Published online May 28, 2008. doi: 10.3748/wjg.14.3207
Revised: April 16, 2008
Accepted: April 23, 2008
Published online: May 28, 2008
AIM: To find occult metastases during hepatectomy in patients with colorectal cancer liver metastases (CRCLM), contrast-enhanced intraoperative ultrasonography (CE-IOUS) was performed using a new microbubble agent, sonazoid, which provides a parenchyma-specific contrast image based on its accumulation in the Kupffer cells.
METHODS: Eight patients with CRCLM underwent CE-IOUS using sonazoid before hepatectomy. The liver was investigated during a late Kupffer-phase imaging, which is a valuable characteristic of sonazoid.
RESULTS: CE-IOUS using sonazoid provided the early vascular- and sinusoidal-phase images for
10 min followed by the late Kupffer-phase image up to 30 min after the injection of sonazoid. IOUS did not provide new findings of metastatic lesion in the 8 patients. However, during the late Kupffer-phase image of sonazoid, a metastatic lesion was newly found in two of the 8 patients. These newly detected lesions were removed by an additional hepatectomy and histopathologically diagnosed as a metastasis.
CONCLUSION: CE-IOUS using sonazoid can allow surgeons to investigate the whole liver with enough time and to find new metastases intraoperatively.
- Citation: Nakano H, Ishida Y, Hatakeyama T, Sakuraba K, Hayashi M, Sakurai O, Hataya K. Contrast-enhanced intraoperative ultrasonography equipped with late Kupffer-phase image obtained by sonazoid in patients with colorectal liver metastases. World J Gastroenterol 2008; 14(20): 3207-3211
- URL: https://www.wjgnet.com/1007-9327/full/v14/i20/3207.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.3207
Hepatic resection is the only treatment offering a chance of long-term survival to patients with colorectal cancer liver metastases (CRCLM)[1–4]. However, a total of 75% of patients with CRCLM who undergo liver resection will develop recurrence and the main site of recurrence is the liver[5]. In addition, 65% to 85% of all recurrences appear within the first 2 years[5]. Therefore, occult liver metastases may present at the time of hepatectomy and can be undetected preoperatively by computed tomography (CT), magnetic resonance image (MRI), or positron emission tomography (PET)[6].
Intraoperative ultrasound (IOUS) is now considered as a standard method to determine the resection margin or to find preoperatively undetected tumors[78]. Recently some authors reported that a contrast-enhanced IOUS (CE-IOUS) was more sensitive than conventional IOUS to identify new lesions and subsequently to influence surgical management[910].
Sonazoid (perfluorobutane, GE Helthcare, Oslo, Norway) is a new microbubble agent[11] that provides a parenchyma-specific contrast image based on its accumu-lation in the Kupffer cells in the liver[12–14]. Sonazoid was recently approved for clinical use in Japan, and it presents with a late Kupffer-phase image with a long duration following a vascular- and a sinusoidal-phase images[14]. SonoVue (Bracco spA, Milan, Italy) has been already used as a microbubble agent in CE-IOUS[910], but it does not have the Kupffer-phase image[13]. The present brief clinical report shows our experience of CE-IOUS using sonazoid in patients with CRCLM.
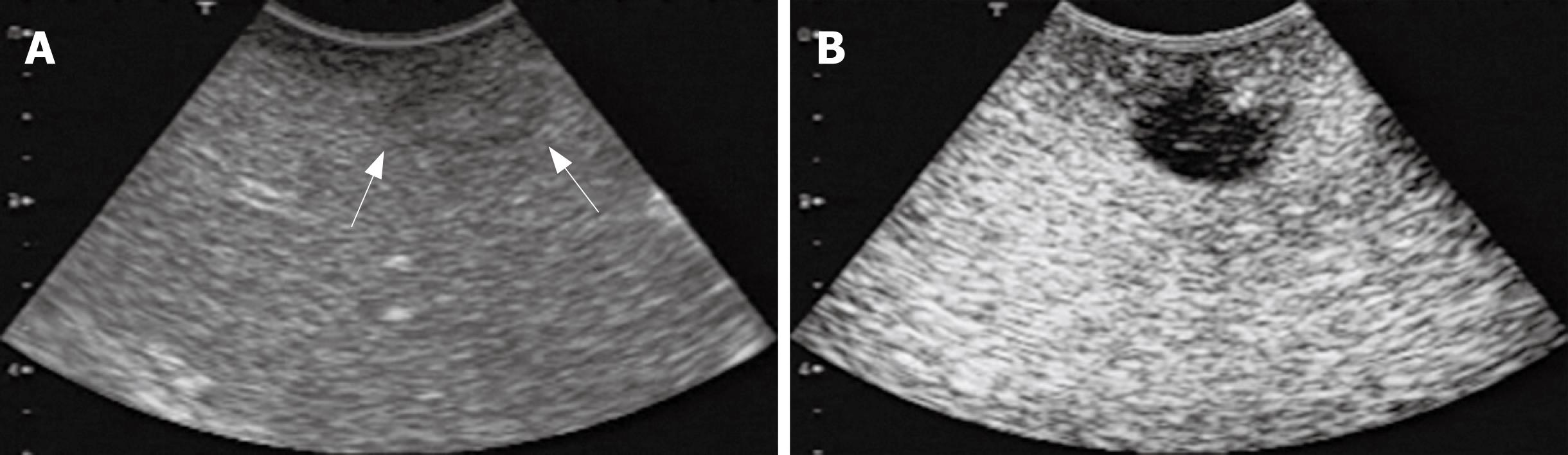
Examination of IOUS and CE-IOUS was performed using an Aplio-XV (Toshiba, Tokyo, Japan) and a micro-convex probe (PVT-375BT, 3.5 MHz, Toshiba). CE-IOUS was performed under a pulse inversion harmonic (PIH) imaging capability (Toshiba). A bolus intravenous injection of sonazoid [0.015 mL/kg body weight (0.12 &mgr;L microbubble/kg body weight as perflubutane microbubble)] was performed via the peripheral venous line followed by 10 mL of normal saline flush. Immediately after the administration of sonazoid, the portal veins, hepatic veins, and the normal liver parenchyma were uniformly enhanced. Hepatic metastases were identified as a dark contrast free filling defect during an early vascular phase image lasting 3 min after the injection of sonazoid. Approximately 10 min after the injection, the liver was scanned again to observe a late Kupffer-phase image. The hepatic metastases were identified as filling defects clearer than those observed at the vascular phase (Figure 1). The late Kupffer-phase image lasted at least for 30 min.
Eight patients with CRCLM underwent CE-IOUS in addition to IOUS. The number and size of metastases identified on preoperative CT, MR, and percutaneous contrast-enhanced ultrasonography (CE-US) were compared with those detected by IOUS and CE-IOUS.
CE-IOUS using sonazoid provided the early vascular- and sinusoidal-phase images for 10 min followed by the late Kupffer-phase image up to 30 min after the injection of sonazoid. Figure 1 shows IOUS and CE-IOUS images of a metastasis at the Segment 8. The lesion was detected as an unclear slightly hypoechoic mass by IOUS (Figure 1A), but the lesion was shown as a clear hypoechoic mass during the late Kupffer-phase (Figure 2B).
Between December 2007 and February 2008, eight patients underwent CE-IOUS. Preoperatively detected sites of liver metastases by CT, MRI, and CE-US were listed, and some differences among CT, MRI and CE-US existed as shown in Table 1. Preoperative CT seemed superior to MRI (patient No. 1 and 4). In addition, preoperative CE-US did not seem useful for finding metastases at the Segment 7 (patient No. 1, 2, and 5). Mainly based on the preoperative findings of CT, surgical methods were planned preoperatively in the eight patients (Table 2). IOUS did not provide new findings of metastatic lesion in the eight patients. Indeed, IOUS could not show some metastatic lesions detected by CT or MRI (Table 1, patient No. 1 and 2). However, CE-IOUS confirmed all hepatic lesions detected by CT or MRI. In addition, metastatic lesions were newly found by CE-IOUS in two of the eight patients. These newly detected lesions were removed by an additional hepatectomy and histopathologically diagnosed as a metastasis.
| Patient | Preoperatively diagnosed metastases | Intraoperatively found lesions | ||||
| No. | By CT | By MRI | By CE-US | By IOUS | By CE-IOUS | By CE-IOUS |
| 1 | S5, S5-6 | S5, S5-6, S7 | S5, S5-6 | S5, S5-6 | S5, S5-6, S7 | S4 |
| 2 | S3, S7 | S3, S7 | S3 | S3 | S3, S7 | S6 |
| 3 | S6, S6 | S6, S6 | S6, S6 | S6, S6 | S6, S6 | (-) |
| 4 | S6-7, S1 | S6-7 | S6-7, S1 | S6-7, S1 | S6-7, S1 | (-) |
| 5 | S7 | S7 | (-) | S7 | S7 | (-) |
| 6 | S7 | S7 | S7 | S7 | S7 | (-) |
| 7 | S7-6, S3 | S7-6, S3 | S7-6, S3 | S7-6, S3 | S7-6, S3 | (-) |
| 8 | S3, S4, S8 | S3, S4, S8 | S3, S4, S8 | S3, S4, S8 | S3, S4, S8 | (-) |
| Patient No. | Preoperatively planned surgery | Mainly performed operative procedures | Methods of additional surgery based the findings of CE-IOUS |
| 1 | Enucleations at S5, S5-S6, and S7 | Bisegmentectomy of S5 and S6 | Enucleation at S4 |
| Enucleation at S7 | |||
| 2 | Left lateral sectionectomy, partial resection of S7 | Left lateral sectionectomy, partial resection of S7 | Enucleation at S6 |
| 3 | S6 segmentectomy | S6 segmentectomy | (-) |
| 4 | Right posterior sectionectomy, S1 partial resection | Right posterior sectionectomy, S1 partial resection | (-) |
| 5 | S7 partial resection | S7 partial resection | (-) |
| 6 | S7 segmentectomy | S7 segmentectomy | (-) |
| 7 | Posterior sectionectomy, left lateral sectionectomy | Posterior sectionectomy, left lateral sectionectomy | (-) |
| 8 | Enucleations at S3, S4, and S8 | Enucleations at S3, S4, and S8 | (-) |
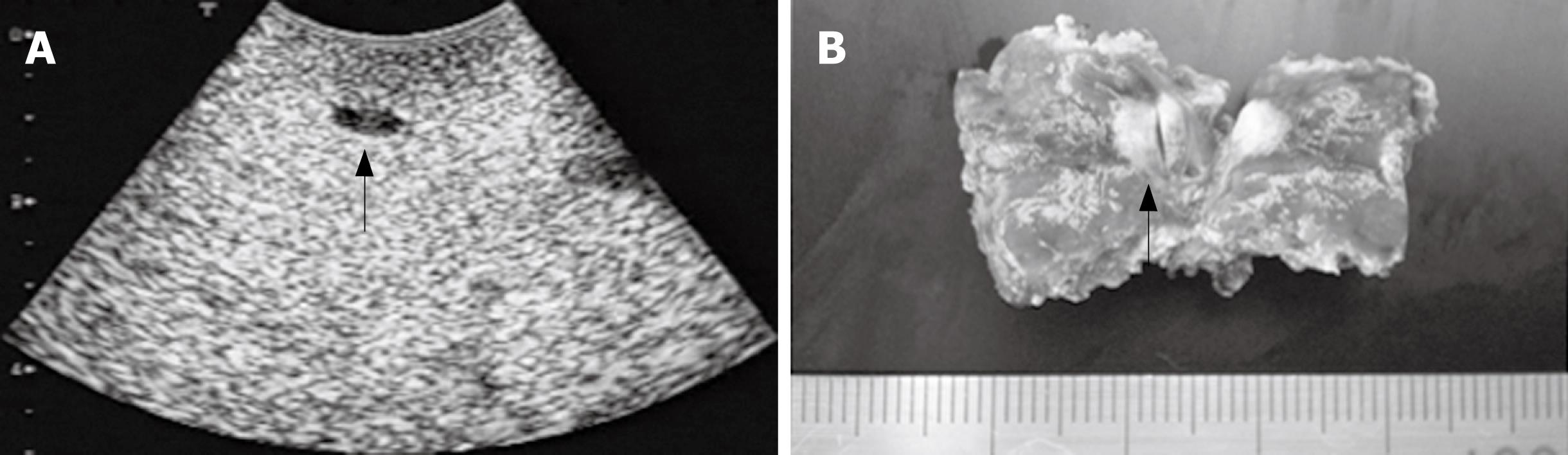
In the patient No. 1 (Table 1), a small hypoechoic lesion with 6 mm in diameter at the Segment 4 was newly detected by the CE-IOUS at the late Kupffer-phase view (Figure 2A) although IOUS did not show this lesion. This small lesion was resected and histopathologically confirmed as a metastatic nodule (Figure 2C).
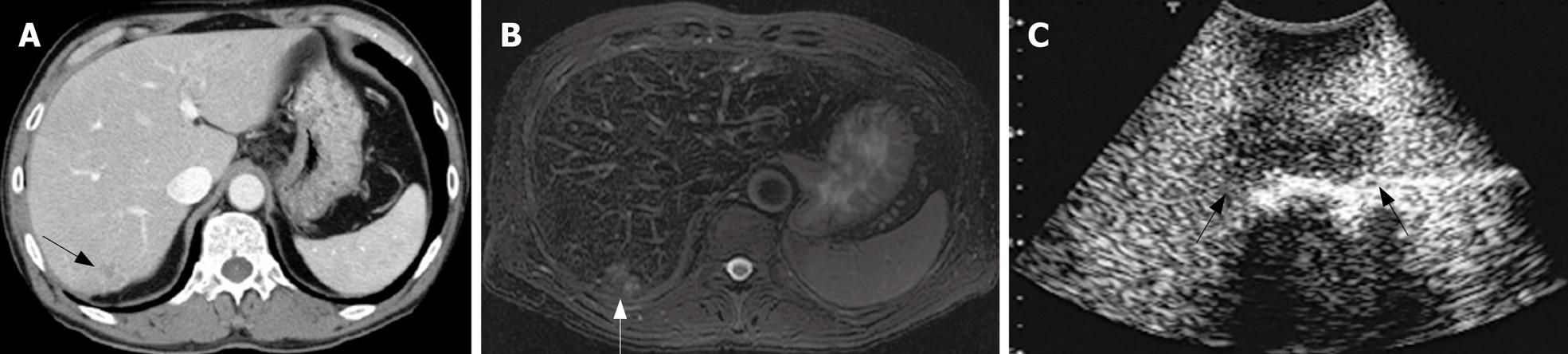
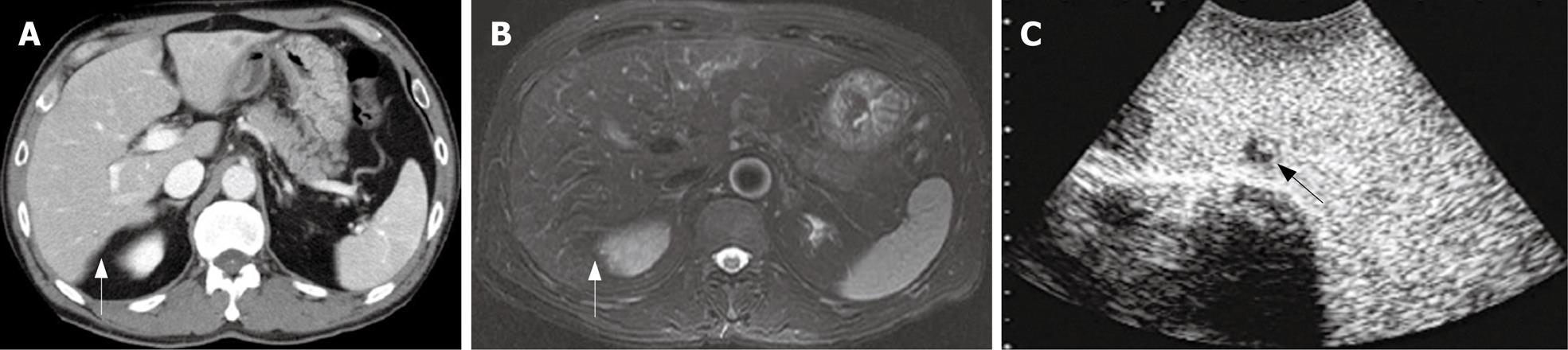
In the patient No. 2 who preoperatively presented with liver metastases at the Segment 3 (Table 1), another lesion at the Segment 7 was pointed out as a metastasis with an ill-defined mass by preoperative CT and MRI (Figure 3A and B). Preoperative percutaneous CE-US using sonazoid could not show the lesion at the Segment 7 because of the attenuation of echogenecity. During the surgery, IOUS did not show the metastasis at the segment 7, but CE-IOUS showed a well-demarcated mass at the Segment 7 (Figure 3C). This lesion was resected by a partial hepatectomy and histopathologically confirmed as a metastasis. In addition, CE-IOUS detected a new small lesion at the Segment 6 which was not pointed out by CT or MRI preoperatively (Figure 4). This lesion at the Segment 6 was also removed and histopathologically confirmed as an occult metastasis.
The importance of CE-IOUS in patients with CRCLM has been shown by two recent studies[910]. Indeed, Torzilli et al reported that new metastatic lesions, which were not detected by preoperative examinations and IOUS, were detected in 5 out of 24 patients (21%) using CE-IOUS[9]. They also reported that the modification rate of hepatectomy by CE-IOUS alone was 21% in the patients with CRCLM. Leen et al showed that additional new hepatic metastases were detected in 11 out of 57 patients (19%) and the planned surgical methods were converted in these patients[10]. In the present study, an occult metastatic lesion was newly detected in two of the eight patients using CE-IOUS and removed by an additional hepatectomy. These metastatic lesions were not detected by preoperative CT, MRI, preoperative percutaneous CE-US, or IOUS.
Sonazoid is a novel microbubble-based ultrasound contrast agent, and is classified as a second-generation agent in which the perfluorocarbon gas has enough intravascular stability in vivo[1516]. Watanabe et al showed that microbubbles of sonazoid were taken up by Kupffer cells immediately after intravenous injection and existed as microbubbles for 30 min within Kupffer cells, and that the hepatic parenchyma-specific contrast by sonazoid was due to the distribution of the microbubbles in Kupffer cells[14]. Therefore, sonazoid has a unique “late Kupffer-phase image” in addition to “early-vascular phase image” and “sinusoidal-phase image”. This late Kupffer-phase image can provide high echogenic contrast enhancement in the liver parenchyma[14]. On the other hand, other microbubble contrast agents such as Imavist and SonoVue provide parenchyma-specific contrast by transient mechanical slowdown of microbubbles within the sinusoid, and these two contrast agents are hardly phagocytosed by the Kupffer cells[101317]. Therefore, Imavist and SonoVue cannot provide the late Kupffer-phase image, and the parenchyma-specific contrast image of these two microbubble agents can be seen during only 3 min to 5 min after the injection. Therefore, as previously described, SonoVue needs repeated injections during CE-IOUS to perform a whole liver examination[910]. Therefore, sonazoid seems a superior microbubble contrast agent for CE-IOUS in patients with CRCLM since a whole precise liver investigation by CE-IOUS, in which determination of surgical margin and examination of occult metastases should be investigated. Therefore, CE-IOUS needs more than 5 min, and the present brief clinical experience of CE-IOUS confirmed the usefulness of sonazoid during surgery in patients with CRCLM. In addition, the duration of the approximately 30 min of the late-Kupffer phase image using sonazoid seems useful to perform preoperative percutaneous CE-US compared to SonoVue because the limiting time of SonoVue image (5 min) does not seem convenient to perform preoperative CE-US. Indeed, a routine preoperative CE-US in our institution, in which only the late Kupffer-phase image is performed, can be performed between 10 min and 30 min after the injection of sonazoid. However, based on our experience, small metastases at the Segment 7 seem hardly visualized by percutaneous CE-US using sonazoid because of the attenuation of echogenecity as shown in the present case No. 2.
Sonazoid has been reported as a safe medicine. Indeed, the incidence of adverse effects of sonazoid was shown in 25 out 397 patients (6.3%) in a clinical phase II study performed in Japan. The main side effects were headache (1.0%) and diarrhea (1.0%), but no anaphylactic shock due to sonazoid was reported unlike with contrast-enhanced CT. The image mechanism of CE-IOUS using sonazoid seems similar to superpara-magnetic iron oxide-enhanced magnetic resonance image (SPIO-MRI) because both images are based on the phagocytosis by Kupffer cells. However, sonazoid is much less expensive compared to SPIO-MRI. Regardless of sensitivity rate of sonazoid for detecting small metastases compared to SPIO-MRI, CE-IOUS is useful to perform intraoperative liver biopsy of newly detected lesions and to determine an additional hepatectomy.
In conclusion, CE-IOUS using sonazoid can allow surgeons to investigate the whole liver with enough time (at least 30 min of the late Kupffer-phase image) and to find new metastases intraoperatively.
Contrast-enhanced intraoperative ultrasonography (CE-IOUS) seems more sensitive than conventional IOUS to identify new occult lesions during hepatectomy in patients with colorectal cancer liver metastases (CRCLM). Sonazoid (perfluorobutane, GE Helthcare, Oslo, Norway) is a new microbubble agent that provides late Kupffer-phase image, which cannot be obtained by conventional contrast mediums.
No study has investigated the intraoperative efficacy of the late Kupffer-phase image of sonazoid in patients with CRCLM.
CE-IOUS using sonazoid enabled whole liver investigation at least for 30 min of the late Kupffer-phase image. Occult metastases, which had not been detected preoperatively, were newly found in some patients and removed by an additional hepatectomy.
CE-IOUS using sonazoid can reduce intrahepatic recurrence after hepatectomy in patients with CRCLM.
This article presented the clinical significance of CE-IOUS using sonazoid during hepatectomy for colorectal cancer liver metastases. CE-IOUS for detection of liver metastases requires stable image for enough time to perform repeated whole liver scans. Sonazoid seems to be suitable for this purpose. This article is worthy for publication in WJG with minor revision.
| 1. | Nordlinger B, Jaeck D, Guiguet M, Vaillant JC, Balladur P. Surgical resection of hepatic metastases: Multicentric retrospective study by the French Association of Surgery. Treatment of hepatic metastases of colorectal cancer. Springer-Verlag: Paris 1992; 129-146. |
| 2. | Adam R, Pascal G, Azoulay D, Tanaka K, Castaing D, Bismuth H. Liver resection for colorectal metastases: the third hepatectomy. Ann Surg. 2003;238:871-883; discussion 883-884. |
| 3. | Jaeck D, Bachellier P, Nakano H, Oussoultzoglou E, Weber JC, Wolf P, Greget M. One or two-stage hepatectomy combined with portal vein embolization for initially nonresectable colorectal liver metastases. Am J Surg. 2003;185:221-229. |
| 4. | Weber JC, Nakano H, Bachellier P, Oussoultzoglou E, Inoue K, Shimura H, Wolf P, Chenard-Neu MP, Jaeck D. Is a proliferation index of cancer cells a reliable prognostic factor after hepatectomy in patients with colorectal liver metastases? Am J Surg. 2001;182:81-88. |
| 5. | Fong Y, Cohen AM, Fortner JG, Enker WE, Turnbull AD, Coit DG, Marrero AM, Prasad M, Blumgart LH, Brennan MF. Liver resection for colorectal metastases. J Clin Oncol. 1997;15:938-946. |
| 6. | Finlay IG, McArdle CS. The role of occult hepatic metastases in staging colorectal carcinoma. Scand J Gastroenterol Suppl. 1988;149:150-154. |
| 7. | Torzilli G, Montorsi M, Donadon M, Palmisano A, Del Fabbro D, Gambetti A, Olivari N, Makuuchi M. "Radical but conservative" is the main goal for ultrasonography-guided liver resection: prospective validation of this approach. J Am Coll Surg. 2005;201:517-528. |
| 8. | Jarnagin WR, Bach AM, Winston CB, Hann LE, Heffernan N, Loumeau T, DeMatteo RP, Fong Y, Blumgart LH. What is the yield of intraoperative ultrasonography during partial hepatectomy for malignant disease? J Am Coll Surg. 2001;192:577-583. |
| 9. | Torzilli G, Del Fabbro D, Palmisano A, Donadon M, Bianchi P, Roncalli M, Balzarini L, Montorsi M. Contrast-enhanced intraoperative ultrasonography during hepatectomies for colorectal cancer liver metastases. J Gastrointest Surg. 2005;9:1148-1153; discussion 1153-1154. |
| 10. | Leen E, Ceccotti P, Moug SJ, Glen P, MacQuarrie J, Angerson WJ, Albrecht T, Hohmann J, Oldenburg A, Ritz JP. Potential value of contrast-enhanced intraoperative ultrasonography during partial hepatectomy for metastases: an essential investigation before resection? Ann Surg. 2006;243:236-240. |
| 11. | Forsberg F, Piccoli CW, Liu JB, Rawool NM, Merton DA, Mitchell DG, Goldberg BB. Hepatic tumor detection: MR imaging and conventional US versus pulse-inversion harmonic US of NC100100 during its reticuloendothelial system-specific phase. Radiology. 2002;222:824-829. |
| 12. | Kindberg GM, Tolleshaug H, Roos N, Skotland T. Hepatic clearance of Sonazoid perfluorobutane microbubbles by Kupffer cells does not reduce the ability of liver to phagocytose or degrade albumin microspheres. Cell Tissue Res. 2003;312:49-54. |
| 13. | Yanagisawa K, Moriyasu F, Miyahara T, Yuki M, Iijima H. Phagocytosis of ultrasound contrast agent microbubbles by Kupffer cells. Ultrasound Med Biol. 2007;33:318-325. |
| 14. | Watanabe R, Matsumura M, Munemasa T, Fujimaki M, Suematsu M. Mechanism of hepatic parenchyma-specific contrast of microbubble-based contrast agent for ultrasonography: microscopic studies in rat liver. Invest Radiol. 2007;42:643-651. |
| 15. | Hagen EK, Forsberg F, Aksnes AK, Merton DA, Liu JB, Tornes A, Johnson D, Goldberg BB. Enhanced detection of blood flow in the normal canine prostate using an ultrasound contrast agent. Invest Radiol. 2000;35:118-124. |
| 16. | Yao J, Teupe C, Takeuchi M, Avelar E, Sheahan M, Connolly R, Ostensen J, Pandian NG. Quantitative 3-dimensional contrast echocardiographic determination of myocardial mass at risk and residual infarct mass after reperfusion: experimental canine studies with intravenous contrast agent NC100100. J Am Soc Echocardiogr. 2000;13:570-581. |