aliquots in protein samples were precipitated with 10 volumes of HCl-acetone (3:100) and washed with 5 mL of 10% TCA solution. Pellets were re-suspended in 500 &mgr;L buffer solution and reacted with 500 &mgr;L of 10 mmol/L DNPH (in 2 mol/L HCl) by vortexing for 15 min. To remove the un-reacted DNPH, the centrifuged pellets were washed with 5 mL of 20% TCA and 5 mL of ethanol: ethylacetate mixture (v/v = 1:1). The final precipitate was resolved in 1 mL of 6 mol/L guanidine HCl, and the absorbance at 380 nm was determined for the sample treated with DNPH and HCl, which was subtracted as a background and compared with a known protein carbonyl standard.
The amount of stable nitrite (nitrite and nitrate), the end product of NO in gastric mucosa, was determined by colorimetric assay as described previously[20]. Briefly, 50 &mgr;L of gastric mucosa homogenate was mixed with an equal volume of Griess reagent consisting of 1% sulfanilamide, 0.1% naphthyl ethylenediamine dihydrochloride and 2.5% H3PO4, and incubated at room temperature for 10 min. The absorbance was read at 540 nm on a microplate reader (Elx800, Bio-TEK Ins, USA). The amount of nitrite was calculated from a NaNO2 standard curve.
Gastric mucosa was homogenized on ice in the prepared solution (20 mmol/L Tris-HCl containing 1% NP-40, 100 mmol/L NaF, 137 mmol/L NaCl, 5 mmol/L EDTA, 0.1 mol/L PMSF, 1% proteinase inhibitor, and 10% glycerol, pH 7.5) for 30 min at 4°C. The homogenate was centrifuged at 12 000 r/min for 20 min to remove cellular debris. Protein concentration was determined using a BCA protein assay reagent kit.
Nitrotyrosine levels were quantified as previously described[21]. In short, assay was performed in 96-well plates coated with 5 mg/L of nitrotyrosine-BTG conjugate, which was blocked with gelatin to prevent nonspecific binding. A standard curve was plotted by incubating serial dilutions of NT with biotin labeled anti-nitrotyrosine Fab’ in PBS containing 0.1% gelatin for 1 h. Subsequently, plates were incubated with a strepavidin peroxidase conjugate followed by o-phenylenediamine (OPD). The reaction was terminated after 20-30 min by addition of 4 mol/L H2SO4. Data on the standard curve were fitted to a logistic plot and the levels of NT were measured. All samples and standards were assayed in triplicate.
Anti-nitrotyrosine monoclonal antibody used in ELISA was a kind gift from Dr. Yang TB (Institute of Space Medico-Engineering, Beijing, China). The study of cross-reaction with nitrotyrosine-like compounds showed that the antibodies have a high specificity for NT[21].
The stomach was homogenized on ice in a buffer containing 50 mmol/L Tris-Cl, 150 mmol/L NaCl, 0.02% NaN2, 100 mg/L phenylmethanesulfonyl fluoride, 1 mg /L aprotitin, and 1% Triton X-100. Lysates were centrifuged at 12 000 r/min for 25 min at 4°C. The supernatant was used for nNOS and inducible NOS (iNOS) determined by Western blot analysis.
Proteins were detected by the Bradford method using bovine serum albumin as a standard. An equal amount of 40 &mgr;g protein from each sample was run per lane on 10% sodium dodecyl sulphate polyarcylantide gel electrophoresis (SDS-PAGE) and electroblotted to nitrocellulose membranes. The membranes were blocked by overnight incubation in 5% dry milk at 4°C, and thereafter incubated with primary antibodies (1:200-1000 dilution) for 3 h at room temperature. Each blot was probed with monoclonal anti-PCNA, anti-Cyclin D1 (Santa Cruz Biotech, USA), polyclonal anti-β-actin (Upstate, USA), anti-nNOS, and anti-iNOS (Santa Cruz Biotech, USA). The membranes were washed and incubated with horseradish peroxidase-conjugated goat anti-mouse or anti-rabbbit IgG (1:2000 dilution) (Upstate, USA) for 1 h. Immune complexes were visualized with an ECL kit (Pierce; Rockford, IL, USA) according to the manufacturer’s protocol. Signal intensity was quantified using a Bio-Rad image analysis system and the results were normalized to the signal intensity of β-actin for each blot.
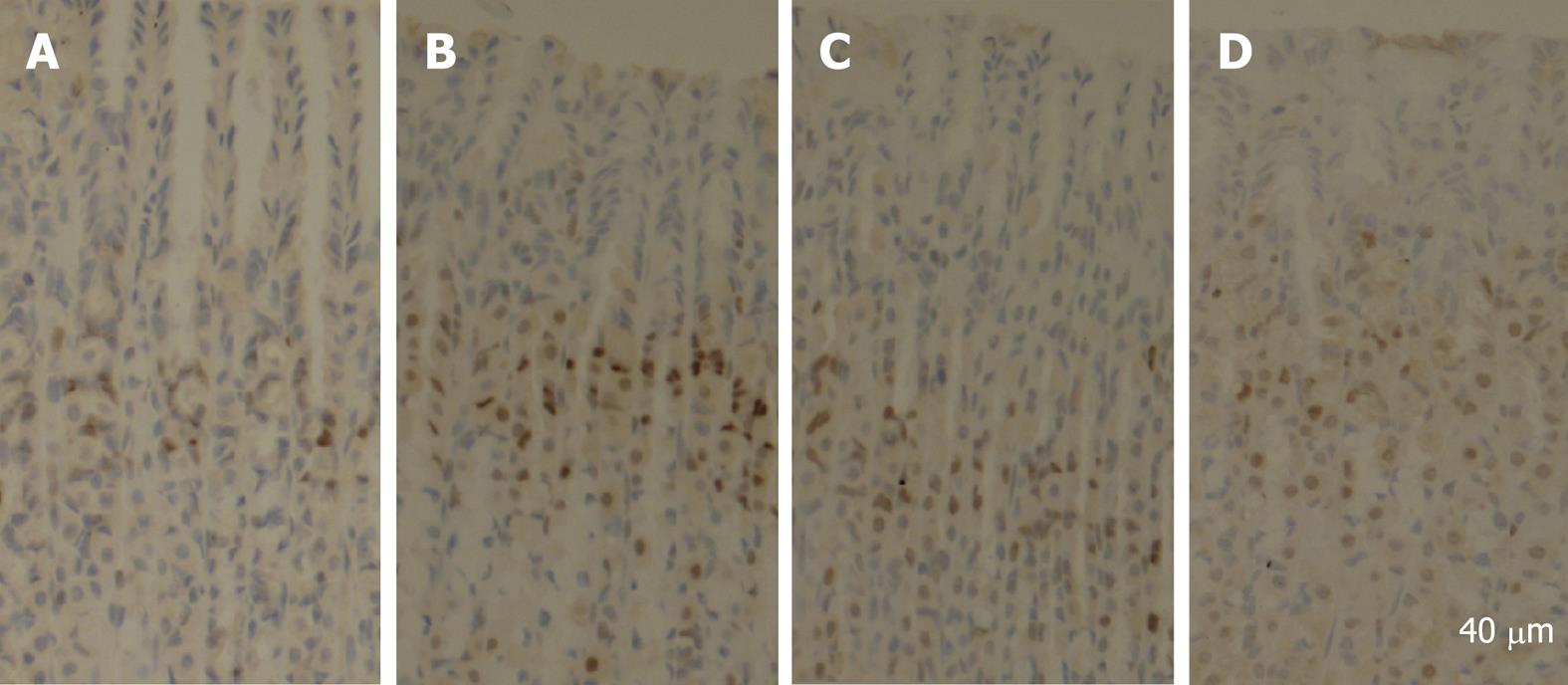
Stomachs were excised from three rats in each group and fixed in 10% neutral buffered formalin, embedded in paraffin and sectioned at 5 &mgr;m for immunohistochemical staining. Staining was performed according to the routine standard procedures. Briefly, the sections were deparaffinized in xylene, cleared in graded ethanol to PBS, then quenched in 3% hydrogen peroxide (H2O2) containing 0.1% sodium azide to suppress the endogenous peroxidase activity and placed in 10 mmol/L citrate buffer (pH 6.0) for 15 min at 100°C for antigen retrieval. A routine streptavidin-biotin protocol using the DAKO LSAB + kits (Dako Japan, Kyoto, Japan) was applied. The tissue sections mounted on glass slides were incubated in PBS containing 0.5% BSA to reduce nonspecific protein binding, and sequentially incubated to react with monoclonal anti-PCNA primary antibody overnight at 4°C. The antibody was then linked with streptavidin conjugated to horseradish peroxidase (HRP). HRP sites were visualized with 3,3’-diaminobenzidine (DAB) and H2O2, counterstained with hematoxylin. The presence of PCNA was detected by light field microscopy as a dark brown reaction product in cell nuclei. Some sections were reacted with normal mouse IgG instead of the specific antibody as a negative control. An image analysis system (NYD100) was used for quantitative analysis of cell density (cell number/view field) of the PCNA-positive cells in the rat stomach. Four sections from four rats were used. PCNA-positive cells per section were counted in five randomly selected view fields at a magnification of × 400. At least, 20 fields in each group were analyzed.
All experiments were done in triplicate and stomach tissues were excised from three rats in each group. One-way analysis of variance was used to estimate the overall significance followed by post hoc Tukey’s test corrected for multiple comparisons. Data are presented as mean ±SD. P < 0.05 was considered statistically significant.
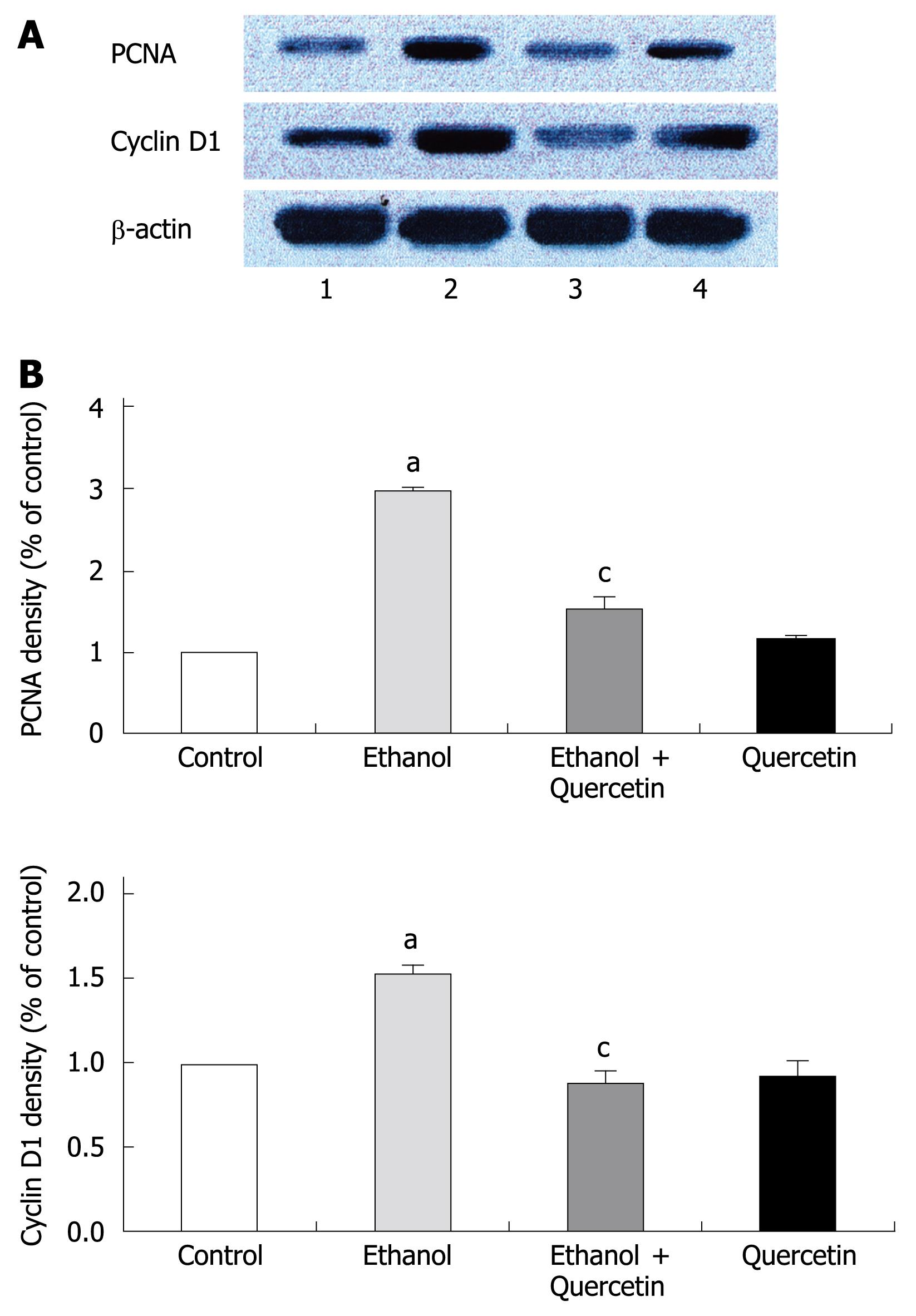
PCNA is a polypeptide that specifically increases in nuclei during G1 and S phases of the cell cycle. It is considered to be an essential cofactor for the activation of DNA polymerase during DNA replication. Therefore, PCNA-positive nuclei indicate that cells replicate DNA and undergo proliferation. It is well known that Cyclin D1 promotes G1 phase progression. The levels of PCNA and Cyclin D1 were higher in gastric mucosa exposed to 6% ethanol for 7 d than in normal control rats, while the expression of PCNA and Cyclin D1 was reduced after treatment with quercetin in this study (Figure 1). PCNA immunohistochemistry and computer image analysis showed, a significantly increased number of PCNA positive cells in the fundic gland of rats treated with ethanol for 7 d. The number of PCNA positive cells in ethanol + quercetin and quercetin treated rats was very analogous to that in the control rats (Figure 2, Table 1).
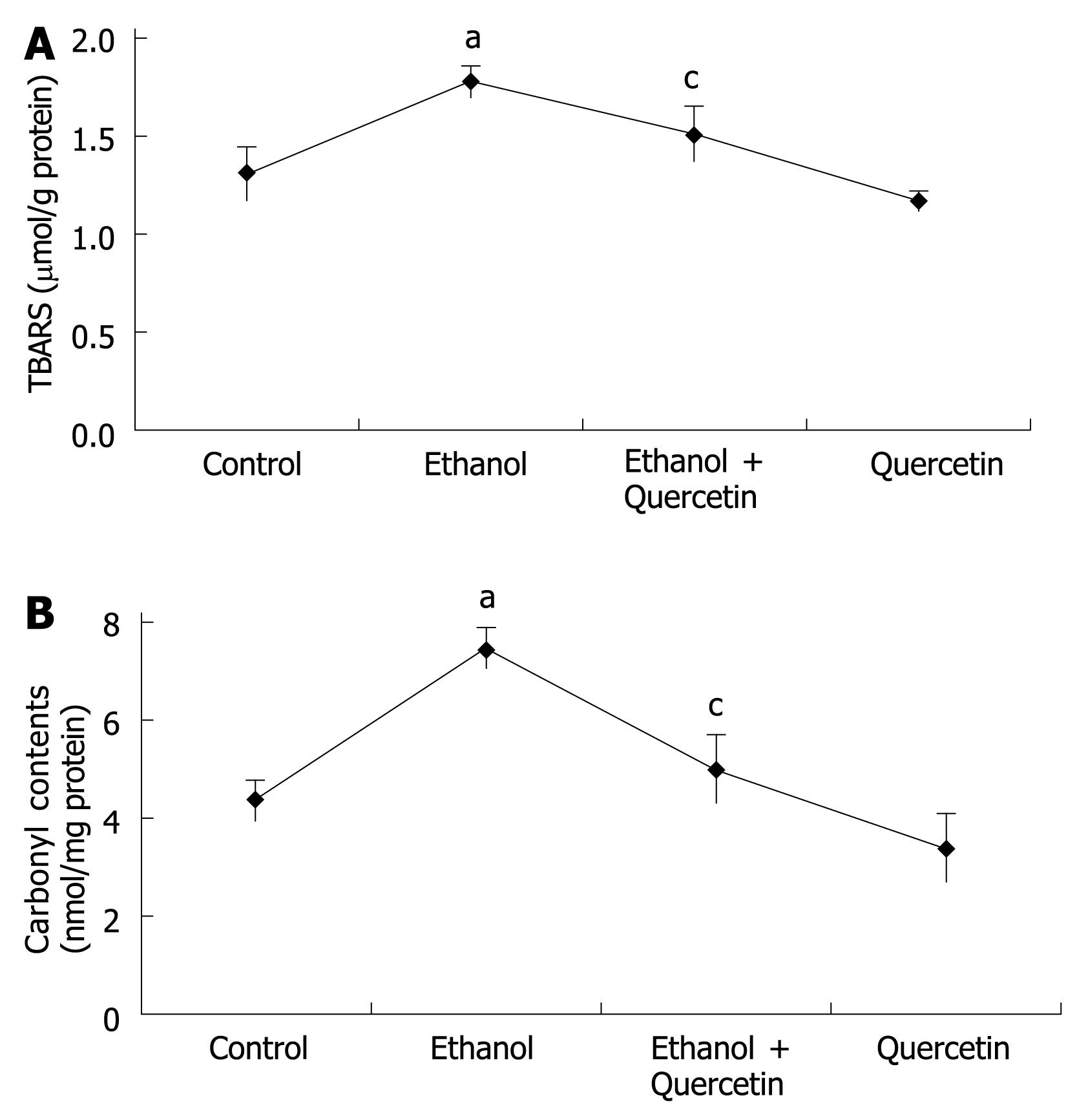
As TBASR shown in Figure 3, ethanol-induced ROS may increase lipid peroxidation. Quantitative measurement of TBASR in gastric mucosa revealed a significant effect of ethanol treatment on ethanol-induced lipid peroxidation and protein oxidation in gastric mucosa (1.772 &mgr;mol/g protein) compared to the normal control rats (1.298 &mgr;mol/g protein), which was reduced to 1.500 &mgr;mol/g protein (P < 0.05). TBARS was slightly decreased in the rats treated with quercetin (Figure 3A), suggesting that quercetin can decrease lipid peroxidation in gastric mucosa. The mean values of carbonyl contents in gastric tissue are shown in Figure 3B, revealing a similar pattern of TBARS in each group of rats.
The nitrite/nitrate content in gastric mucosa was determined using the Griess method. As shown in Table 1, the nitrite/nitrate content in the group treated with 6% ethanol for 7 d was significantly lower than that in the control group (P < 0.01) and significantly higher in rats treated with combined ethanol and quercetin than that in rats treated with ethanol only (P < 0.01). The gastric nNOS level was slightly increased in rats treated with quercetin, suggesting that quercetin treatment can prevent ethanol-induced decrease of nitrite/nitrate content in rat gastric mucosa.
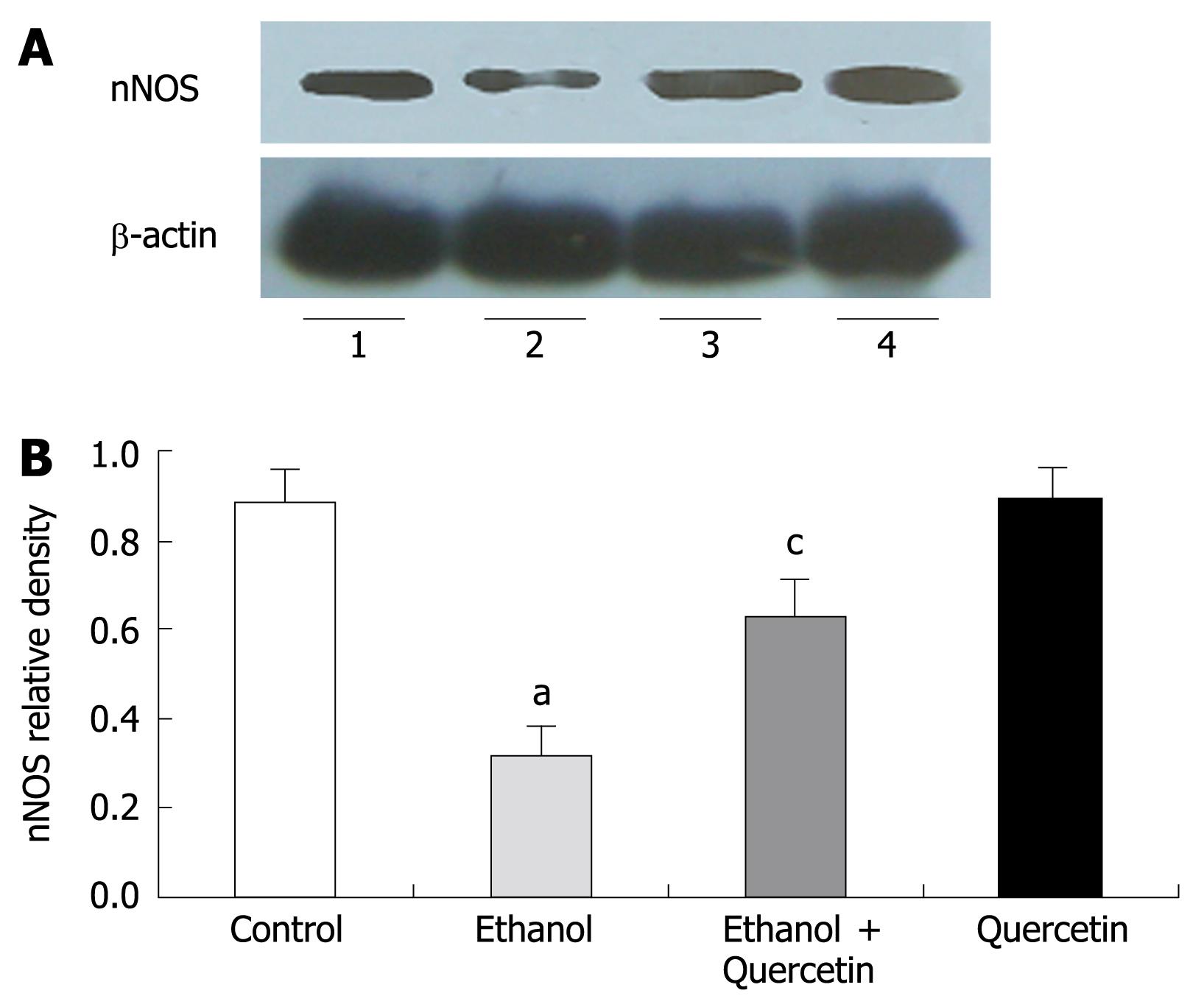
NO produced by nNOS was detected by Western blot in gastric mucosa (Figure 4). Quantitative analysis revealed a significant effect of ethanol treatment on ethanol-induced decrease in nNOS levels. The gastric nNOS level in rats treated with combined ethanol and quercetin was significantly higher than that in rats treated with ethanol only. The gastric nNOS level was slightly increased in rats treated with quercetin, suggesting that quercetin can prevent ethanol-induced decrease in nNOS, which is in agreement with the data on nitrite/nitrate (Table 1) in rat gastric mucosa. No iNOS expression was detected in each group.
Ultimately, increased NO, nitrite/nitrate, and peroxynitrite resulted in production of protein-bound 3-NT in gastric mucosa (Table 1). Quantitative analysis revealed a significant effect of ethanol on ethanol-induced decrease in protein-bound 3-NT. Gastric 3-NT levels in rats treated with combined ethanol and quercetin were significantly higher than those in rats treated with ethanol only. The level of 3-NT was slightly increased in rats not treated with ethanol, suggesting that quercetin treatment can prevent ethanol-induced decrease in 3-NT, which is in agreement with the data on nitrite/nitrate (Table 1) and nNOS level in rat gastric mucosa (Figure 4).
ROS, such as superoxide anion radical (O2-.), hydroxyl radical (.OH), lipid peroxidation and nitric oxide (NO), are involved both in the regulation of cell proliferation and apoptosis and in macromolecular damage to gastric cells, leading to increased oxidative stress and stress-induced senescence[2223]. ROS are oxygen-containing molecules having either unpaired electrons or ability to abstract electrons from other molecules. Lipids are modified by ROS and visualized as a thiobarbituric acid-reactive substance (TBARS). Oxidative damage to proteins generates increased carbonyl groups due to oxidation of sensitive amino acids, such as histidine, proline, arginine and lysine[24]. We measured the TBARS and protein carbonyls to serve as an indicator for intracellular oxidation in gastric mucosa. NO formation in cells is rapidly converted to nitrite. After reducing nitrate to nitrite with bacterial nitrate reductase, nitrite levels can be determined as an indicator for NO synthesis based on the Griess reaction[20]. O2- reacts with NO to produce peroxynitrite (ONOO-), which is considered a more powerful oxidant than O2-[25], entering the cells rapidly. A variety of nitrate macromolecules are chiefly at the aromatic rings[26]. The nitration of tyrosyl residues on proteins is considered the stable “foot print” of RNS stress both in vitro and in vivo[27]. In this study, we also measured the levels of NOX and 3-NT to provide an index of NO in gastric mucosa. Using these indicators, the effect of quercetin on chronic ethanol-induced generation of ROS and NO was detected.
The present study demonstrated a clear enhancement of cell proliferation in gastric mucosa of animals subjected to ethanol treatment for 7 d, which is similar to that in our previous studies[7]. Since PCNA and Cyclin D1 were strongly up-regulated (Figure 1), and the number of PCNA positive cells was increased in gastric mucosa (Figure 2 and Table 1). This enhancement function was accompanied with an increase in ROS generation and abolished by co-administration of quercetin and ethanol, which was accompanied with a decrease in ROS level. Quercetin has the ability to directly block the cell cycle at the G1/S transition in colon and gastric cancer cells[28] as well as in human leukemic T cells[29]. However, the protein levels of PCNA and Cyclin D1 were similar to the control values irrespective of quercetin administration alone in our study. These results show that an excessive amount of ROS can induce enhanced cell proliferation in gastric mucosa of rats in vivo.
In addition to ROS, RNS in the form of NO has also been implicated in regulation of cellular proliferation, but its role as a proliferative signal is not well defined, because it appears to depend on the cell type responsible for its release and the NOS isoforms within cells, as well as on the concentration of released NO and the composition of intracellular milieu[3031]. The neuronal and endothelial isoforms are thought to be responsible for production of low levels of NO[32] and both isoforms have been identified in gastric mucosa[3334]. NO is a lipophilic radical, which can exert beneficial effects by reacting with O2- when produced in a small amount and, in this manner, behaves as an antioxidant. A low level of NO could protect against ROS and inhibit gastric cancer cell proliferation[23]. NO donors retard gastric wound healing by inhibiting cell migration and proliferation and inducing cell apoptosis in a dose- and time-dependent manner[35]. However, excess NO produced by inducible NOS (iNOS) plays a potent role as a cytotoxic agent during infection and inflammation, with essential involvement of chronic inflammation, especially increased rates of cell proliferation, in H pylori-associated glandular stomach carcinogenesis[36]. Suppression of NO generation by iNOS inhibitors (aminoguanidine, AG) could also suppress cancer cell proliferation in gastric cancer xenografts[37].
In the present study, iNOS expression was not detectable in gastric mucosa. After treatment with 6% ethanol for 7 d, the expression of nNOS and the levels of NOx (nitrite/nitrate) and NT in gastric homogenates were decreased, suggesting that the nNOS activity is decreased. These results are consistent with the reported data[38]. Surprisingly, the decreased nNOS activity could be abolished by co-administration of quercetin and ethanol. Without further examination, we cannot rule out the mechanism of quercetin-enhanced activity of nNOS. It was reported that resveratrol, another kind of flavonoids, can inhibit gastric cancer cell proliferation by stimulating the activity of NOS in vitro[23], suggesting that quercetin may play a role as resveratrol in the inhibition of gastric cell proliferation in vivo.
In conclusion, our findings indicate that the antioxidant action of quercetin resides depends in part, on its ability to stimulate nNOS and increase production of NO that would interact with endogenously produced reactive oxygen to inhibit hyper-proliferation of gastric mucosal cells in rats that have chronic ethanol consumption.
Chronic ethanol consumption resulting in hyper-regenerative gastrointestinal mucosa has an increased susceptibility to chemical carcinogens and thus influences carcinogenesis. Some studies indicate that ethanol-associated gastric cell proliferation may involve oxidative stress. It was reported that quercetin, a 3,3’,4’,5,7-pentahydroxy flavone, has effects on oxidative damage to ethanol-induced gastric lesions due to its antioxidant properties of potent anti-oxidant. We hypothesized that quercetin has an effect on gastric mucosal cell proliferation in rats that have chronic ethanol consumption, thus inhibiting gastric cancer.
In this study, we developed an animal model by continuous ethanol ingestion for 7 d. By using this model, we investigated the relationship between chronic ethanol intake and gastric mucosal cell proliferation, which is related to reactive oxygen species (ROS) and reactive nitrogen species (RNS).
Our findings indicate that the antioxidant action of quercetin resides, depends in part, on its ability to stimulate nNOS and increase production of NO that would interact with endogenously produced reactive oxygen to inhibit gastric mucosal cell roliferation in rats that have chronic ethanol consumption.
This animal model established by continuous ethanol ingestion for 7 d is a useful tool for studying the mechanism of gastric mucosal cell proliferation in vivo. We will investigate the signal transduction of ROS in gastric mucosal cell proliferation.
ROS are oxygen-containing molecules having either unpaired electrons or ability to abstract electrons from other molecules. Reactive RNS are forms of NO.
This is an interesting paper, in which the authors showed that ethanol could induce gastric mucosal cell proliferation in their animal model. The ROS/RNS pathway may be involved. Further study is needed to show signal transduction of ROS in gastric mucosal cell proliferation.
| 1. | Seitz HK, Maurer B, Stickel F. Alcohol consumption and cancer of the gastrointestinal tract. Dig Dis. 2005;23:297-303. |
| 2. | Franke A, Teyssen S, Singer MV. Alcohol-related diseases of the esophagus and stomach. Dig Dis. 2005;23:204-213. |
| 3. | Kountouras J, Chatzopoulos D, Zavos C. Reactive oxygen metabolites and upper gastrointestinal diseases. Hepatogastroenterology. 2001;48:743-751. |
| 4. | La Casa C, Villegas I, Alarcon de la Lastra C, Motilva V, Martin Calero MJ. Evidence for protective and antioxidant properties of rutin, a natural flavone, against ethanol induced gastric lesions. J Ethnopharmacol. 2000;71:45-53. |
| 5. | Santos FA, Rao VS. 1,8-cineol, a food flavoring agent, prevents ethanol-induced gastric injury in rats. Dig Dis Sci. 2001;46:331-337. |
| 6. | Bilici D, Suleyman H, Banoglu ZN, Kiziltunc A, Avci B, Ciftcioglu A, Bilici S. Melatonin prevents ethanol-induced gastric mucosal damage possibly due to its antioxidant effect. Dig Dis Sci. 2002;47:856-861. |
| 7. | Ge YB, Du J, Fan LL, Li YC, Gu L. Chronic ethanol feeding alters the epithelial cell proliferation and apoptosis in rat gastric mucosa. Histol Histopathol. 2007;22:185-190. |
| 8. | Hernandez-Munoz R, Montiel-Ruiz C, Vazquez-Martinez O. Gastric mucosal cell proliferation in ethanol-induced chronic mucosal injury is related to oxidative stress and lipid peroxidation in rats. Lab Invest. 2000;80:1161-1169. |
| 9. | Forman HJ, Torres M, Fukuto J. Redox signaling. Mol Cell Biochem. 2002;234-235:49-62. |
| 10. | Bronner C, Landry Y. Kinetics of the inhibitory effect of flavonoids on histamine secretion from mast cells. Agents Actions. 1985;16:147-151. |
| 11. | Reutrakul V, Ningnuek N, Pohmakotr M, Yoosook C, Napaswad C, Kasisit J, Santisuk T, Tuchinda P. Anti HIV-1 flavonoid glycosides from Ochna integerrima. Planta Med. 2007;73:683-688. |
| 12. | Hanasaki Y, Ogawa S, Fukui S. The correlation between active oxygens scavenging and antioxidative effects of flavonoids. Free Radic Biol Med. 1994;16:845-850. |
| 13. | Plumb GW, Price KR, Williamson G. Antioxidant properties of flavonol glycosides from green beans. Redox Rep. 1999;4:123-127. |
| 14. | Fiorani M, De Sanctis R, Menghinello P, Cucchiarini L, Cellini B, Dacha M. Quercetin prevents glutathione depletion induced by dehydroascorbic acid in rabbit red blood cells. Free Radic Res. 2001;34:639-648. |
| 15. | Morand C, Crespy V, Manach C, Besson C, Demigne C, Remesy C. Plasma metabolites of quercetin and their antioxidant properties. Am J Physiol. 1998;275:R212-R219. |
| 16. | Kahraman A, Erkasap N, Koken T, Serteser M, Aktepe F, Erkasap S. The antioxidative and antihistaminic properties of quercetin in ethanol-induced gastric lesions. Toxicology. 2003;183:133-142. |
| 17. | Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302-310. |
| 18. | Ambrosio G, Flaherty JT, Duilio C, Tritto I, Santoro G, Elia PP, Condorelli M, Chiariello M. Oxygen radicals generated at reflow induce peroxidation of membrane lipids in reperfused hearts. J Clin Invest. 1991;87:2056-2066. |
| 19. | Fagan JM, Sleczka BG, Sohar I. Quantitation of oxidative damage to tissue proteins. Int J Biochem Cell Biol. 1999;31:751-757. |
| 20. | Grisham MB, Johnson GG, Gautreaux MD, Berg RD. Measurement of nitrate and nitrite in extracellular fluids: A window to systemic nitric oxide metabolism. Methods:ACompanion to Methods in Enzymology. Academic Press: San Diego 1995; 84-90. |
| 21. | Qu LN, Yang TB, Yuan YH, Zhong P, Yang B, Zhao H. A novel competitive ELISA for both free and protein-bound nitrotyrosine. Hybrid Hybridomics. 2003;22:401-406. |
| 22. | Kim H. Oxidative stress in Helicobacter pylori-induced gastric cell injury. Inflammopharmacology. 2005;13:63-74. |
| 23. | Holian O, Wahid S, Atten MJ, Attar BM. Inhibition of gastric cancer cell proliferation by resveratrol: role of nitric oxide. Am J Physiol Gastrointest Liver Physiol. 2002;282:G809-G816. |
| 24. | Young J, McKinney SB, Ross BM, Wahle KW, Boyle SP. Biomarkers of oxidative stress in schizophrenic and control subjects. Prostaglandins Leukot Essent Fatty Acids. 2007;76:73-85. |
| 25. | Huie RE, Padmaja S. The reaction of no with superoxide. Free Radic Res Commun. 1993;18:195-199. |
| 26. | Beckman JS, Chen J, Ischiropoulos H, Crow JP. Oxidative chemistry of peroxynitrite. Methods Enzymol. 1994;233:229-240. |
| 27. | Radi R, Peluffo G, Alvarez MN, Naviliat M, Cayota A. Unraveling peroxynitrite formation in biological systems. Free Radic Biol Med. 2001;30:463-488. |
| 28. | Yoshida M, Sakai T, Hosokawa N, Marui N, Matsumoto K, Fujioka A, Nishino H, Aoike A. The effect of quercetin on cell cycle progression and growth of human gastric cancer cells. FEBS Lett. 1990;260:10-13. |
| 29. | Yoshida M, Yamamoto M, Nikaido T. Quercetin arrests human leukemic T-cells in late G1 phase of the cell cycle. Cancer Res. 1992;52:6676-6681. |
| 30. | Lane P, Gross SS. Cell signaling by nitric oxide. Semin Nephrol. 1999;19:215-229. |
| 31. | Wallace JL, Miller MJ. Nitric oxide in mucosal defense: a little goes a long way. Gastroenterology. 2000;119:512-520. |
| 32. | Stuehr DJ. Mammalian nitric oxide synthases. Biochim Biophys Acta. 1999;1411:217-230. |
| 33. | Beck KF, Eberhardt W, Frank S, Huwiler A, Messmer UK, Muhl H, Pfeilschifter J. Inducible NO synthase: role in cellular signalling. J Exp Biol. 1999;202:645-653. |
| 34. | Akiba Y, Nakamura M, Nagata H, Kaunitz JD, Ishii H. Acid-sensing pathways in rat gastrointestinal mucosa. J Gastroenterol. 2002;37 Suppl 14:133-138. |
| 35. | Kiviluoto T, Watanabe S, Hirose M, Sato N, Mustonen H, Puolakkainen P, Ronty M, Ranta-Knuuttila T, Kivilaakso E. Nitric oxide donors retard wound healing in cultured rabbit gastric epithelial cell monolayers. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1151-G1157. |
| 36. | Cao X, Tsukamoto T, Nozaki K, Tanaka H, Cao L, Toyoda T, Takasu S, Ban H, Kumagai T, Tatematsu M. Severity of gastritis determines glandular stomach carcinogenesis in Helicobacter pylori-infected Mongolian gerbils. Cancer Sci. 2007;98:478-483. |
| 37. | Wang GY, Ji B, Wang X, Gu JH. Anti-cancer effect of iNOS inhibitor and its correlation with angiogenesis in gastric cancer. World J Gastroenterol. 2005;11:3830-3833. |
| 38. | Nam SY, Kim N, Lee CS, Choi KD, Lee HS, Jung HC, Song IS. Gastric mucosal protection via enhancement of MUC5AC and MUC6 by geranylgeranylacetone. Dig Dis Sci. 2005;50:2110-2120. |