Published online May 21, 2008. doi: 10.3748/wjg.14.3069
Revised: January 24, 2008
Published online: May 21, 2008
AIM: To investigate the clinical significance of Bcl-xL gene in the pathogenesis of human colon carcinoma.
METHODS: Fifty-six pair tissue samples from patients with colon cancer were collected, and protein level of the Bcl-xL gene was measured by immunohistochemistry method. The correlation of Bcl-xL expression with clinical index was evaluated. After human colon cancer cell line HT29 was transfected with Bcl-xL small interfering RNA (siRNA), the anchorage-independent growth of cancer cells was detected by colony formation in soft agar and invasion ability of cancer cells was determined by a transwell model.
RESULTS: The Bcl-xL expression was higher in cancerous tissue samples than in normal tissue samples (38.78 ±11.36 vs 0.89 ± 0.35, P < 0.001), and was associated with the pathological grade, lymphnode metastasis and Duke’s stage of colorectal carcinoma. Transfection with Bcl-xL siRNA inhibited the colony formation and invasion ability of human colon cancer cell line HT29 in vitro.
CONCLUSION: Bcl-xL gene plays an important role in carcinogenesis of human colorectal carcinoma and is associated with malignant biological behaviors of human colorectal carcinoma.
- Citation: Zhang YL, Pang LQ, Wu Y, Wang XY, Wang CQ, Fan Y. Significance of Bcl-xL in human colon carcinoma. World J Gastroenterol 2008; 14(19): 3069-3073
- URL: https://www.wjgnet.com/1007-9327/full/v14/i19/3069.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.3069
Colorectal cancer is the third most common malignant neoplasm worldwide[1] and the second leading cause of cancer-related death[2]. Despite recent advances in diagnostic and therapeutic measures, the prognosis of colorectal cancer patients with distant metastasis still remains poor. Enhanced understanding of the signaling mechanisms that regulate metastasis of colon cancer may provide important insights into more effective therapeutic strategies.
Cells harboring multiple genetic alterations are normally eliminated by apoptosis. Diminished apoptosis plays a critical role in tumor initiation, invasion, metastasis, progression, and drug resistance. Results of numerous scientific and clinical studies link altered expression of apoptosis-regulatory proteins to the development of a lot of cancer cells. Among them, the Bcl-2 family of genes, which share sequence homology domains, plays a key role in the regulation of apoptotic cell death induced by a wide variety of therapeutic stimuli[3]. These genes can form homodimers and/or heterodimers that modulate one another’s function, whereby their relative concentrations function as a rheostat for the apoptotic program[4]. Of them, Bcl-xL gene has been well characterized as a potential gene involved in the apoptotic signal pathway.
Bcl-xL, a mitochondrial membrane protein, promotes cell survival by regulating the electrical and osmotic homeostasis of mitochondria in response to a variety of stimuli[56]. Over-expression of Bcl-xL is reported to confer a multidrug resistance phenotype[78]. Moreover, inhibition of Bcl-xL expression by some ways results in an altered ratio of BAX to Bcl-xL and subsequent mitochondria-mediated cell death[9]. Thus, Bcl-xL might serve as an ideal molecular target of anticancer therapy
However, previous studies about Bcl-xL gene have mainly focused on the regulation of apoptosis and drug resistance. There is little information about the linkage of Bcl-xL with invasion in cancer cells. Increasing data show that Bcl-xL over-expression might be related to invasion and metastasis of some solid tumors, such as breast cancer[1011], hepatocellular carcinoma[12], ovarian cancer[13], glioma[14], and lung carcinoma[15]. We have found in previous works that human colon cancer cells transfected with signal transducer and activator of transcription 3 (STAT3) small interfering RNA (siRNA) can inhibit the invasion ability of cancer cells. Meanwhile, expression of Bcl-xL protein is also markedly down-regulated in transfected cancer cells[16]. However, the possible role of Bcl-xL in invasion of human colon cancer is not clear.
In the present study, we investigated the linkage of Bcl-xL with the invasion of human colon cancer in vivo and in vitro.
A total of 56 paired colon cancer tissue and distant normal colon tissue samples were obtained from 56 patients undergone surgical operation. Tumor histotype and grade of differentiation were defined according to the WHO criteria. The clinical and pathological stages were defined according to Duke’s staging. These patients did not receive any chemotherapy or radiotherapy before operation. This study was approved by the Medical Ethical Committee of Affiliated Hospital of Jiangsu University, and all patients provided their written informed consent to participate in the study. All the specimens were fixed in 10% neutral-buffered formalin, dehydrated in ascending series of ethanol and routinely embedded in paraplast. Sections were cut at 4 &mgr;m, stained with hematoxylin and eosin for histopathological and immunohistochemical evaluation. The clinicopathological parameters are summarized in Table 1.
| Characteristic | n | Bcl-xL PU | P |
| Sex | > 0.05 | ||
| Male | 30 | 39.22 ± 11.35 | |
| Female | 26 | 37.36 ± 12.18 | |
| Age (yr) | > 0.05 | ||
| ≤ 55 | 25 | 39.89 ± 15.78 | |
| > 55 | 31 | 38.66 ± 12.56 | |
| Tumor differentiation | < 0.05 | ||
| Well | 12 | 31.58 ± 12.69 | |
| Moderate | 19 | 39.77 ± 16.55 | |
| Poor | 25 | 53.95 ± 17.89 | |
| Lymph node metastasis | < 0.05 | ||
| Negative | 27 | 32.19 ± 13.35 | |
| Positive | 29 | 56.36 ± 11.95 | |
| Duke’s staging | < 0.05 | ||
| A + B | 28 | 31.55 ± 12.39 | |
| C + D | 28 | 58.78 ± 11.68 |
All the tissue sections were deparaffinized, rehydrated and incubated in a citrate buffer (0.01 mol/L, pH 6.0) for 1 min at 121°C. The endogenous peroxidase activity was blocked by covering the sections with 3% H2O2/methanol for 15 min. The sections were then incubated in a 1:100 dilution of goat antihuman Bcl-xL IgG at 4°C overnight. After washed with PBS containing 0.05% Tween, the tissue sections were incubated in a 1:50 dilution of biotinylated donkey anti-goat IgG (Santa Cruz) for 30 min. The SABC reagents were used to amplify the immunoreactivity that was detected using 3’-diaminobenzidine according to the manufacturer’s instructions. The sections were counterstained with hematoxylin. The positive uniT (PU) represents the relative concentration of positive staining according to previous data[17]. Each section was observed randomly at five areas and the mean PU was assembled and calculated.
The anti-sense sequence of siRNA (5’-CTCTGATATGCTGTCCCTG-3’) corresponding to Bcl-xL mRNA with dTdT on 3’-overhangs was designed and chemically synthesized according to the recommendation of the manufacturer (Dharmacon Research, USA). The scrambled siRNA served as a control, and its sequences are 5'-UUCUCCGAACGUGUCACGUTdTd-3' and 5'-ACGUGACACGUUCGGAGAATdTdT-3'.
Human colon cancer cell line HT29 (Institute of Cell Biology, Shanghai, China) was cultured in RPMI 1640 (Invitrogen, Inc.) supplemented with 10% fetal bovine serum (FBS) in an atmosphere containing 50 mL/L CO2 at 37°C. siRNA was transfected with a commercial reagent, oligofectamine (Invitrogen, USA) in 6-well plates following its manufacturer’s instructions. Briefly, On the day before transfection, confluent layers of cells were trypsinized, counted and re-suspended. Cells (1 × 105) were plated into each well of the 6-well plates, so that they could become about 70% confluence next day at the time of transfection. Oligofectamine was diluted in serum-free RPMI 1640 and mixed with siRNA at a 1:2 ratio (4 &mgr;L of 20 &mgr;mol/L of siRNA formulated with 8 &mgr;L of oligofectamine). The cells were then incubated for other 48 h. The number of cells was determined using a hemocytometer before subsequent assays.
Total cellular RNA was isolated from cancer cell lines using Trizol. Final RNA pellets were dissolved in 20 &mgr;L of diethyl pyrocarbonate-treated water. RNA yield was determined by spectroscopy. For complementary DNA (cDNA) synthesis, 2 &mgr;g of total RNA was transcribed with cDNA transcription reagents using 0.2 &mgr;g of the oligo(dT)18 primer for subsequent quantitative, real-time polymerase chain reaction (RT-PCR).
Real-time RT-PCR analyses were performed on an ABI Prism 7700 sequence detection system (Perkin-Elmer Applied Biosystems, Foster City, CA). For Bcl-xL amplification, primers with the sequences 5’-TCCTTGTCTACGCTTTCCACG-3’ and 5’-GGTCGCATTGTGGCCTTT-3’ were used in combination with a sequence 5’-ACAGTGCCCCGCCGAAGGAGA-3’. Primers, Taqman and TaqMan probes were designed by the Primer Express TM 1.0 (Applied Biosystems) software and the probes were labeled at 5’ end with the reporter dye molecule FAM (6-carboxy-fluorescein) and at 3’ end with the quencher dye molecule TAMARA (6-carboxytetramethyl-rhodamine). Real-time PCR was conducted in a total volume of 50 &mgr;L with 1 × TaqMan Master Mix (Applied Biosystems) and primers. Thermal cycle parameters included one cycle at 95°C for 3 min, and 45 cycles involving denaturation at 95°C for 30 s, annealing at 52°C for 45 s, extension at 72°C for 45 s, followed by a final extension at 72°C for 10 min. The relative amount of each cDNA in each sample was calculated by dividing the CT value with the corresponding value of the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH). All reactions were performed in triplicate.
HT29 cells were harvested and lysed in a buffer containing 10 mmol/L Tris-HCl (pH 8.0), 150 mmol/L NaCl, 1% NP40, 0.5% sodium deoxycholate, 0.1% SDS, 1 mmol/L EDTA (pH 8.0), 2 mmol/L phenylmethylsulfonyl fluoride, 2 mg/L aprotinin, 2 mg/L leupeptin, and 1 mmol/L Na3VO4. For Western blotting analysis, 30 &mgr;g of total extracted proteins was applied per lane before SDS-PAGE. Following transfer to nitrocellulose membranes, protein expression levels were detected using anti-Bcl-xL (Alpha Diagnostics International, TX). The expression of β-actin (Sigma-Aldrich, MO) was used as a normalization control for protein loading.
For the anchorage-independent growth experiments, HT29 cells (8 × 103 cells/well) were seeded in 0.3% Difco Bactoagar (Difco, MI) supplemented with a complete culture medium. This suspension was layered over 0.5 mL of 0.8% agar-medium base layer in 24 multiwell cluster dishes (Becton Dickinson, Italy). After 15 d, the colonies were stained with nitroblue tetrazolium, and colonies larger than 50 &mgr;m were acquired with a micro-Scopeman camera system (Moritex Europe Ltd, Italy) and analyzed with Image-Pro Plus (Media Cybernetics, MD) computer program.
Transwell invasion assays were performed using HT29 cells cultured in 12-well plates containing either 8 &mgr;m pore matrigel-coated inserts according to the manufacturer’s instructions (Becton Dickinson, Bedford, MA). The membranes were rehydrated with warm serum-free (SF) Dulbecco’s modified Eagle’s medium (DMEM) (1.0 mL/chamber) for 2 h. The upper chamber was filled with 1 × 105 cells in L-15 medium containing 5% FBS. The lower chamber was filled with L-15 medium containing 25% FBS as a chemo-attractant. After the chambers were incubated for 24 h at 37°C in an atmosphere containing 50 mL/L CO2, non-invading cells were removed from the upper surface of the membrane by scrubbing, and invading cells on the lower surface of the membrane were fixed and stained with HE. The number of cells penetrating the filter was counted by a technician blinded to the experimental settings in four microscopic fields of each filter, under × 20 magnification. The percentage of invasion was expressed as the ratio of the mean cell number from the invasion chamber to the mean cell number from the control chamber according to the manufacturer’s recommendation.
All analyses were performed with t test and ANOVA using SPSS 11.5 software (Statistical Package for Social Science). P < 0.05 was considered statistically significant.
Bcl-xL expression was rarely expressed in normal large intestinal mucosa. However, Bcl-xL was mainly expressed in cytoplasm of the para-cancerous or cancer cells. The nuclei were stained brownish yellow, located sporadically or in the form of sheets. Quantitative immunohistochemistry analysis is summarized in Table 1. Bcl-xL PU was significantly higher in cancerous tissue samples than in normal tissue samples (38.78 ± 11.36 vs 0.89 ± 0.35, P < 0.001).
Correlation of Bcl-xL expression with clinicopathological parameters was evaluated. Bcl-xL PU, positive lymph nodes and Duke’s C/D stage were higher in cancerous tissue samples with low differentiation than in cancerous tissue samples with high differentiation (P < 0.05, Table 1). However, Bcl-xL expression was not correlated with sex, age of the patients.
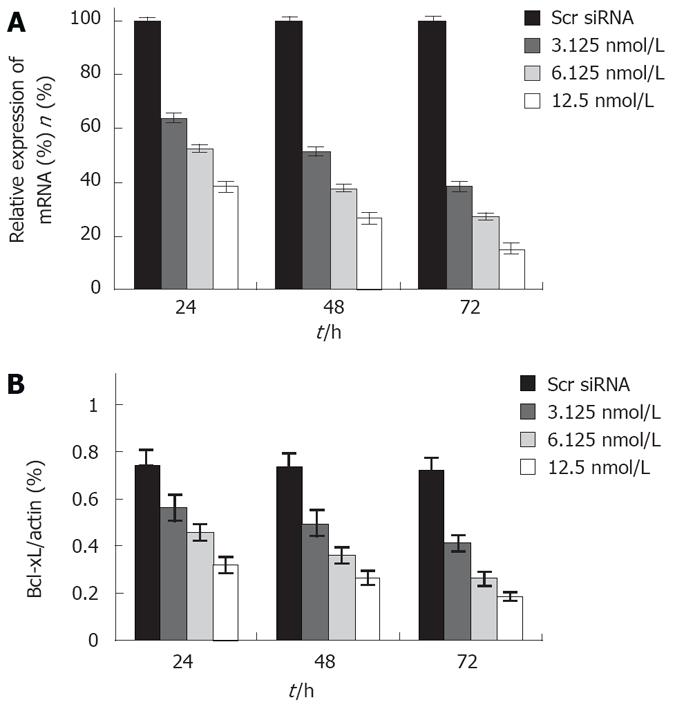
To further clarity the role of Bcl-xL gene, siRNA was used to knockdown the Bcl-xL expression in human colon cancer cells. Bcl-xL siRNA was transfected into the colon cancer cell line HT29. The ability of siRNA to down-regulate Bcl-xL expression was quantified by real time RT-PCR analysis and Western blot assay, respectively. siRNA significantly reduced the Bcl-xL mRNA and protein level in a dose- and time- dependent manner (Figure 1). However, the control scrambled siRNA treatment had no effect on Bcl-xL expression, thus supporting the specificity of Bcl-xL siRNA.
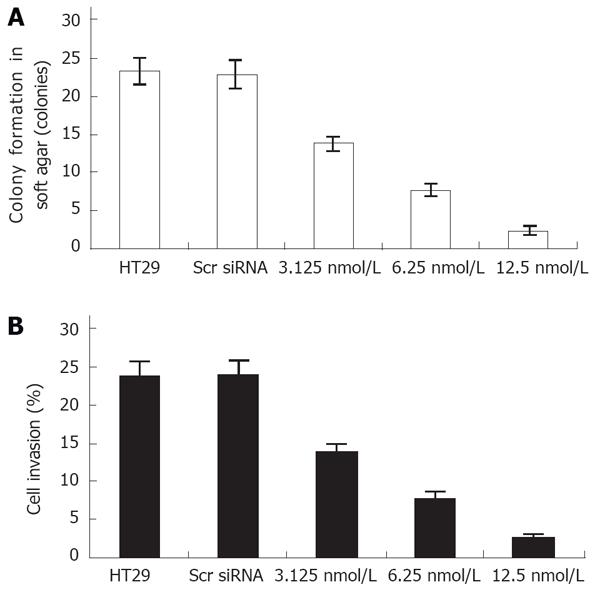
Next we evaluated the biological effects of Bcl-xL suppression on human colon cancer HT29 cells using several different types of assays. Colony formation in soft agar is a property closely associated with malignancy. Treatment with Bcl-xL siRNA significantly inhibited the anchorage-independent growth of human colon cancer cells in a dose-dependent manner (Figure 2A).
Given the known role of Bcl-xL siRNA in down-regulation of anchorage-independent HT29 cell growth, we attempted further to evaluate whether the Bcl-xL gene contributes to cell invasion of colon cancer cells. Cell invasion studies were performed using the matrigel matrix assays. The results showed that Bcl-xL siRNA treatment resulted in a dramatic low level of invasion potential of HT29 cells (Figure 2B), but not scrambled siRNA treatment.
The Bcl-2 family is characterized by the presence of Bcl-2 homology domains and falls into two main groups: anti-apoptotic proteins, such as Bcl-2, Bcl-xL, Bcl-w, Mcl-1, A1, and proapoptotic proteins, such as Bax, Bak, Bad, Bid, and Bcl-xS[6]. The Bcl-x gene encodes two proteins, a long form (Bcl-xL) and a short form (Bcl-xS), through an alternative splicing mechanism. Bcl-xL, displaying remarkable amino acids and an overall structural homology to Bcl-2, can effectively block apoptosis, whereas Bcl-xS, lacking 63 amino acids in Bcl-xL, is a dominant inhibitor of Bcl-2 activity and thereby acts as a proapoptotic factor[9].
Although there is evidence of cell apoptosis and Bcl-xL gene, the relationship between Bcl-xL and invasion of malignant tumors remains unclear. It was reported that Bcl-xL is related to the invasion and metastasis of some solid tumors. Zhang et al[18] and Takada et al[19] have reported the inhibition of invasion of cancer cells after treated with the Bcl-xL gene. Our previous study showed that STAT3 siRNA tranfection inhibits the invasion ability of human colon cancer cell line HT29 and that the Bcl-xL protein is significantly inhibited in transfected cancer cells[16], suggesting that Bcl-xL contributes to the invasion of human colon cancer cells. To verify it, we studied the relationship between Bcl-xL and invasion of human colon cancer in vivo and in vitro.
The expression of Bcl-xL protein in human colon cancer was determined by immunohistochemistry assay, showing that Bcl-xL protein was over-expressed in colon cancer tissue samples compared to normal tissue samples (P < 0.001). Meanwhile, Bcl-xL expression had no significant correlation with sex and age of the patients, but was greatly correlated with differentiation stage, lymph node metastasis, and Duke’s stage of colorectal carcinoma (P < 0.05), indicating that Bcl-xL over-expression is related to the development and invasion of human colon cancer.
In order to further investigate the relationship between Bcl-xL and invasion of human colon cancer cells, we studied the effects of Bcl-xL down-regulated by siRNA on the invasion ability of human colon cancer cells. SiRNA is a short oligonucleotide consisting of 21-23 nucleotides that can be used in vitro to induce sequence specific gene silencing of mammalian cells[20]. To elucidate the role of Bcl-xL gene in human colon cancer, siRNA was used to knockdown the Bcl-xL expression in human colon cancer cell line HT29. Real time RT-PCR and Western blot analysis showed that the expression of Bcl-xL in HT29 cancer cells transfected with siRNA was significantly reduced in a dose- and time-dependent manner. In addition, tranfection of human colon cancer HT29 cells with Bcl-xL siRNA decreased the invasion ability and anchorage-independent growth of human colon cancer cells. The data in vitro suggest that the Bcl-xL gene plays an important role in regulating the invasion of human colon cancer cell.
In conclusion, the Bcl-xL gene is relevant to the invasion and progression of human colon cancer, and can be used in evaluating the carcinogenesis of human colon cancer. However, the precise mechanism of Bcl-xL underlying the carcinogenesis of human colon cancer is still unclear, and further study is needed.
Despite recent advances in diagnostic and therapeutic measures, the prognosis of colorectal cancer patients with distant metastasis still remains poor. Enhanced understanding of the signaling mechanism underlying metastasis of colon cancer may provide important insights into more effective therapeutic strategies.
The results of this study indicate that the Bcl-xL gene plays an important role in the carcinogenesis of human colorectal carcinoma and is associated with the malignant biological behaviors of colorectal carcinoma.
The results of the present study suggest that the Bcl-xL gene is relevant to the invasion and progression of human colon cancer and can be used in evaluating the carcinogenesis of human colon cancer.
The paper helps to clarify the mechanism underlying the invasion and metastasis of colon cancer and contributes to the choice of therapeutic strategies.
This interesting article indicates that the Bcl-xL gene is relevant to the invasion and progression of human colon cancer, and might be used in evaluating the carcinogenesis of human colon cancer.
| 1. | Shike M, Winawer SJ, Greenwald PH, Bloch A, Hill MJ, Swaroop SV. Primary prevention of colorectal cancer. The WHO Collaborating Centre for the Prevention of Colorectal Cancer. Bull World Health Organ. 1990;68:377-385. |
| 2. | Winawer SJ, Fletcher RH, Miller L, Godlee F, Stolar MH, Mulrow CD, Woolf SH, Glick SN, Ganiats TG, Bond JH. Colorectal cancer screening: clinical guidelines and rationale. Gastroenterology. 1997;112:594-642. |
| 3. | Nunez G, Clarke MF. The Bcl-2 family of proteins: regulators of cell death and survival. Trends Cell Biol. 1994;4:399-403. |
| 4. | Chao DT, Korsmeyer SJ. BCL-2 family: regulators of cell death. Annu Rev Immunol. 1998;16:395-419. |
| 5. | Vander Heiden MG, Chandel NS, Williamson EK, Schumacker PT, Thompson CB. Bcl-xL regulates the membrane potential and volume homeostasis of mitochondria. Cell. 1997;91:627-637. |
| 6. | Gottlieb E, Vander Heiden MG, Thompson CB. Bcl-x(L) prevents the initial decrease in mitochondrial membrane potential and subsequent reactive oxygen species production during tumor necrosis factor alpha-induced apoptosis. Mol Cell Biol. 2000;20:5680-5689. |
| 7. | Minn AJ, Rudin CM, Boise LH, Thompson CB. Expression of bcl-xL can confer a multidrug resistance phenotype. Blood. 1995;86:1903-1910. |
| 8. | Kharbanda S, Pandey P, Schofield L, Israels S, Roncinske R, Yoshida K, Bharti A, Yuan ZM, Saxena S, Weichselbaum R. Role for Bcl-xL as an inhibitor of cytosolic cytochrome C accumulation in DNA damage-induced apoptosis. Proc Natl Acad Sci USA. 1997;94:6939-6942. |
| 9. | Zhang L, Yu J, Park BH, Kinzler KW, Vogelstein B. Role of BAX in the apoptotic response to anticancer agents. Science. 2000;290:989-992. |
| 10. | Fernández Y, España L, Mañas S, Fabra A, Sierra A. Bcl-xL promotes metastasis of breast cancer cells by induction of cytokines resistance. Cell Death Differ. 2000;7:350-359. |
| 11. | España L, Fernández Y, Rubio N, Torregrosa A, Blanco J, Sierra A. Overexpression of Bcl-xL in human breast cancer cells enhances organ-selective lymph node metastasis. Breast Cancer Res Treat. 2004;87:33-44. |
| 12. | Watanabe J, Kushihata F, Honda K, Sugita A, Tateishi N, Mominoki K, Matsuda S, Kobayashi N. Prognostic significance of Bcl-xL in human hepatocellular carcinoma. Surgery. 2004;135:604-612. |
| 13. | Frankel A, Rosen K, Filmus J, Kerbel RS. Induction of anoikis and suppression of human ovarian tumor growth in vivo by down-regulation of Bcl-X(L). Cancer Res. 2001;61:4837-4841. |
| 14. | Weiler M, Bahr O, Hohlweg U, Naumann U, Rieger J, Huang H, Tabatabai G, Krell HW, Ohgaki H, Weller M. BCL-xL: time-dependent dissociation between modulation of apoptosis and invasiveness in human malignant glioma cells. Cell Death Differ. 2006;13:1156-1169. |
| 15. | Sánchez-Ceja SG, Reyes-Maldonado E, Vázquez-Manríquez ME, López-Luna JJ, Belmont A, Gutiérrez-Castellanos S. Differential expression of STAT5 and Bcl-xL, and high expression of Neu and STAT3 in non-small-cell lung carcinoma. Lung Cancer. 2006;54:163-168. |
| 16. | Fan Y, Zhang YL, Wu Y, Zhang W, Wang YH, Cheng ZM, Li H. Inhibition of signal transducer and activator of transcription 3 expression by RNA interference suppresses invasion through inducing anoikis in human colon cancer cells. World J Gastroenterol. 2008;14:428-434. |
| 17. | Tan HY, Liu J, Wu SM, Luo HS. Expression of a novel apoptosis inhibitor-survivin in colorectal carcinoma. World J Gastroenterol. 2005;11:4689-4692. |
| 18. | Zhang X, Xu Q, Saiki I. Quercetin inhibits the invasion and mobility of murine melanoma B16-BL6 cells through inducing apoptosis via decreasing Bcl-2 expression. Clin Exp Metastasis. 2000;18:415-421. |
| 19. | Takada Y, Kobayashi Y, Aggarwal BB. Evodiamine abolishes constitutive and inducible NF-kappaB activation by inhibiting IkappaBalpha kinase activation, thereby suppressing NF-kappaB-regulated antiapoptotic and metastatic gene expression, up-regulating apoptosis, and inhibiting invasion. J Biol Chem. 2005;280:17203-17212. |