Published online Jan 14, 2008. doi: 10.3748/wjg.14.248
Revised: November 29, 2007
Published online: January 14, 2008
AIM: To assess the prevalence of mild gastrointestinal disorders in milk-fed infants in paediatric practice, and to evaluate the effectiveness and satisfaction with dietetic treatment.
METHODS: A cross-sectional epidemiological study was first carried out. A total of 285 paediatricians included 3487 children seen during a period of one week. In a second phase an observational, prospective and multicentre study was conducted and 2069 milk-fed infants with mild gastrointestinal disorders (colic, constipation, regurgitation and diarrhoea) were included. There was a baseline visit (start of treatment) and a final visit four weeks later. The effectiveness of the various Novalac formulas, as well as the satisfaction of the parents/tutors and paediatricians with the dietetic treatment were assessed at the final visit.
RESULTS: The prevalence of mild gastrointestinal disorders was 27.8% of all paediatrician consultations (9.2%, 7.8%, 6.1% and 4.6% in relation to colic, constipation, regurgitation and diarrhoea, respectively). The several Novalac adapted milk formulas resolved 88.4% of the mild gastrointestinal disorders. Depending on the type of disorder, differences in response rate were observed. The highest effectiveness was recorded with respect to diarrhoea (92.6%), followed by constipation (91.6%), colic (87.6%) and regurgitation (81%). Overall, 91% of the paediatricians and 88.8% of the parents/tutors were satisfied or very satisfied with the Novalac adapted milk formulas.
CONCLUSION: Mild gastrointestinal disorders show a high prevalence in paediatric practice. The Novalac adapted milk formulas have been shown to be effective in treating mild gastrointestinal disorders in milk-fed infants in the context of routine clinical practice.
- Citation: Pina DI, Llach XB, Ariño-Armengol B, Iglesias VV. Prevalence and dietetic management of mild gastrointestinal disorders in milk-fed infants. World J Gastroenterol 2008; 14(2): 248-254
- URL: https://www.wjgnet.com/1007-9327/full/v14/i2/248.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.248
Human milk is a complete and complex species-specific food that provides all the nutrients needed for the growth of infants born to term during the first 4-6 mo of life. There is total agreement that maternal milk is the feeding method of choice for infants. However, at present, 25% of our population does not breastfeed, while 50% of women do so for only a reduced period of time (3-4 mo). In the event of contraindication or refusal to breastfeed, artificial nursing measures must be adopted. In many of these cases the infant may suffer mild gastrointestinal disorders (MGDs) during the period of lactation[1] including colic[2], regurgitation[3], diarrhoea[4] and constipation[5]. These symptoms cause discomfort for the infant and are a source of concern for parents. Acute diarrhoea cannot be considered a dysfunction as such; however, since it can be treated by dietetic measures, it has been included among these MGDs.
Interest among paediatricians to solve these disorders and improve knowledge of their physiopathology has led the infant food industry to develop specific formulas for these conditions. In many cases, dietetic adjustments are the first link in the treatment chain. At present, a broad range of artificial formulas have been developed[6] for feeding “healthy” infants. These formulas can partially or completely replace human milk, covering the normal nutritional requirements of the infant. In the case of infants with MGDs, adapted formulas have been developed[78] in which some of the nutrients have been specifically modified with the purpose of minimizing or resolving the digestive disorder while ensuring optimum infant growth. These formulas are manufactured according to the recommendations of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN)[9–11], the European Commission[1213], and the Royal Decrees of the Spanish Ministry of Health[1415].
The present study investigates the prevalence of MGDs in Spanish infants under four months of age and seen in paediatric practice. An evaluation is also made of the effectiveness of the specifically elaborated formulas belonging to the Novalac line of products (United Pharmaceuticals, France; Chiesi España S. A, Spain) [Novalac Anti-Colic (low lactose, adapted formula), Novalac Anti-Regurgitation (thickened with starch and enriched amylopectins), Novalac Anti-Diarrhoea (lactose-free and with adapted concentration of electrolytes), or Novalac Anti-Constipation (adapted concentration of magnesium and lactose)], in relation to the number of avoided episodes of colic, regurgitation, diarrhoea and constipation among infants exclusively fed with artificial milk formulas. Lastly, satisfaction with the Novalac line of products among both parents/tutors and paediatricians, was evaluated along with its relation to resolution or reduction of the symptoms associated with MGDs.
The present study comprises of two sub-studies. The first sub-study is a cross-sectional data-collecting study involving a 7-d period to estimate the prevalence of infants less than four months of age with MGDs such as colic, constipation, regurgitation and diarrhoea, among the global infant population seen in paediatric practice for any reason. The second sub-study in turn corresponds to a prospective longitudinal survey of the effectiveness of dietetic treatment with the Novalac range of formulas specifically developed for such MGDs.
A total of 285 paediatricians throughout Spain (except Ceuta and Melilla) participated in the study, under real-world conditions of clinical practice. Representation of the nursing infant population by Spanish Autonomous Communities was maintained. To investigate the prevalence of infants with MGDs in paediatric practice, each investigator included all infants less than four months of age seen in the clinic for any reason, during a period of one week. The infants of the cross-sectional study presenting MGDs with artificial milk formulas, and who according to medical criterion could be fed with the Novalac range of formulas, were enrolled in the longitudinal study. During one week, each investigator consecutively included 10 infants that met the selection criteria. This period was prolonged for two weeks until the full quota designated to each investigator was covered.
The selection criteria were: (1) infants up to four months of age fed with artificial milk formulas; (2) the presence of MGDs; (3) the possibility of feeding the infants with some product of the Novalac line of formulas; and (4) continuation of these formulas on an exclusive basis for at least 30 d (with no incorporation of other foods to the diet).
The cross-sectional study included 3487 infants to assess the prevalence of MGDs. The prospective study for evaluating the efficacy of the Novalac line of products involved 2069 patients with MGDs. The patients were visited on two occasions: at the time of inclusion and after four weeks. In the case of infants with diarrhoea, the final visit took place after 4-7 d. A total of 1441 infants completed follow-up.
During the initial visit, data were collected relating to sociodemographic (age and sex) and clinical variables (gestational age, weight and height at birth and at the time of the visit, and the type and duration of the disorder). A questionnaire addressing the different symptoms and their intensity was designed for each disorder: (1) Constipation: number of stools/d, consistency, irritability, need for external help to defecate; (2) Regurgitations-vomiting: number/d, duration of the feedings, volume ingested; (3) Colic: excess gases, duration of crying, duration of bottle feeding; (4) Diarrhoea: number of stools and their characteristics.
The effectiveness of the dietetic treatment was evaluated on the final visit. Anthropometric data were collected (weight, height), along with the number of days to disappearance or improvement of the disorder, the evolution of symptoms, adverse events, and satisfaction of the parents/tutors and paediatricians with the treatment. Also documented was paediatrician contact after 15 d with the parents by telephone, to know the condition of the infant (in those cases where the paediatrician considered the call relevant).
The satisfaction of the parents/tutors and paediatrician with the Novalac formulas used for the MGDs was assessed on occasion of the final visit by means of a Likert-type scale with five possible answers (from very satisfied to very dissatisfied).
Duplicate data introduction was carried out, with validation of the database. Analysis of the prevalence of MGDs was based on a descriptive analysis of the sociodemographic and clinical characteristics of the study population. Continuous variables were reported as the mean and standard deviation (SD), while categorical variables were expressed as the number and percentage of infants per response category. The description of the characteristics of the infants was made for the total study sample, with stratification according to MGD presence.
A descriptive analysis was made of the number and percentage of infants seen in paediatric practice for MGDs, calculating the confidence interval (CI) of prevalence with a level of significance of 0.05. In order to assess variability in the Spanish setting, comparative studies were made of the prevalence of MGDs among the different regions in the country, based on the chi-square test.
Calculation was made of the number of days needed for disappearance or improvement of the disorder, along with the mean number (95% confidence interval) of days of MGD persistence.
A descriptive analysis was made of satisfaction as expressed by the parents/tutors and paediatricians. To this effect we used descriptive indicators such as the mean, standard deviation, median and percentiles 25 and 75. A study was made of the relationship between parent/tutor satisfaction with the Novalac line of products and their effectiveness, based on the Student t-test for independent data.
The SAS version 8.02 statistical package for Microsoft Windows was used throughout. In all the statistical tests a level of significance of α = 0.05 was used.
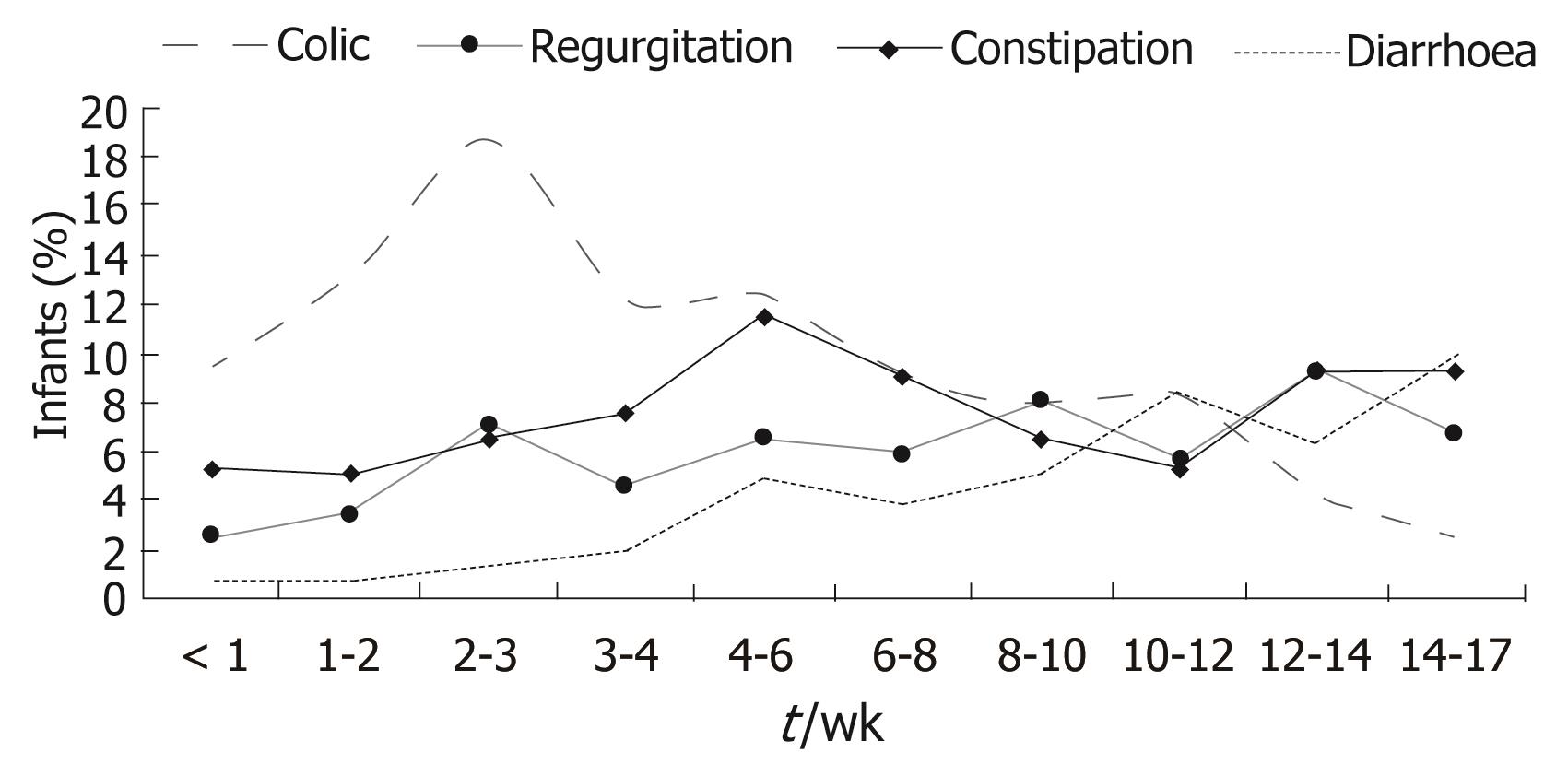
The results of the cross-sectional study were obtained from the infant population included in the period of one week commented above, and involved 3487 infants. Boys predominated slightly over girls (52.2% vs 47.8%). Patient age at consultation was between under one week and 17 wk. Colic manifested at earlier ages (6.2 wk on average), followed by constipation (7.6 wk), regurgitation (8.6 wk) and diarrhoea (10.4 wk) (Figure 1). Gestational age was 39 wk on average (SD: 1.6 wk), with a mean body weight at birth of 3.2 (SD 0.5) kg and a height of 49.5 (SD: 2.3) cm.
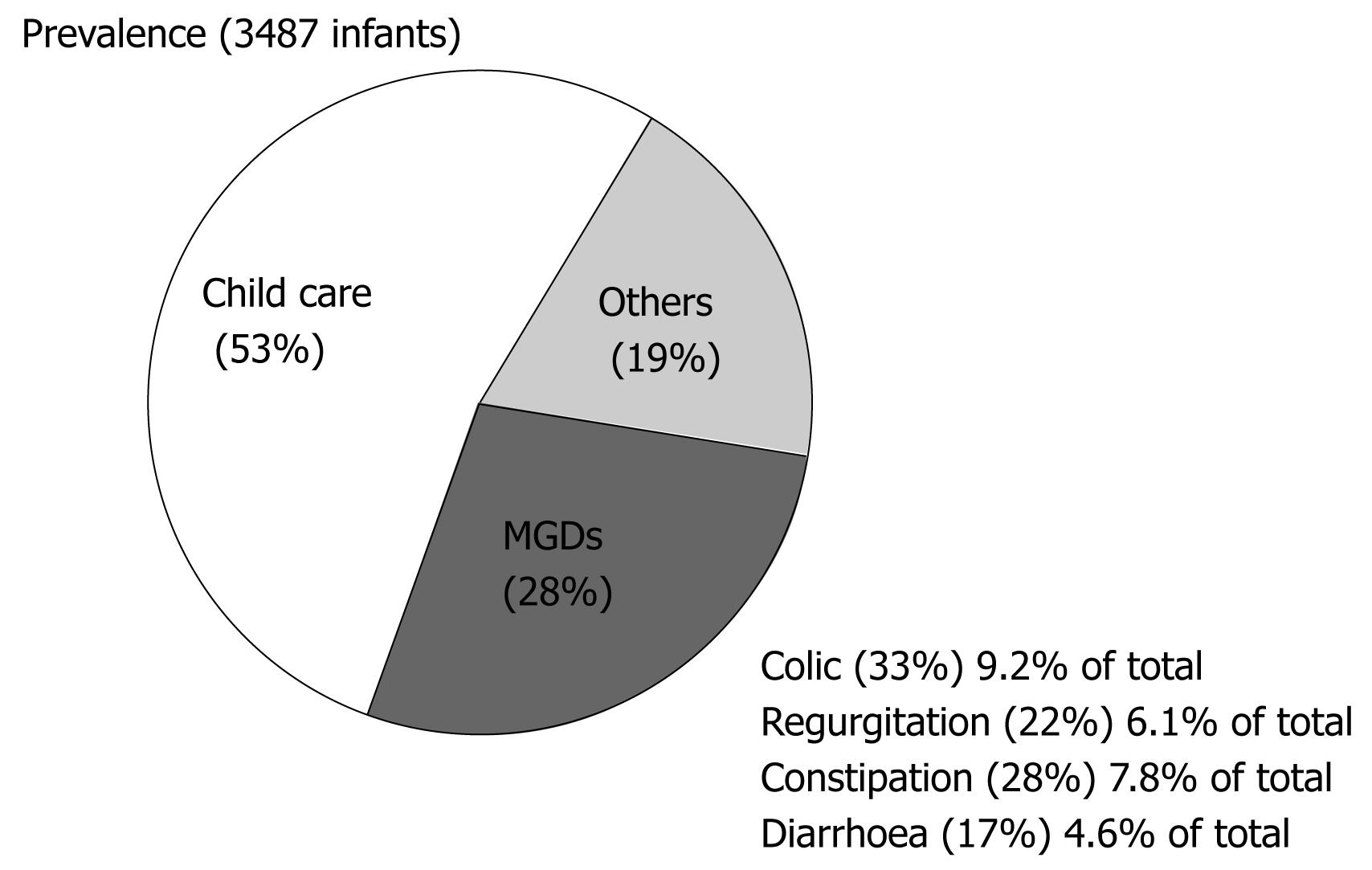
The prevalence of MGDs was 27.8% (95% CI: 26.3-29.2); of these disorders, 9.2% (95% CI: 8.3-10.2) corresponded to colic, 7.8% (95% CI: 6.9-8.7) to constipation, 6.1% (95% CI: 5.3-6.9) to regurgitation -vomiting, and 4.6% (95% CI: 3.9-5.3) to diarrhoea. Only 0.6% of the infants presented with other digestive disorders (anorexia/hyporexia and eating disorders), while 19.0% (95% CI: 17.8-20.4) were diagnosed with some other non-digestive problem (upper airways infection, bronchitis and dermatitis). Of the study series, 52.6% consulted for aspects related to child care (Figure 2). The prevalence considering only the 27.8% of infants with MGDs was: colic 31.2% (95% CI: 30.3-36.2), constipation 29.2% (95% CI: 25.3-30.9), regurgitation 21.3% (95% CI: 19.5-24.7) and diarrhoea 18.3% (95% CI: 14.2-18.9).
The longitudinal study included 2069 infants with MGDs: 646 infants with colic, 441 with regurgitation, 604 with constipation and 378 with diarrhoea. The effectiveness of dietetic treatment for these disorders was evaluated among the 1441 infants that completed follow-up. Premature study termination among some infants was due to adverse events in 2.7% of cases, parent decision in 6.9%, loss to follow-up in 1.64%, protocol violations in 2.46%, and non-specified reasons in 16.62%.
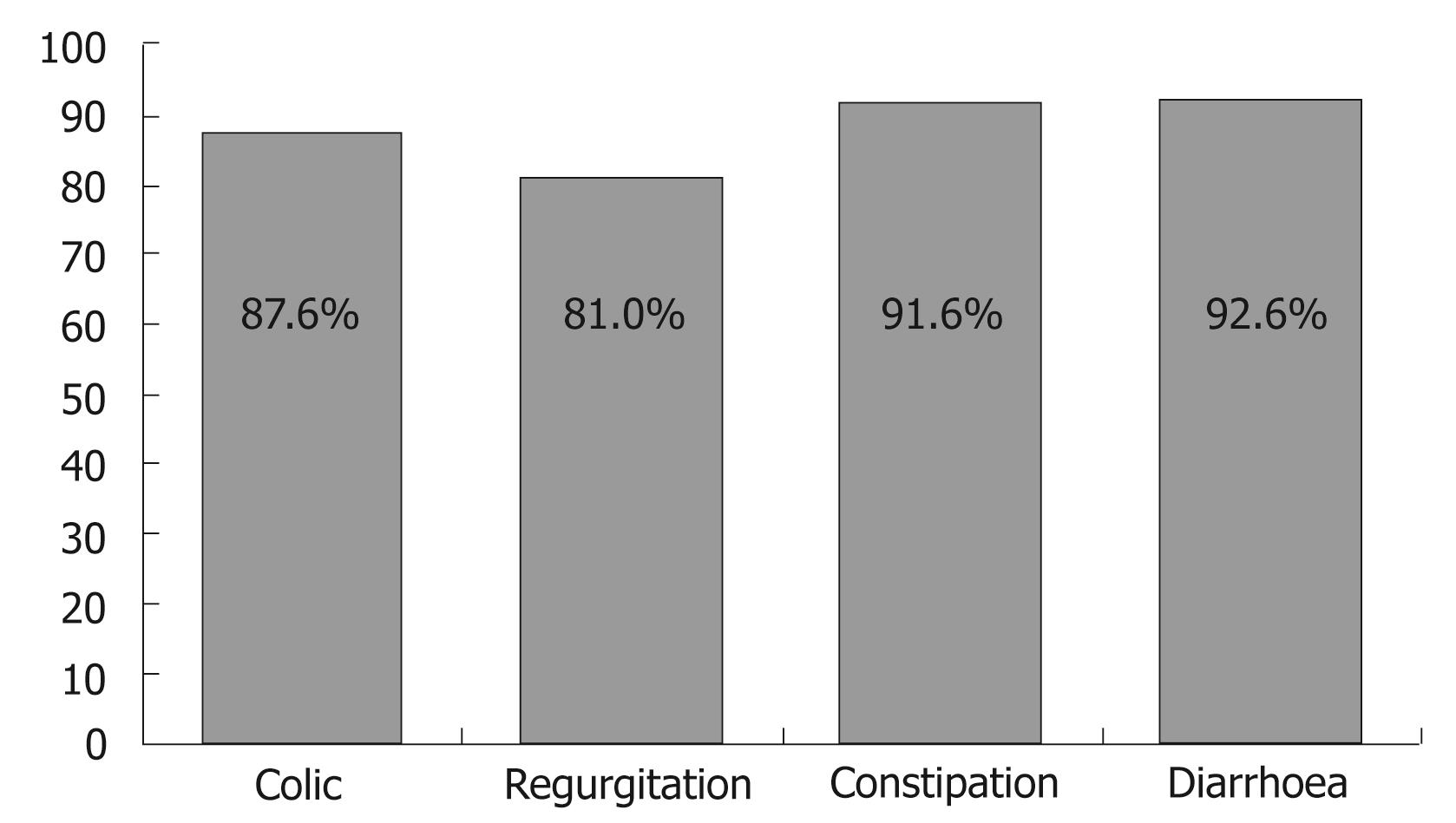
Effectiveness in resolving the different disorders, after feeding with the specific Novalac formulas under conditions of routine clinical practice, is reported in Table 1 and Figure 3.
| Baseline | 30 d | ||
| Colic | Excess gases | 82.90% | 25.80% |
| Duration of crying | |||
| < 1 h/d | 12.10% | 85.20% | |
| 1-3 h/d | 53.00% | 13.30% | |
| > 3 h/d | 34.80% | 1.60% | |
| Regurgitations | Regurgitation and/or vomiting per day [mean (SD)] | 6.9 (4.5) | 2.4 (1.8) |
| Mean number of daily ingestions | 6.0 | 5.6 | |
| Duration of daily ingestions | |||
| Increases | - | 24.50% | |
| Without change | - | 22.40% | |
| Decreases | - | 53.10% | |
| Volume of daily ingestion | |||
| Increases | - | 61.90% | |
| Without change | - | 18.70% | |
| Decreases | - | 19.40% | |
| Constipation | Type of stools | ||
| Normal | 33.40% | 95.60% | |
| Hard | 66.60% | 4.40% | |
| Presence of pain or discomfort | |||
| Yes | 90.00% | 10.40% | |
| No | 10.00% | 89.60% | |
| External help needed for defecation | |||
| Yes | 76.10% | 8.80% | |
| No | 23.90% | 91.20% | |
| Baseline | 7 d | ||
| Diarrhoea | Presence of fever | ||
| Yes | 21.40% | 1.50% | |
| No | 78.60% | 98.50% | |
| Number of stools per day [mean (SD)] | 5.4 (1.8) | 2.2 (1) | |
| Type of stools | |||
| With mucus and/or blood | 5.20% | 1.10% | |
| Liquid | 45.00% | 4.80% | |
| Semiliquid | 49.80% | 14.40% | |
| Normaly | 0.00% | 79.70% |
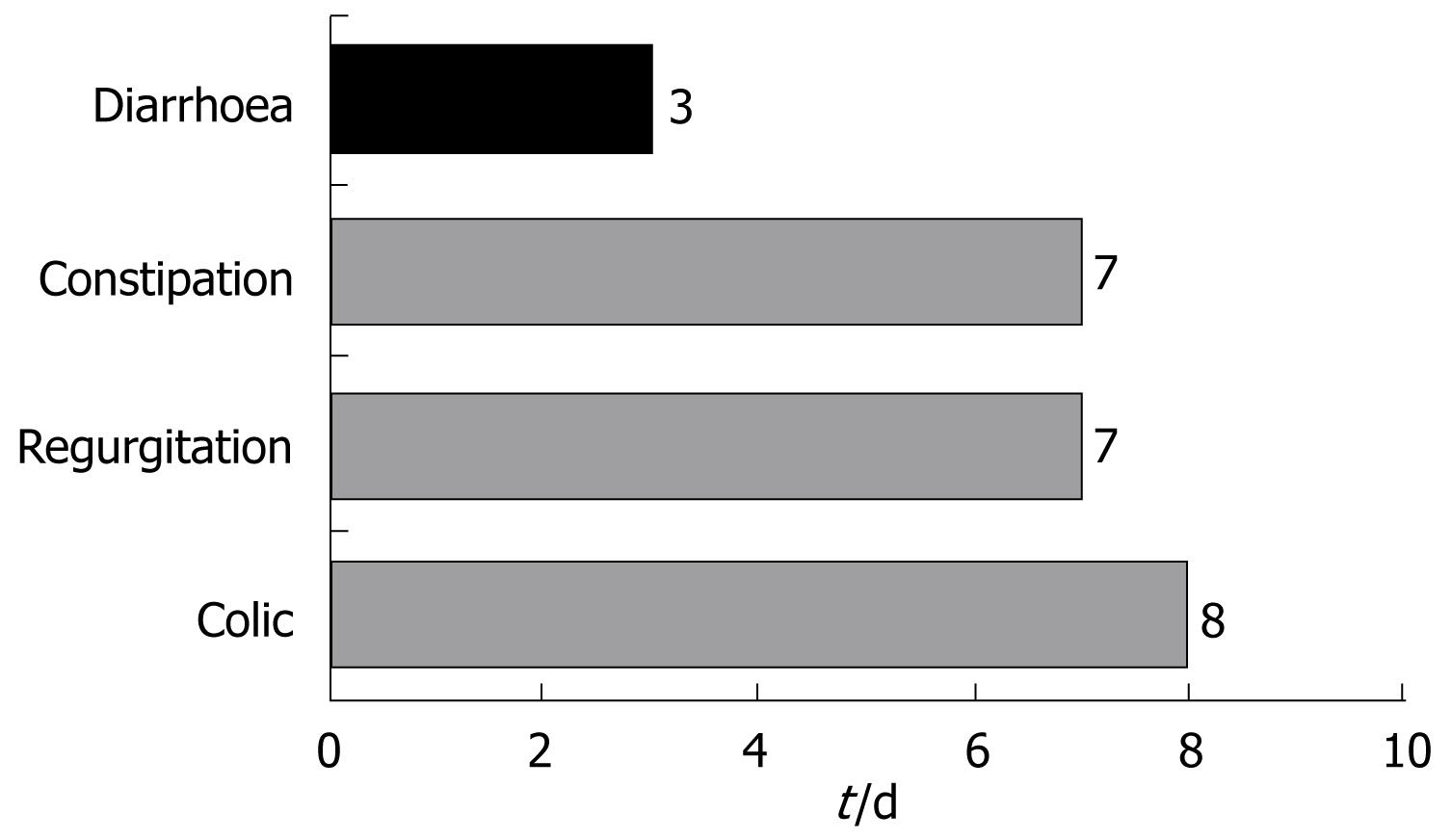
Colic was resolved among 87.6% of the infants after a median of 8 d - the proportion of infants with normal bottle feeding increasing from 33.9% at baseline to 69.2%. Likewise, a considerable reduction was observed in excess gases (from 82.9% at baseline to 25.8%). The duration of crying decreased in all infants.
Regurgitation was resolved in 81% of the infants after a median of 6 d of follow-up. The figure decreased from six regurgitations and/or vomiting episodes a day to only two, and the time required for bottle feeding decreased 53% - with an increase in the volume of daily ingestions in 61.9% of the infants.
A full 91.6% of the cases of constipation were resolved within 7 d. The number of daily stools among the infants with constipation increased from 0.6 (SD 0.7) stools/d to 1.7 (SD 0.8) stools/d. At the end of follow-up, the stools were found to be normal in 95.6% of the infants, while 89.6% presented no pain or discomfort, and 91.2% required no external help in defecating.
The infants with diarrhoea in turn, showed a decrease (mean [SD]) from 5.4 (1.8) to 2.2 (1) stools/d. Fever was absent in 98.5% of the cases, and 79.7% of the infants showed normal defecation at the end of treatment. A full 92.6% of the cases of diarrhoea were resolved within three days (Figure 4).
Development of all infants fed with the Novalac formulas was within the normal range.
Ninety-one percent (91%) of the paediatricians were satisfied or very satisfied with the dietetic regimen, though statistically significant differences were observed between groups (P < 0.001) - increased satisfaction being recorded among the paediatricians that treated diarrhoea and constipation versus those that treated colic and regurgitations. The greatest levels of satisfaction corresponded to the treatment of diarrhoea, with 95.9% of the paediatricians claiming to be satisfied, followed by constipation (92.8%), colic (88.5%) and regurgitation (87.8%).
As to parent satisfaction with the treatment (P < 0.001), 88.8% claimed to be satisfied with the course of the disorder - the specific figures being 93.7% in the case of diarrhoea, followed by constipation (90.0%), colic (88.1%) and regurgitation (83.3%).
Regarding the adverse events recorded during the study, only 3.9% of the infants receiving Novalac formulas suffered some adverse event. The most frequent affected the digestive tract (1.4%), including diarrhoea and constipation, and respiratory apparatus (0.7%) (e.g. bronchitis and bronchiolitis). Ten infants (0.5%) required hospital admission for different reasons: septicaemia (n = 1), dehydration (n = 2), vomiting (n = 1), hernia (n = 1) and bronchitis or bronchiolitis (n = 2).
Maternal breast milk is the usual food source for infants, though when breastfeeding is not possible, artificial lactation can be provided in the form of starting formulas, with the introduction of follow-up formulas 4-6 mo later, to ensure optimum nutritional development. During this period mild gastrointestinal disorders (MGDs) are not uncommon, and may be treated with dietetic measures.
This study for the first time provides information on the prevalence of these disorders in Spain, and on the efficacy of dietetic treatment using the different formulas of the Novalac product range. The results of the prevalence study indicate that colic in infants is the MGD most often seen in paediatric clinics (9.2%), representing 33.3% of all MGDs.
Infant “colic” is an ill-defined term that is often used in reference to different situations found in paediatric practice[16]. Due to the lack of a clear definition, prevalence and treatment studies characteristically include heterogeneous groups of infants. These are usually healthy children that in the first four months of life develop paroxysmal crying without any apparent cause, and that gradually subsides. The underlying aetiology has not been clearly established, though the disorder has been related to alterations in motility[8], prostaglandin dysfunction, abnormal serotonin concentrations, and neuropeptide immaturity. Other authors more inclined to seek behavioural explanations tend to relate infant colic to alterations in the family environment, or even consider it a “normal” form of behaviour in infants with a more irritable temperament.
Human milk contains 7 g of lactose per 100 mL. However, in the first few weeks of life, infants present physiological or functional lactase insufficiency that limits absorption of these amounts. The non-hydrolyzed lactose reaches the colon, where it ferments to yield lactic acid, short-chain fatty acids, methane, carbon dioxide and hydrogen. This exerts a beneficial effect in that the stools become semiliquid as a result. However, is some cases such fermentation gives rise to excess gas[17–19], which favours the development of colic. In effect, different studies have related excess crying with excess intestinal gas[20].
The Novalac Anti-Colic formula offers an important reduction of the symptoms. Colic was resolved in 87% of the cases within one week, with a reduction in crying time, improvement in feeding continuity, and a reduction in the amount of intestinal gas.
In this study, constipation was the cause of consultation in 7.8% of the infants (representing 28% of all MGDs). A recent article published by Quinlan et al[21] studied the stool characteristics of 844 infants between 7-15 d old. Hard stools were recorded in 17% of the formula-fed infants, and in none of the breastfed infants. Breastfeeding entails a series of compensating physiological mechanisms that avoid constipation[22]. The Novalac Anti-Constipation formula bases its therapeutic efficacy partially on its high lactose content (8.1 mg/100 mL). The non-hydrolyzed lactose reaches the colon, where it is metabolized by the anaerobic flora, producing an osmotic laxative effect, since it attracts water into the intestinal lumen[17–19]. The magnesium contained in the formula (within the authorized maximum limits: 9.1 mg/100 mL) also enhances the laxative effect due to its osmotic action, and stimulates bowel motility by inducing cholecystokinin (CCK) secretion[23–25]. The efficacy of such treatment is reflected by an increase in the number of stools, and a reduction in the number of hard depositions, associated discomfort and the need for external help in defecation.
The next most prevalent MGD in the present study was regurgitation (22% of the total MGDs). Regurgitation and vomiting are the final manifestation of reflux. The aetiology of reflux in infants is of a multifactorial nature, and has not yet been fully elucidated. Postprandial reflux is accepted to be physiological, and occasionally occurs in almost all infants. However, in some cases the clinical picture is particularly manifest and can produce important discomfort for both the child and family. It is generally agreed that uncomplicated gastro-oesophageal reflux should not receive pharmacological treatment (antacids, prokinetic agents, H2 receptor blockers), though effective measures such as postural and dietetic management are recommended. In any case, the same authors that initially recommended such treatment have recently questioned it[26].
Milk thickening is a therapeutic practice that has been recommended for decades. Increased food viscosity and density reduces the reflux rate but does not modify the rest of parameters indicative of pathological gastro-oesophageal reflux. The products used as thickeners for the most part have been locust bean flour, pectin and cellulose. Cereal starch (rice and corn) has also been proposed, and offers the advantage of avoiding the possible adverse effects associated with the presence of galactomannans[27]. The Novalac Anti-Regurgitation formula contains a specially selected corn starch (having high amylopectin content) in a proportion of 1.9 g per 100 mL. It is pregelatinised and contributes to the increased viscosity in the stomach (on average 10 fold the viscosity in the feeding bottle). The presence of medium-chain fatty acids in turn favours a reduction in gastric emptying[28]. The efficacy of the treatment is reflected by the results obtained in the present study. In effect, the number of regurgitations/vomiting episodes decreased during the 7 d of treatment, and improvements were also seen in the rapidity of bottle ingestion, with a larger ingested volume.
Lastly, acute gastroenteritis represented 16.5% of the global MGDs in our study. Acute gastroenteritis, of a bacterial or viral origin, is characterized by alterations in the normal displacement of water and electrolytes within the intestinal lumen. When diarrhoea is caused by a virus (fundamentally rotavirus) transient lactase deficiency may also result. The duration of this deficiency is usually 7-15 d, though infants that are malnourished or suffer serious intestinal lesions may have persistent diarrhoea for up to 18-24 mo. In those cases in which deficient lactose absorption is suspected, lactose-free formulas are justified[2930]. In most cases a single week of lactose exclusion suffices, after which the usual formula is reintroduced, while monitoring tolerance.
The most recent developments point to the advisability of introducing soluble fibre[31]. The use of amylase-resistant carbohydrates is an innovation that takes advantage of the functional properties of the colon - specifically, the metabolic activity of the anaerobic flora and the absorption capacity of the colon mucosa. The purported mechanism involves use by the colon mucosa of the short-chain fatty acids produced by bacterial metabolism of non-absorbed carbohydrates, to favour the absorption of water and electrolytes[32]. In the colon, many carbohydrates are fermented by the anaerobic flora, resulting in short-chain fatty acids: propionate, acetate and butyrate. In the mucosa, these fatty acids stimulate the absorption of water and electrolytes.
The Novalac Anti-Diarrhoea formula contains no lactose, and moreover incorporates a series of novelties. The presence of electrolytes within the accepted maximum range (31 mg of Na/100 mL, 83 mg of K/100 mL and 49 mg of Cl/100 mL) ensures an increased mineral supply for those patients who after discontinuing oral rehydration remain at risk of excessive losses. On the other hand, the presence of pectin (1.5 g per 100 mL) offers the advantage versus other fructo-oligosaccharides of being totally fermented in the colon, since it is a larger and more viscous molecule - thereby favouring short-chain fatty acid production.
The present study for the first time offers information on the prevalence of mild gastrointestinal disorders in Spanish infants under four months of age seen in paediatric clinical practice. Dietetic intervention with the Novalac formulas has been shown to be effective in resolving these disorders in the routine clinical setting - with a significant reduction in associated symptoms. A close relationship has been found between satisfaction among the parents/tutors and paediatricians and the effectiveness of the dietetic treatment provided. In turn, the low prevalence of treatment-related adverse events reflects the good tolerability of the formulas belonging to the Novalac range of products.
Different milk-feed infant formulae have appeared in the market during the last decades for the nutritional treatment of different entities such as colic, constipation, regurgitation and acute diarrhoea, called “mild gastrointestinal disorders” (MGD), There are few data about the prevalence or these MGD and its evolution after the intake of nutritional formulae.
This study investigated the MGD prevalence, MGD physiopathology, International Committees and Scientific Societies position in front of new formulations composition, as well as clinical response with these new formulae.
In this article we have published, for the first time in our country, relevant data regarding the MGD prevalence and dietetic clinical response in a huge number of milk-fed infants (n = 2069). Among international publications one can find lots of information about recommendations of how these formulations have been made and its usages indications and efficacy, but never in a population as big as this.
This study allows the real acknowledgment about MGD prevalence, and also explains why new formulae composition have to be used and its clinical response. It also increases paediatricians possibility of use of these formulations, giving them confidence regarding clinical efficacy and nutritional safety.
Mild gastrointestinal disorders: colic, constipation, regurgitation and acute diarrhoea.
The authors are to be congratulated on an important and well presented study.
| 1. | Pediatr integral. Continuous Training Program for Outpatient Paediatrics. Available from: URL: http://www.sepeap.es/Revista/PI7_1.pdf. |
| 2. | Lucassen PL, Assendelft WJ, Gubbels JW, van Eijk JT, van Geldrop WJ, Neven AK. Effectiveness of treatments for infantile colic: systematic review. BMJ. 1998;316:1563-1569. |
| 3. | Carbajo AJ. Vomiting and regurgitations. Gastro-oesophagical reflux. Pediatr Integral. 2003;7:15-23. |
| 4. | Available from: URL: http: //escuela.med.puc.cl/paginas/publicaciones/Pediatria/ManualGastro/dag.html. |
| 5. | Marina C. Constipation and Encopresis. Pediatr Integral. 2003;VII:55-60. |
| 6. | Practice guideline on pediatric nutrition. Infant nutrition. Spanish Society of Gastroenterology, Hepatology and Pediatric Nutrition. An Esp Pediatr. 2001;54:145-159. |
| 7. | Indications for anti-regurgitation formulas. Committee on Nutrition of the AEP. An Esp Pediatr. 2000;52:369-371. |
| 8. | Tormo R, Potau N, Infante D, Moran J, Martin B, Bergada A. Protein in infant formulas. Future aspects of development. Early Hum Dev. 1998;53 Suppl:S165-S172. |
| 9. | ESPGAN Committee on Nutrition. Guidelines on infant nutrition. I. Recommendations for the composition of an adapted formula. Acta Paediatr Scand Suppl. 1977;(262):1-20. |
| 10. | ESPGAN Committee on Nutrition. Guidelines on infant nutrition. II. Recommendations for the composition of follow-up formula and Beikost. Acta Paediatr Scand Suppl. 1981;287:1-25. |
| 11. | ESPGAN Committee on Nutrition. Committee report. Comment on the composition of cow's milk based follow-up formula. Acta Paediatr Scand. 1990;79:250-254. |
| 12. | Commission of European Communities. Commission Directive 91/321/EEC of 14 May 1991 on infant formulae and follow-on formulae. Official journal for European Communities, 4 July 1991. Available from: URL: http: //eur-lex.europa.eu/LexUriServ/site/en/consleg/1991/L/01991L0321-20040501-en.pdf. |
| 13. | Commission of European Communities. Commission Directive 96/4/EC, Euratom of 16 February 1996 amending Directive 91/321/EEC on infant formulae and follow-on formulae. Official journal for European Communities, 28 Feb, 1996. Available from: URL: http: //eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CELEX: 31996L0004: EN: HTML. |
| 14. | Royal Decree 145/1997, 31 January, that approves the definitive positive list of additives not artificial colourings nor sweeteners, for its use in the manufacture of food products and its terms of use. B.O.E. no 70, 22 March 1997. Available from: URL: http: //www.boe.es/boe/dias/1997/03/22/pdfs/A09378-09418.pdf. |
| 15. | Royal Decree 72/1998, 23 January, that approves the specific regulation for formulas and continuity preparations for unweaned babies. B.O.E. No 30, 4 February 1998. Available from: URL: http: //www.boe.es/boe/dias/1998/02/04/pdfs/A03772-03780.pdf. |
| 17. | Barr RG, Hanley J, Patterson DK, Wooldridge J. Breath hydrogen excretion in normal newborn infants in response to usual feeding patterns: evidence for "functional lactase insufficiency" beyond the first month of life. J Pediatr. 1984;104:527-533. |
| 18. | Fomon S. Carbohydrate. Nutrition of Normal Infants. St. Louis: Mosby 1993; 178-180. |
| 19. | Roggero P, Mosca F, Motta G, Mangiaterra V, Perazzani M, Offredi ML, Marini A, Careddu P. Sugar absorption in healthy preterm and full-term infants. J Pediatr Gastroenterol Nutr. 1986;5:214-219. |
| 20. | Tormo R, Bertaccini A, Conde M, Infante D, Cura I. Methane and hydrogen exhalation in normal children and in lactose malabsorption. Early Hum Dev. 2001;65 Suppl:S165-S172. |
| 21. | Quinlan PT, Lockton S, Irwin J, Lucas AL. The relationship between stool hardness and stool composition in breast- and formula-fed infants. J Pediatr Gastroenterol Nutr. 1995;20:81-90. |
| 22. | Lopez A, Castellote A. I, Campoy C, Rivero M, Tormo R, Infante D y cols. The influence of dietary palmiitic acid triacylglyceride position on the fatty acid, calcium and magnesium contents of at term newborn faeces. Early Hum Dev. 2001;65:S83-S94. |
| 23. | Nores JM, Rochette L. Magnesium Physiology, Cahiers de Nutrition et de Dietetique. Nutrition and dietetic notebooks. Louis: Mosby 1988; 379-384. |
| 24. | Binder HJ, Donowitz M. A new look at laxative action. Gastroenterology. 1975;69:1001-1005. |
| 25. | Moritz M, Finkelstein G, Meshkinpour H, Fingerut J, Lorber SH. Effect of secretin and cholecystokinin on the transport of electrolyte and water in human jejunum. Gastroenterology. 1973;64:76-80. |
| 26. | Sondheimer JM. Gastroesophageal reflux in children. Clinical presentation and diagnostic evaluation. Gastrointest Endosc Clin N Am. 1994;4:55-74. |
| 27. | Tormo R, Bertaccini A, Conde M, Infante D, Cura I. Methane and hydrogen exhalation in normal children and in lactose malabsorption. Early Hum Dev. 2001;65 Suppl:S165-S172. |
| 28. | Sutphen JL, Dillard VL. Medium chain triglyceride in the therapy of gastroesophageal reflux. J Pediatr Gastroenterol Nutr. 1992;14:38-40. |
| 29. | Lifshitz F, Fagundes Neto U, Garcia Olivo CA, Cordano A, Friedman S. Refeeding of infants with acute diarrheal disease. J Pediatr. 1991;118:S99-S108. |
| 30. | Lifshitz F, Maggioni A. The nutritional management of acute diarrhea in young infants. J Pediatr Gastroenterol Nutr. 1994;19:148-150. |
| 31. | Oli MW, Petschow BW, Buddington RK. Evaluation of fructooligosaccharide supplementation of oral electrolyte solutions for treatment of diarrhea: recovery of the intestinal bacteria. Dig Dis Sci. 1998;43:138-147. |
| 32. | Desjeux JF. Can malabsorbed carbohydrates be useful in the treatment of acute diarrhea? J Pediatr Gastroenterol Nutr. 2000;31:499-502. |