Published online Jan 14, 2008. doi: 10.3748/wjg.14.307
Revised: October 13, 2007
Published online: January 14, 2008
AIM: To evaluate the arguments for and against the possible roles of H pylori in hepatocellular carcinoma (HCC).
METHODS: We performed a systematic review of all relevant studies published in the literature. A total of 103 clinical trials and reports were identified, but only 10 trials qualified under our selection criteria. A meta-analysis was carried out by a biostatistician according to the Cochrane Reviewers’ Handbook recommended by The Cochrane Collaboration.
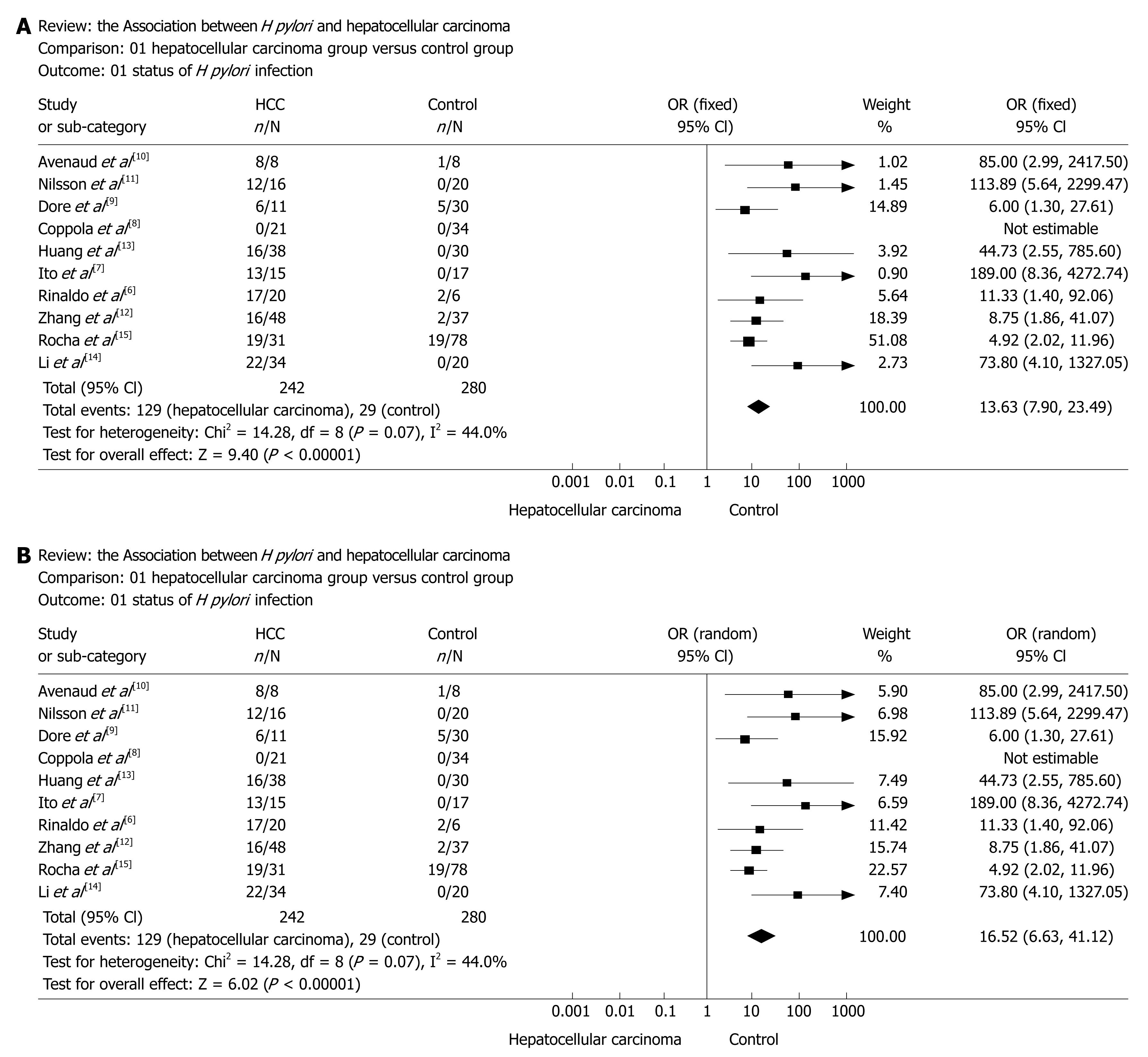
RESULTS: Nine case-control studies and one retrospective cross sectional study were included in the final analysis. Overall the prevalence of H pylori infection was 53.3% (129 of 242) in cases and 10.4% (29 of 280) in controls, and the summary odds ratio for the association of H pylori infection with the risk for HCC (using the fixed-effects model, which accounted for the homogeneity across the 10 studies) was determined to be 13.63 (95% CI, 7.90-23.49).
CONCLUSION: Our analysis showed a positive association between H pylori infection and the risk of HCC, with an indication of possible publication bias and possible confounders due to study designs that showed results of less pronounced associations.
-
Citation: Xuan SY, Xin YN, Chen AJ, Dong QJ, Qiang X, Li N, Zheng MH, Guan HS. Association between the presence of
H pylori in the liver and hepatocellular carcinoma: A meta-analysis. World J Gastroenterol 2008; 14(2): 307-312 - URL: https://www.wjgnet.com/1007-9327/full/v14/i2/307.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.307
The profound impact of hepatocellular carcinoma (HCC) on human health is known worldwide[1]. Persistent hepatitis B virus (HBV) and hepatitis C virus (HCV) infections and aflatoxins are the main causes of HCC[2]. The real risk factors for HCC may be far more numerous than the known causes. The infectious agent Helicobacter hepaticus (H hepaticus), for example, first described by Ward et al in 1994, has recently received new attention for its role in causing chronic active hepatitis and associated liver tumors l[3].
Since H pylori was first cultivated from a human gastric biopsy specimen in 1982, it has become apparent that H pylori infection is correlated with gastric cancer and mucosa-associated lymphoid tissue lymphoma. More recently, researchers have reported that Helicobacter spp. have been identified in liver tissue resected from patients with HCC[4]. Experimental infection by Helicobacter hepaticus in mice causes chronic hepatitis and HCC. It is highly noteworthy that H pylori was found in liver tissues resected from patients with HCC[5–7].
The question of whether H pylori could play a role in the development of HCC remains controversial. Many conflicting reports have been published to date; thus, we performed a systematic rev iew of all of the relevant studies published in the literature to evaluate the arguments for and against the possible roles of H pylori in HCC.
We searched different databases, including the Cochrane Controlled Trials Register on The Cochrane Library Issue 1, 2007, MEDLINE (January, 1989-March, 2007), EMBASE.com (January, 1989-March, 2007) and the China Biological Medicine Database (CBMdisc) (January, 1989-March, 2007), for the terms ‘hepatocellular carcinoma’ and ‘H pylori’. The reference lists of pertinent reviews and retrieved articles were also checked for the identification of additional studies. We also performed a full manual search from the bibliographies of selected papers. Studies were identified by two researchers, independently; the two lists were compared and discrepancies were resolved. We also contacted the authors of studies containing relevant information, who did not report the results necessary for this analysis. Unpublished data were also accepted if an abstract was available and further information was obtained from the author.
In the meta-analysis, the following inclusive selection criteria were set and reviewed by two independent investigators: (1) each trial is an independent case-controlled study; (2) the purpose of all studies and statistical methods is similar; (3) the numbers of cases, controls and positive rate of helicobacter (the presence of H pylori DNA sequences in human liver or other standard methods for definition of H pylori infection in the liver) are concrete; (4) the study groups have definite HCC by pathology, histology and operation; (5) only studies that selected HCC patients as cases and subjects with other liver diseases or normal liver tissue specimens as controls are included.
The following exclusive selection criteria were set: (1) incomplete raw data; (2) repetitive reports (if more than one version of the same study was retrieved, only the most recent is used); (3) the positive rate of helicobacter is not detected by the presence of H pylori DNA sequences or other standard methods for detection of H pylori infection in liver (that is, all subjects are tested for the presence in serum of IgG antibodies against H pylori).
A total of 103 clinical trials and reports were identified and only 10 trials[6–15] qualified on the basis of our selection criteria. The studies were independently evaluated by two researchers. Discrepancies in the evaluations of some studies were resolved by discussion between the reviewers. The main features of the trials included in the meta-analysis are shown in Table 1.
| Reference | Country, year of publication | H pyloripositivity/total subjects | Type of controls (n) | Age range (mean), yr | ||
| Cases | Controls | Cases | Controls | |||
| Pellicano et al[6] | Italy, 2004 | 17/20 | 2/6 | Metastatic cancer (n = 6) | NA | NA |
| Ito et al[7] | Japan, 2004 | 13/15 | 0/17 | Cirrhotic liver tissue specimens (n = 10), normal liver tissue specimens (n = 7) | 36-73 (59.2) | NA |
| Coppola et al[8] | Italy, 2003 | 0/21 | 0/34 | Metastatic liver carcinoma (n = 7), chronic hepatitis (n = 27) | NA | NA |
| Dore et al[9] | Italy, 2002 | 6/11 | 5/30 | Chronic viral hepatitis without (n = 18) or with (n = 12) cirrhosis | 19-78 (54.9) | 49-78 (65.2) |
| Avenaud et al[10] | France, 2000 | 8/8 | 1/8 | Patients without primary liver carcinoma (n = 8) | NA | NA |
| Nilsson et al[11] | Sweden, 2001 | 12/16 | 0/20 | Metastatic liver carcinoma (n = 20) | NA | NA |
| Zhang et al[12] | China, 2004 | 16/48 | 2/37 | Liver cirrhosis (n = 12), pericacinomatous tissues (n = 10), benign tumor of liver (n = 9), chronic hepatitis (n = 6) | 25-67 (46.5) | 35-65 (42.5) |
| Huang et al[13] | China, 2004 | 16/38 | 0/30 | Liver cirrhosis (n = 15), benign tumor of liver (n = 15) | NA | NA |
| Li N et al[14] | China, 2006 | 22/34 | 0/20 | Liver external injury (n = 5) giant hemangioma (n = 5), macrosis hepatic cyst (n = 3), ntrahepatic bile duct stone (n = 7) | 28-71 (52) | 30-68 (48) |
| Rocha et al[15] | France, 2005 | 19/31 | 19/78 | Non-cirrhotic chronic hepatitis C (n = 24), HCV Positive cirrhosis without HCC (n = 29), HCV Positive cirrhosis and HCC (n = 25) | NA | NA |
Data extracted included year of publication, country of origin, number of cases and controls, characteristics of controls, age of participants, prevalence of H pylori infection in cases and controls, and reported odds ratios (OR). All available studies were reviewed by two investigators independently. Reference 18 is a retrospective cross-sectional study; the others are case-controlled studies.
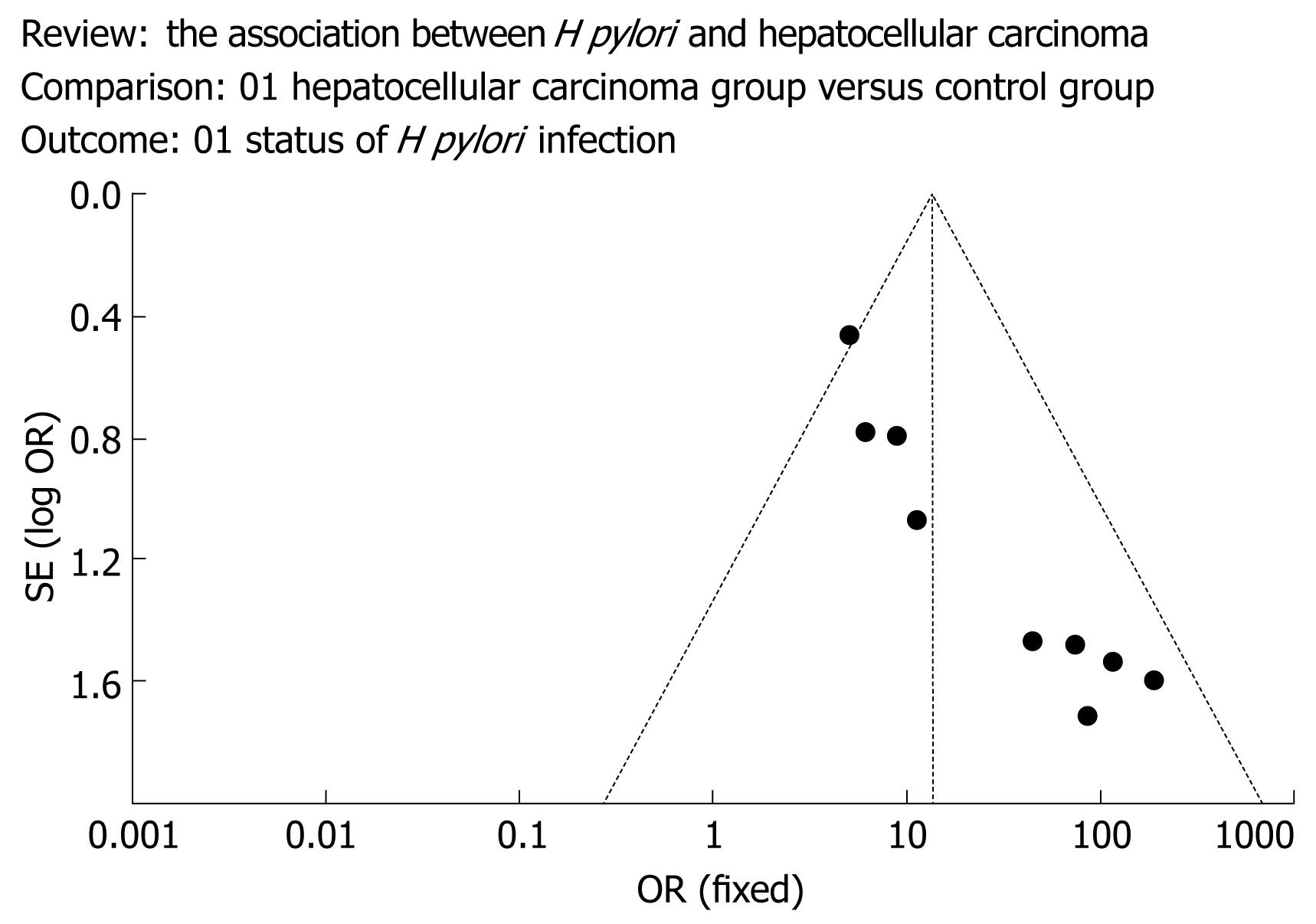
The meta-analysis was carried out by a biostatistician (Chen AJ) according to the Cochrane Reviewers’ Handbook recommended by The Cochrane Collaboration. First, a pooled OR was calculated using the fixed-effect model. The heterogeneity of the studies was examined using the DL Q statistic[16]. Because the results were homogeneous (P = 0.07), a fixed-effects model was employed using the DerSimonian and Laird (DL) methods. A pooled OR was presented as a standard plot with 95 percent confidence intervals (CI). Begg and Mazumdar’s proposed adjusted rank correlation test[17] and Egger’s linear regression approach[10] were used to measure publication bias, which was shown as a funnel plot (Figure 1). Fixed (Figure 2A) and random-effects models (Figure 2B) were also used to perform sensitivity-analysis to assess the reliability of meta-analysis. The statistical package RevMan version 5.0 (provided by The Cochrane Collaboration, Oxford, England) was used for statistical analyses.
Nine case-controlled studies and one retrospective cross-sectional study were were identified and reviewed, as shown in Table 1[6–15].
In the meta-analysis, the overall prevalence of H pylori infection was 53.3% (129 of 242) in cases and 10.4% (29 of 280) in controls, and a summary OR for the association of H pylori infection with the risk for hepatocellular carcinoma (using the fixed-effects model, which accounted for the homogeneity across the 10 studies) was determined to be 13.63 (95% CI, 7.90-23.49) (Figure 2A). Figure 2A shows the ORs and 95% CIs of each study, and the summary OR determined by meta-analysis. The proportion of the total variation in study estimates, because of heterogeneity, was 44.0% (heterogeneity test statistics χ2 = 14.28, on 8 df, P = 0.07, I2 = 44.0%). A random-effects model was also used to perform sensitivity-analysis to assess the reliability of meta-analysis, as shown in Figure 2B.
Publication bias was assessed for all pooled ORs with confidence intervals using Begg’s test[1718]. This bias is shown as a funnel plot in Figure 1.
The funnel plot method was used to assess the possible presence of publication bias[1920]. It consists of plotting each study’s OR on a logarithmic scale (horizontal axis) against its SE (vertical axis), with an informal visual examination of the funnel plot graph to check for funnel plot asymmetry as an indication of potential publication bias. Studies of smaller size normally have a wider distribution of results than larger studies, because of a higher degree of random variation, and the ORs scatter more widely at the bottom of such a graph, with the spread narrowing with increasing precision among larger studies. In the absence of a publication bias, the graph appears as a symmetrical inverted funnel, as the risk estimates should be symmetrically distributed around the midpoint value (that is, the summary OR). Publication bias may occur if smaller studies showing no significant results remain unpublished, leading to an asymmetrical appearance of the funnel plot with a gap at the bottom of the graph.
H pylori infection is a classical model with which to study cancer development as a consequence of chronic inflammation. The estimated total of infection-attributed malignancies per year is 1.9 million cases or 17.8% of the global cancer burden[21]. Among the principal carcinogenic agents, H pylori is a leading factor, being responsible for 5.5% of all cancers. H pylori was classified as a type I carcinogen by the International Agency for Research on Cancer in 1994[22]. A striking finding indicated by Ward et al is that bacterial infection of the liver in healthy A/JCr male mice is capable of inducing a strong inflammatory change in the parenchyma (for example, hepatitis) leading to HCC.
We analyzed the published evidence investigating the association between H pylori infection and HCC. Studies concerning this possible association have been undertaken since the early 2000s. To our knowledge, this is the first published meta-analysis investigating this association. The summary OR for the association of evidence of H pylori infection and the risk for HCC was estimated to be 13.63 with a 95% CI from 7.90 to 23.49, with a confound of study design (lower for studies of prospective design and higher for retrospective case-controlled studies).
In our study, only publications in English or Chinese were used for evaluation. ‘Meta-analytical’ research on 29 meta-analyses investigating language bias has provided evidence that the OR estimated in meta-analyses from non-English publications are on average 0.8-fold (95% CI, 0.7-1.0) the OR estimates from English-written publications[23]. Therefore, even if we had not searched for non-English publications, this might have introduced only a small bias in the overall findings, which, in our opinion, would not have altered our main conclusions.
Several other points should be considered when interpreting the results of our study.
First, the positive rate of helicobacter of the most studies was detected by the presence of H pylori DNA sequences. Polymerase chain reaction (PCR) amplification using two sets of primers located in the 16S ribosomal DNA (rDNA) was used to detect the presence of bacteria, but this is not a ‘gold standard’ method for detecting H pylori infection in the liver. Histology with standard stains and culturing maybe more precise than 16S rDNA; however, false negatives are likely. Research in this area has been limited by the lack of a gold standard for the diagnosis of these organisms in the liver. Most published data to date have been based on molecular techniques that detect the DNA of Helicobacter species in liver tissues, rather than evidence of viable organisms in the liver.
Secondly, Reference 18 is a retrospective cross-sectional study, whereas the other studies are case-controlled studies. Such studies are generally lower in quality for use as prospective design studies and higher in quality as retrospective case-controlled studies. These observational studies are more prone to bias than randomized clinical trial (RCT) studies.
Third, in this analysis, graphical and statistical methods for testing and adjusting for a possible publication bias and a test for potential heterogeneity between studies were performed. A graphical funnel plot of the 10 published studies was asymmetrical, which may suggest the probable existence of publication bias, although this may occur if some studies showing no significant results remained unpublished or if such negative studies are few.
Additionally, because the information used in our research was based on data from observational studies, the characteristics of each study population and the different methodologies of these studies should be taken into account when interpreting the results of our analysis. For example, different inclusion criteria for selection of the participants might have influenced the results of this research. Differences in the age distribution, different countries and different types of control groups (cirrhotic patients, patients with chronic viral hepatitis without or with cirrhosis, patients without primary liver carcinoma, patients with metastatic liver carcinoma, pericacinomatous tissues, benign tumors of the liver, liver external injuries, giant hemangiomas, macrosis hepatic cysts, and intrahepatic bile duct stones) could also be among the potential causes of variation in the studies’ estimates.
Most studies did not control for the matching variables in the analysis, and the risk for HCC was not controlled for possible confounders such as HBV or HCV, except in Reference 18.
Only the articles of Dore et al[9] and Rocha et al[15] demonstrated the association of Helicobacter species with hepatitis C cirrhosis and HCC. These two studies included a large series of patients and examined both tumor and cirrhotic liver tissue samples from patients with HCV-positive HCC. Helicobacter DNA was found in a small percentage of liver biopsies from controls as well as from patients with chronic hepatitis C (4.2% and 3.5%, respectively). However, the prevalence of Helicobacter species was high in patients with HCV-positive cirrhosis and in those with cirrhosis and HCC (68% and 61%, respectively). In nearly all cancer tissues, Helicobacter DNA was detected and identified as Helicobacter pullorum or H pylori-like organisms. The authors suggested a possible causal role of these bacteria in the progression of chronic hepatitis C and the development of HCC.
Furthermore, although we tried to maximize our efforts to identify all relevant published studies in peer-reviewed journals, it is possible that some escaped our attention.
There is some evidence to suggest that Helicobacter infection may be associated with an increased risk of extra-gastric malignancies[24]. The presence of Helicobacter species in liver tissues from patients with different liver diseases, including hepatic neoplasias, has been reported by numerous authors. The most intriguing hypothesis is that these bacteria might play a role in the development of HCC[25].
Despite its limitations, the present analysis has some implications: as relatively few studies are available in this field and current evidence remains limited, the necessity to conduct large studies with an adequate methodological quality, properly controlling for possible confounds in order to obtain valid results, should be emphasized; for example, tissue from unaffected liver metastatic carcinoma could be used as a suitable control.
In conclusion, our analysis showed a positive association between H pylori infection and the risk of HCC. We obtained from our meta-analysis a summary OR of 13.63 for the association of H pylori infection and HCC, with an indication of possible publication bias and confounds of study design, with less pronounced associations in prospective studies than in retrospective studies. Therefore, this risk increase should be interpreted with caution. Better designed and better controlled studies are needed to clarify the strength of this association and the possible causal role of H pylori infection in patients with HCC, and further prospective studies are required to prove this hypothesis. Given the importance of this potential association, further verification is warranted.
The real risk factors for hepatocellular carcinoma (HCC) may be far more than the known causes. A new infectious agent, Helicobacter hepaticus (H hepaticus), causing chronic active hepatitis and associated liver tumors, has been described by Ward et al. The question of whether H pylori could play a role in the development of HCC remains controversial. Many conflicting reports have been published to date, so we performed a systematic review of all of the relevant studies published in the literature to evaluate the arguments for and against the possible roles of H pylori in HCC.
Since H pylori was first cultivated from human gastric biopsy specimens in 1982, it has become apparent that H pylori infection is correlated with gastric cancer and mucosa-associated lymphoid tissue lymphoma. More recently, researchers have reported that Helicobacter spp. were identified in liver tissue resected from patients with HCC. Experimental infection by Helicobacter hepaticus in mice causes chronic hepatitis and HCC. It is highly noteworthy that H pylori was found in liver tissue resected from patients with HCC.
To our knowledge, this is the first published meta-analysis investigating the association between H pylori and hepatocellular carcinoma risk.
Our analysis showed a positive association between H pylori infection and the risk of hepatocellular carcinoma, with an indication of possible publication bias and confound of study design, with less pronounced associations in prospective studies than in retrospective studies. Therefore, this risk increase should be interpreted with caution; better designed and better controlled studies are needed to clarify the strength of the association and the possible causal role of H pylori infection in hepatocellular carcinoma. Further prospective studies are requested to prove this hypothesis; given the importance of this potential association, further verification is warranted.
Meta analysis: In statistics, a meta-analysis combines the result of several studies that address a set of related research hypotheses. H pylori: H pylori is a bacteria that can cause digestive illnesses, including gastritis and peptic ulcer disease. More recently, researchers have reported that Helicobacter spp. were identified in liver tissue resected from patients with hepatocellular carcinoma.
Authors evaluated the arguments for and against the possible roles of H pylori in HCC through a systematic review of all relevant studies published in the literature. The analysis showed a positive association between H pylori infection and the risk of HCC.
| 1. | Sun HC, Tang ZY. Preventive treatments for recurrence after curative resection of hepatocellular carcinoma--a literature review of randomized control trials. World J Gastroenterol. 2003;9:635-640. |
| 3. | Ward JM, Fox JG, Anver MR, Haines DC, George CV, Collins MJ Jr, Gorelick PL, Nagashima K, Gonda MA, Gilden RV. Chronic active hepatitis and associated liver tumors in mice caused by a persistent bacterial infection with a novel Helicobacter species. J Natl Cancer Inst. 1994;86:1222-1227. |
| 4. | Verhoef C, Pot RG, de Man RA, Zondervan PE, Kuipers EJ, IJzermans JN, Kusters JG. Detection of identical Helicobacter DNA in the stomach and in the non-cirrhotic liver of patients with hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2003;15:1171-1174. |
| 5. | Huang Y, Fan XG, Wang ZM, Zhou JH, Tian XF, Li N. Identification of helicobacter species in human liver samples from patients with primary hepatocellular carcinoma. J Clin Pathol. 2004;57:1273-1277. |
| 6. | Pellicano R, Mazzaferro V, Grigioni WF, Cutufia MA, Fagoonee S, Silengo L, Rizzetto M, Ponzetto A. Helicobacter species sequences in liver samples from patients with and without hepatocellular carcinoma. World J Gastroenterol. 2004;10:598-601. |
| 7. | Ito K, Nakamura M, Toda G, Negishi M, Torii A, Ohno T. Potential role of Helicobacter pylori in hepatocarcinogenesis. Int J Mol Med. 2004;13:221-227. |
| 8. | Coppola N, De Stefano G, Marrocco C, Scarano F, Scolastico C, Tarantino L, Rossi G, Battaglia M, Onofrio M, D'Aniello F. Helicobacter spp. and liver diseases. Infez Med. 2003;11:201-207. |
| 9. | Dore MP, Realdi G, Mura D, Graham DY, Sepulveda AR. Helicobacter infection in patients with HCV-related chronic hepatitis, cirrhosis, and hepatocellular carcinoma. Dig Dis Sci. 2002;47:1638-1643. |
| 10. | Avenaud P, Marais A, Monteiro L, Le Bail B, Bioulac Sage P, Balabaud C, Megraud F. Detection of Helicobacter species in the liver of patients with and without primary liver carcinoma. Cancer. 2000;89:1431-1439. |
| 11. | Nilsson HO, Mulchandani R, Tranberg KG, Stenram U, Wadstrom T. Helicobacter species identified in liver from patients with cholangiocarcinoma and hepatocellular carcinoma. Gastroenterology. 2001;120:323-324. |
| 12. | Zhang SQ, Bao Y, Zu MH. The correlation between Helicobacter infection and hepatocellular carcinoma. Zhongguo Zhongliu Linchuang. 2004;31:761–764. |
| 13. | Huang Y, Fan XG, Zhou JH. Immunohistochemistry of Helicobacter pylori in primary liver carcinoma tissues. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2004;29 Feb:15-17. |
| 14. | Li N, Zhang SH, Xuan SY, Qiang X. Study on Helicobacter infection in liver tissue from hepatocellular carcinoma. Zhonghua Liuxingbingxue Zazhi. 2006;27:894-896. |
| 15. | Rocha M, Avenaud P, Menard A, Le Bail B, Balabaud C, Bioulac-Sage P, de Magalhaes Queiroz DM, Megraud F. Association of Helicobacter species with hepatitis C cirrhosis with or without hepatocellular carcinoma. Gut. 2005;54:396-401. |
| 16. | DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177-188. |
| 17. | Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088-1101. |
| 18. | Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629-634. |
| 19. | Sterne JA, Egger M, Smith GD. Systematic reviews in health care: Investigating and dealing with publication and other biases in meta-analysis. BMJ. 2001;323:101-105. |
| 20. | Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54:1046-1055. |
| 21. | Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118:3030-3044. |
| 22. | Schistosomes , liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7-14 June 1994. IARC Monogr Eval Carcinog Risks Hum. 1994;61:1-241. |
| 23. | Sterne JA, Juni P, Schulz KF, Altman DG, Bartlett C, Egger M. Statistical methods for assessing the influence of study characteristics on treatment effects in 'meta-epidemiological' research. Stat Med. 2002;21:1513-1524. |
| 24. | Zumkeller N, Brenner H, Zwahlen M, Rothenbacher D. Helicobacter pylori infection and colorectal cancer risk: a meta-analysis. Helicobacter. 2006;11:75-80. |
| 25. | Wu XZ, Chen D. Helicobacter pylori and hepatocellular carcinoma: correlated or uncorrelated? J Gastroenterol Hepatol. 2006;21:345-347. |