Published online May 21, 2008. doi: 10.3748/wjg.14.3015
Revised: April 7, 2008
Published online: May 21, 2008
AIM: To investigate the malignant potential of hepatic stem cells derived from the bone marrow stromal cells (BMSCs) in a mouse model of chemical hepatocarcino-genesis.
METHODS: BMSCs from male BALB/c mice were harvested and cultured, then transplanted into female syngenic BALB/c mice via portal vein. Hepato-carcinogenesis was induced by 6 mo of treatment with diethylnitrosamine (DEN). Six months later, the liver was removed from each treated mouse and evaluated by immunohistochemistry and fluorescence in situ hybridization (FISH).
RESULTS: Twenty-six percent of recipient mice survived and developed multiple hepatocellular carcinomas (HCCs). Immunohistochemically, HCC expressed placental form of glutathione-S-transferase (GST-P) and α-fetoprotein, but did not express cytokeratin 19. Y chromosome positive hepatocytes were detected by fluorescent in situ hybridization (FISH) in the liver of mice treated with DEN after BMSCs transplantation while no such hepatocytes were identified in the liver of mice not treated with DEN. No HCC was positive for the Y chromosome by FISH.
CONCLUSION: Hepatic stem cells derived from the bone marrow stromal cells have a low malignant potential in our mouse model of chemical hepatocarcinogenesis.
- Citation: Zheng JF, Liang LJ. Transplanted bone marrow stromal cells are not cellular origin of hepatocellular carcinomas in a mouse model of carcinogenesis. World J Gastroenterol 2008; 14(19): 3015-3020
- URL: https://www.wjgnet.com/1007-9327/full/v14/i19/3015.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.3015
Hepatocellular carcinoma (HCC) is the fifth most common cancer in the world[1]. Hepatitis B or C virus can induce chronic hepatitis and potentially result in liver cirrhosis and HCC, and these viral infections are frequently seen among HCC patients[2]. However, there is no clear evidence as to which cell is directly involved in the development of HCCs[3–5]. Two cell lineages have been considered as candidates: the first is hepatic stem cell, and the second is mature hepatocyte.
Oval cells are small, oval shaped epithelial cells identified as hepatic stem cells in the adult liver only following severe, repetitive liver injury[6]. There are increasing evidences that oval cells are the cellular targets for transformation in the development of HCC[78]. Oval cells might give rise to HCC as a result of the arrest of stem cell maturation[9]. Previous studies indicated that bone marrow cells can differentiate into oval cells in rodents and that a similar process could possibly take place in humans[1011]. The incidence of plasticity has been shown to be very variable, from extremely rare to a range from 20% to 40%[1213]. Although there is still controversy about which part of bone marrow cells can differentiate into hepatocytes, the present study clearly shows that transplanted bone marrow cells may help restore the hepatic degenerative diseases and reduce CCl4-induced liver fibrosis[14]. Some studies readily demonstrated bone marrow stromal cells (BMSCs) differentiated into hepatocyte-like cells in culture after HGF treatment in vitro[15]. Therefore, BMSCs could be a valuable strategy for future replacement therapy of damaged or malfunctioned hepatocytes, because getting autologous BMSCs is easier than obtaining other tissue-specific stem cells. However, the safety and efficacy of hepatic stem cells derived from bone marrow cells should be adequately confirmed before any such therapies are tested in humans.
Our aim was to study the malignant potential of hepatic stem cells derived from BMSCs in vivo. To identify hepatic stem cells, BMSCs of male mice were transplanted into recipient female mice. After BMSCs transplantation, HCC was induced in the recipients by chemical hepatocarcinogenic compounds and the presence of the Y chromosome was evaluated in HCC.
Six to eight week old BALB/c mice were purchased from the Animal Breeding Center of Sun Yat-Sen University (Guangzhou, China). Mice were bred and maintained in an air-conditioned animal house with specific pathogen-free conditions, using an alternate 12 h cycle of daylight and darkness, and unlimited access to chow and water. All animal experiments were performed in accordance with the guidelines of the Animal Care and Use Committee of Sun Yat-Sen University.
BMSCs were harvested from bone marrow of the femurs and tibias of male mice by inserting a 21-gauge needle into the shaft of the bone and flushing it with DMEM medium supplemented with heparin[16]. The cell suspension was centrifuged over a Ficoll step gradient (density 1.077 g/mL) (Sigma, St. Louis, MO) at 1500 r/min for 10 min. The interface fraction was then collected and cultured in DMEM medium, supplemented with 10% fetal bovine serum, 2 mmol/L L-glutamine, 100 U/mL penicillin, and 100 &mgr;g/mL streptomycin. Isolated cells were grown at 37°C and 5% CO2 for 3 d. After removing the suspended cells, the adherent BMSCs were grown to 90% confluence and used between passages 3 and 4. After serum starvation for 4 h, BMSCs were treated with human recombinant HGF (Sigma-Aldrich, USA) at a concentration of 50 &mgr;g/L. Cultures were maintained by media exchange every 3 d. On d 21, all cells were detached for the next experiment.
The above detached cells were coated on the glass slides and fixed with 4% paraformaldehyde for 10 min at room temperature, followed by methanol for 2 min at -20°C, and permeabilized with 0.1% Triton X-100 for 10 min. Slides were blocked for 30 min using blocking and diluent solution, then incubated with rabbit anti-α-fetoprotein antibody or goat anti-albumin antibody at 4°C overnight. The cells were reincubated sequentially for 30 min with FITC-conjugated secondary antibody or PE-conjugated secondary antibody. The slides were counterstained with 4’,6-diamidino-2-phenylindole (DAPI, Sigma) before mounting and observed under fluorescence microscope.
BMSCs were harvested after cultured for passages 3 or 4 and suspended in DMEM medium supplemented with penicillin/streptomycin. BMSCs were washed twice in DMEM medium before intraportal injection. Cell viability (> 95%) was measured by trypan blue dye exclusion.
Anesthesia was performed with ether and partial hepatectomy used the standard method for two-thirds resection[17]. Briefly, after ligation of the pedicule and resection of the two largest lobes (median and left), the remaining liver was composed of the caudate and epiploic lobes. BMSCs were injected into the female liver via the superior mesenteric vein using insulin syringes after hepatectomy[18]. A total of 106 cells were injected per mouse.
After partial hepatectomy and BMSCs injection, mice were allowed to recover for one week. Thereafter, DEN (Sigma) was continuously administered for 12 wk through drinking water at a final concentration of 100 &mgr;g/L to induce hepatocarcinogenesis[3].
Sixty female BALB/c mice were randomly assigned to three groups. Ten mice in the normal control group were given BMSCs and non-supplemented drinking water. Twenty-five mice in the model group were continuously administered DEN in the drinking water. Twenty-five mice in the experimental group received BMSCs and DEN. The animals were sacrificed at 6 mo after the carcinogen regimen and the livers were fixed in 10% formalin for 24 h and embedded with paraffin. Routine histology was performed with haematoxylin-eosin staining. Serial sections were cut from liver samples with the macroscopically visible liver tumors and the right lobe for pathologic examination.
To identify characteristics of tumors in the liver after BMSCs transplantation and DNE administration, placental form of glutathione-S-transferase (GST-P), α-fetoprotein, and cytokeratin 19 were assayed immunohistochemically for these tumor nodules as previously described[19]. Briefly, after being deparaffinized with xylene, quenched with hydrogen peroxide and blocked with normal serum, the liver tissue sections were incubated for 1 h with rabbit anti-α-fetoprotein polyclonal antibody (dilution 1:100; Santa Cruz, USA), goat anti-cytokeratin 19 monoclonal antibody (dilution 1:100; Santa Cruz), or goat anti-GST-P polyclonal antibody (dilution 1:1000; Stressgen, Canada). FITC-conjugated secondary antibody or PE-conjugated secondary antibody was added. Counterstaining of nuclei was performed with DAPI for fluorescence staining.
Because BMSCs transplantation was performed from male donor mice to female recipient mice, the transplanted bone marrow derived cells could be recognized in the recipient by the presence of the Y chromosome in the nucleus. Therefore, FISH for the mouse Y chromosome was conducted to detect the transplanted bone marrow derived cells according to the Cambio protocol (http://www.cambio.co.uk/). Paraffin-embedded slides were deparaffinized by baking in an oven overnight at 37°C and cleared in xylene three times for 10 min each; and they were then dehydrated and air-dried. Sections were incubated in 1 mol/L sodium thiocyanate for 10 min at 80°C, washed in PBS, and digested in pepsin (0.4% w/v) in 0.1 mol/L HCl at 37°C for 10 min. The protease was quenched in glycine (0.2% v/w) in 2 × PBS, post-fixed in paraformaldehyde (4% w/v) in PBS, dehydrated through graded alcohols and air-dried. A fluorescein isothiocyanate (FITC)-labeled Y-chromosome paint (Cambio, Cambridge, UK) was added to the sections, sealed under glass with rubber cement, heated to 80°C for 10 min, and incubated overnight at 37°C. The slides were washed in formamide (50% w/v)/2 × saline sodium citrate (SSC) at 37°C, washed with 2 × SSC and 4 × SSC/Tween-20 (0.05% w/v) at 37°C. The slides were rinsed in 0.5 × SSC at 37°C. FITC amplification kit (Cambio) was used to amplify fluorescence signal. The slides were counterstained with DAPI before mounting and observed under confocal microscope (Zeiss, German).
Data were presented as mean ± SD. Significant differences were determined using ANOVA in SPSS10.0. P < 0.05 was considered statistically significant.
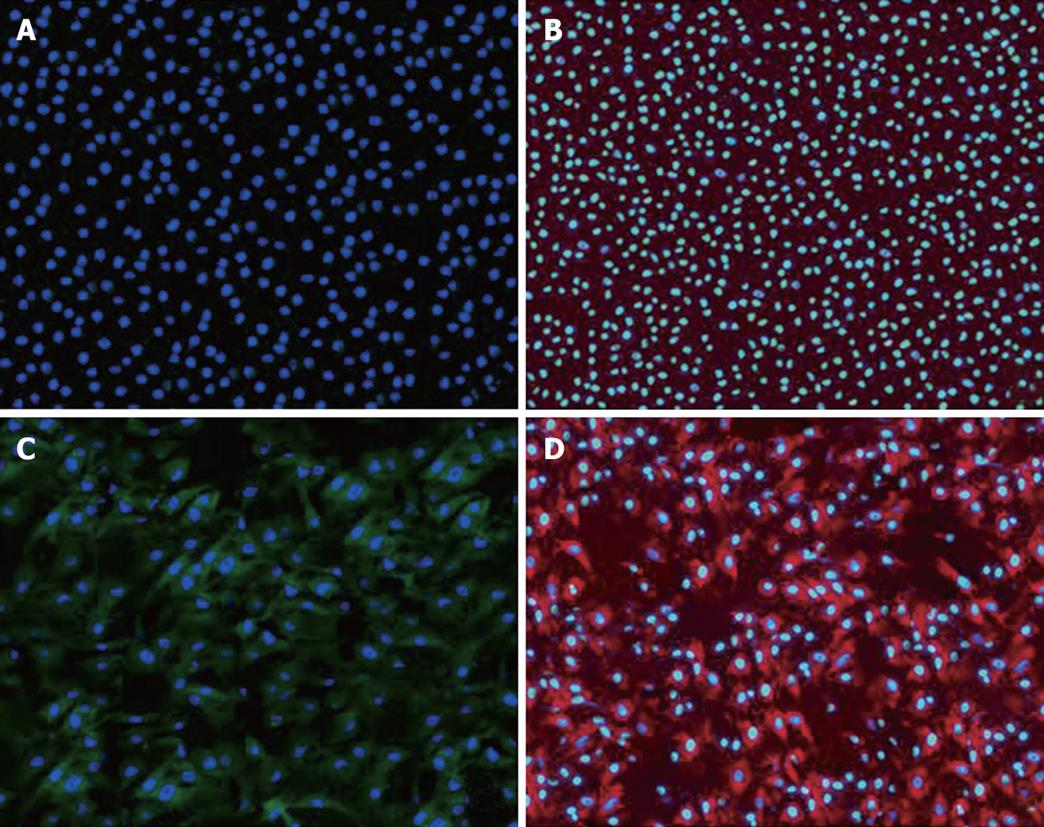
To confirm the differentiation of BMSCs into hepatocytes, we selected cultural BMSCs with or without treatment of HGF for 21 d in culture and examined the expression of α-fetoprotein and albumin by immunofluorescence. We found that cultural BMSCs without treatment of HGF could not express α-fetoprotein and albumin (Figure 1A and B), while differentiated hepatocyte-like BMSCs with treatment of HGF expressed α-fetoprotein and albumin (Figure 1C and D).
We evaluated the survival rate of mice that underwent BMSCs transplantation and/or DEN treatment. All mice that underwent BMSCs transplantation were still alive at the end of the study. Thirteen (26.0%) of 50 mice induced with DEN survived at the end of the 6-month study period, including six mice in the model group and seven in the experimental group. The survival rates were similar between the model group and the experimental group (P > 0.05).
All of the survived recipient mice developed multiple HCCs. Thirteen mice developed HCCs including six mice in the model group and seven mice in the experiment group. These tumors were evenly distributed among the liver lobes of mice. The average sizes of hepatic tumors were not different between the two groups (4.8 ± 1.5 mm vs 4.4 ± 1.1 mm; P > 0.05).
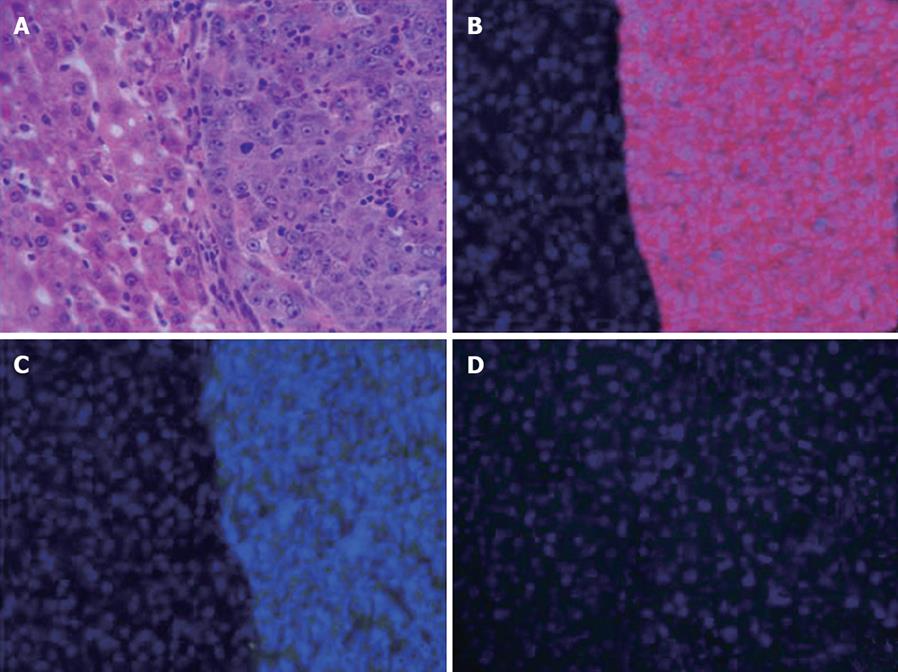
HE stained sections of these tumors confirmed to be HCCs (Figure 2A) expressed GST-P and α-fetoprotein (Figure 2B and C), but not Cytokeratin 19 (Figure 2D). No other types of liver tumors, such as hepatoblastoma or cholangiocellular carcinoma, were noted in our experiment.
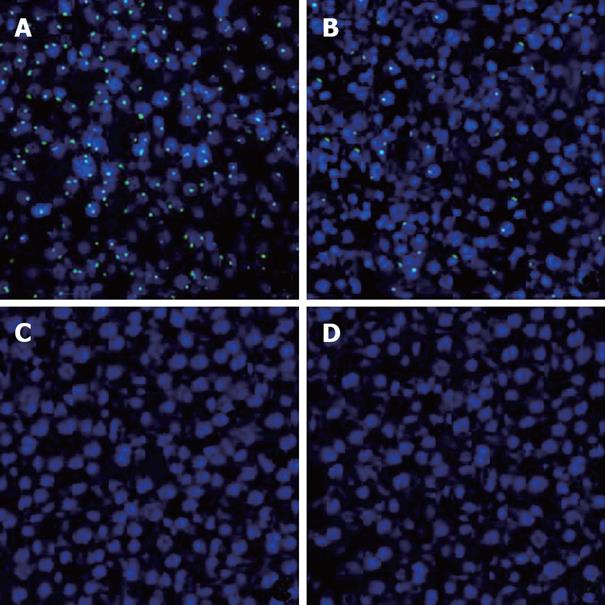
To follow the repopulation and differentiation of BMSCs, we transplanted male BMSCs into the liver of normal and DEN-treated female mice. FISH was performed to detect Y chromosome in the female recipients. A positive FISH signal was detected in the nucleus which was confirmed by counterstaining with DAPI. In male mouse liver, which served as positive control, most of the cells stained positive for Y-chromosome with fluorescein signal in the nuclei (Figure 3A).
We found that male BMSCs infused via portal vein into female syngeneic mouse liver could engraft and differentiate into hepatocytes after induction with DEN using FISH for Y chromosome. Six months after BMSCs transplantation and DEN challenge, FISH revealed that 15% of hepatocyte nuclei were positive for the Y chromosome in the liver of female recipients (Figure 3B). In addition, no nucleus showed two or more signals. However, donor-derived cells were not detected when BMSCs were transplanted to normal recipients without DEN treatment (Figure 3C). Moreover, no HCC was positive for the Y chromosome by FISH (Figure 3D).
The liver is classified as a conditionally renewing tissue and hepatocytes proliferate quiescently and hepatic stem cells are not needed under normal circumstances. Oval cells reside within or adjacent to the canals of Hering and comprise a quiescent compartment of dormant stem cells in adult livers[6]. They can be activated to proliferate and differentiate into hepatocytes or bile duct epithelial cells when there is severe hepatic liver damage and coexistent impaired hepatocyte regeneration.
Accumulated evidence indicates that bone marrow cells can differentiate into specific cell types[20]. It has been reported that 30%-50% liver regeneration with bone marrow-derived cells in the FAH mouse model offers a selective proliferative advantage in the transplanted cells[21]. Bone marrow-derived hepatocytes may originate from the mesenchymal compartment, rather than the hematopoietic compartment[22]. However, other data demonstrate that bone marrow-derived hepatocyte is only a possible but rare event, even in the presence of very strong selection pressure[23]. Several reports have demonstrated that cell fusion is the principal source of bone marrow-derived hepatocytes[24], and bone marrow-derived hepatocytes are primarily of mature myelomonocytic cells which fuse spontaneously with host hepatocytes producing functional liver repopulation[14].
The identity of the specific cell types that differentiate to express hepatocyte characteristics remains undetermined. BMSCs comprise marrow stromal stem cells, sharing characteristics with other multi-potent stem cells such as neural stem cells and hematopoietic stem cells, because they possess the capability of self-renewal and progeny differentiation potentials[25]. Our study demonstrates that cultured BMSCs differentiated hepatocyte-like cells which expressed α-fetoprotein and albumin with the treatment of HGF in vitro. After BMSCs transplantation, Y chromosome positive cells appeared only in mice treated with DEN and not in mice who did not receive DEN. These results suggest that in our model, BMSCs can differentiate into hepatocytes under limited conditions. Bone marrow-derived hepatic stem cells seem not to be required for normal hepatocyte substitution. Indeed, in the present study, we found that in positive hepatocytes, no nucleus had two or more Y chromosomes by FISH. This finding indicates that transdifferentiation, rather than cell fusion, was the main process in our model.
DEN is a DNA alkylating agent that is rapidly metabolized to reactive metabolites. These metabolites interact with DNA to form various DNA adducts, leading to genetic alterations[26]. GST-P is a highly expressed cytoplasmic protein during early and late steps of carcinogenesis and GST-P sensitivity is higher than that of other enzymes for the detection of malignant transformation[27]. Seventy percent of HCCs were stained positively for α-fetoprotein in clinical cases. In our study, chronic exposure to DEN caused multiple HCCs. These HCCs express GST-P and α-fetoprotein, but not cytokeratin 19 which was expressed in cholangiocellular carcinoma.
In this study, we focused our interest on the original cell lineage of carcinogenesis. There are two major nonexclusive hypotheses of the cellular origin of cancer: from stem cells due to maturation arrest or from dedifferentiation of mature cells. Debate has centered on whether hepatocytes are responsible for HCCs through dedifferentiation, or whether oval cells are the prime target for malignant changes after a differential “block”[35]. Oval cells are possibly involved in hepatocarcinogenesis based on the followings: (1) massive existence of oval cells in an animal rodent hepatocarcinogenic model[28]; (2) development of HCC after transformation of oval cells[8]; and (3) occurrence of mixed hepatocellular and cholangiocarcinomatous tumors (oval cell exhibits bipotential developmental ability)[29]. However, the relationship between oval cells and cancer is only circumstantial. In this study, no HCC was positive for Y chromosome after long-term carcinogenic induction. However, as all hepatic stem cells might not be labeled by our method as mentioned above, we cannot completely exclude the stem cell theory. Although our results may be limited to BMSCs transplanted mice treated with DEN, we can state that the malignant potential of the hepatic stem cell derived from bone marrow seems to be low. Further studies are needed to clarify the precise interaction of bone marrow cells with hepatic regeneration and carcinogenesis using other animal models or human studies.
In conclusion, our study demonstrates that cultured BMSCs could differentiate hepatocyte-like cells with HGF treatment in vitro. BMSCs can differentiate into hepatocytes in our model. Hepatic stem cells derived from bone marrow stromal cells are not cellular origin of hepatocellular carcinomas in the DEN model of carcinogenesis. Bone marrow cells may potentially be used in cell based replacement therapy or gene delivery systems. Under these circumstances, our results indicate that hepatic stem cell therapy derived from bone marrow is safe.
Bone marrow stromal cells can differentiate into hepatic stem cells in rodents and in humans. Bone marrow stromal cells could be a valuable strategy for future replacement therapy of damaged or malfunctioned hepatocytes, because getting autologous bone marrow cells is easier than obtaining other tissue-specific stem cells. However, the safety and efficacy of hepatic stem cells derived from bone marrow stromal cells should be adequately confirmed before any such therapies are tested in humans.
There are two major nonexclusive hypotheses of the cellular origin of cancer: from stem cells due to maturation arrest or from dedifferentiation of mature cells. Debate has centered on whether hepatocytes are responsible for hepatocellular carcinoma (HCC) through a process of dedifferentiation, or whether oval cells are the prime target for malignant changes after a differential “block”. There are increasing evidences that oval cells are the cellular targets for transformation in the development of HCC. Accumulating evidences indicate that bone marrow cells can differentiate into specific cell types. It has been reported that 30%-50% liver regeneration with bone marrow-derived cells in the FAH mouse model offers a selective proliferative advantage in the transplanted cells. Bone marrow-derived hepatocytes may originate from the mesenchymal compartment, rather than the hematopoietic compartment.
This study demonstrates that cultured bone marrow stromal cells could differentiate hepatocyte-like cells in vitro. Bone marrow stromal cells can differentiate into hepatocytes. Hepatic stem cells derived from bone marrow stromal cells are not cellular origin of hepatocellular carcinomas in a mouse model of carcinogenesis. Bone marrow cells may potentially be used in cell based replacement therapy or gene delivery systems. The results in this study indicate that hepatic stem cell therapy derived from bone marrow is safe.
Bone marrow stromal cells might be applicable for future replacement therapy of damaged or malfunctioned hepatocytes.
This paper is interesting and the study appears well conducted. The conclusions, although of a limited scope given the design of the study, are in agreement with the results.
| 1. | Kao JH, Chen DS. Changing disease burden of hepatocellular carcinoma in the Far East and Southeast Asia. Liver Int. 2005;25:696-703. |
| 2. | Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45:529-538. |
| 3. | Bralet MP, Pichard V, Ferry N. Demonstration of direct lineage between hepatocytes and hepatocellular carcinoma in diethylnitrosamine-treated rats. Hepatology. 2002;36:623-630. |
| 4. | Gournay J, Auvigne I, Pichard V, Ligeza C, Bralet MP, Ferry N. In vivo cell lineage analysis during chemical hepatocarcinogenesis in rats using retroviral-mediated gene transfer: evidence for dedifferentiation of mature hepatocytes. Lab Invest. 2002;82:781-788. |
| 5. | Lee JS, Heo J, Libbrecht L, Chu IS, Kaposi-Novak P, Calvisi DF, Mikaelyan A, Roberts LR, Demetris AJ, Sun Z. A novel prognostic subtype of human hepatocellular carcinoma derived from hepatic progenitor cells. Nat Med. 2006;12:410-416. |
| 6. | Kofman AV, Morgan G, Kirschenbaum A, Osbeck J, Hussain M, Swenson S, Theise ND. Dose- and time-dependent oval cell reaction in acetaminophen-induced murine liver injury. Hepatology. 2005;41:1252-1261. |
| 7. | Yamamoto T, Uenishi T, Ogawa M, Ichikawa T, Hai S, Sakabe K, Tanaka S, Kato H, Mikami S, Ikebe T. Immunohistologic attempt to find carcinogenesis from hepatic progenitor cell in hepatocellular carcinoma. Dig Surg. 2005;22:364-370. |
| 8. | Dumble ML, Croager EJ, Yeoh GC, Quail EA. Generation and characterization of p53 null transformed hepatic progenitor cells: oval cells give rise to hepatocellular carcinoma. Carcinogenesis. 2002;23:435-445. |
| 9. | Dumble ML, Knight B, Quail EA, Yeoh GC. Hepatoblast-like cells populate the adult p53 knockout mouse liver: evidence for a hyperproliferative maturation-arrested stem cell compartment. Cell Growth Differ. 2001;12:223-231. |
| 10. | Lagasse E, Connors H, Al-Dhalimy M, Reitsma M, Dohse M, Osborne L, Wang X, Finegold M, Weissman IL, Grompe M. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med. 2000;6:1229-1234. |
| 11. | Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168-1170. |
| 12. | Wagers AJ, Sherwood RI, Christensen JL, Weissman IL. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science. 2002;297:2256-2259. |
| 14. | Sakaida I, Terai S, Yamamoto N, Aoyama K, Ishikawa T, Nishina H, Okita K. Transplantation of bone marrow cells reduces CCl4-induced liver fibrosis in mice. Hepatology. 2004;40:1304-1311. |
| 15. | Wang PP, Wang JH, Yan ZP, Hu MY, Lau GK, Fan ST, Luk JM. Expression of hepatocyte-like phenotypes in bone marrow stromal cells after HGF induction. Biochem Biophys Res Commun. 2004;320:712-716. |
| 16. | Luk JM, Wang PP, Lee CK, Wang JH, Fan ST. Hepatic potential of bone marrow stromal cells: development of in vitro co-culture and intra-portal transplantation models. J Immunol Methods. 2005;305:39-47. |
| 17. | Oertel M, Rosencrantz R, Chen YQ, Thota PN, Sandhu JS, Dabeva MD, Pacchia AL, Adelson ME, Dougherty JP, Shafritz DA. Repopulation of rat liver by fetal hepatoblasts and adult hepatocytes transduced ex vivo with lentiviral vectors. Hepatology. 2003;37:994-1005. |
| 18. | Kushida T, Inaba M, Hisha H, Ichioka N, Esumi T, Ogawa R, Iida H, Ikehara S. Crucial role of donor-derived stromal cells in successful treatment for intractable autoimmune diseases in mrl/lpr mice by bmt via portal vein. Stem Cells. 2001;19:226-235. |
| 19. | Vig P, Russo FP, Edwards RJ, Tadrous PJ, Wright NA, Thomas HC, Alison MR, Forbes SJ. The sources of parenchymal regeneration after chronic hepatocellular liver injury in mice. Hepatology. 2006;43:316-324. |
| 20. | Alison MR, Poulsom R, Jeffery R, Dhillon AP, Quaglia A, Jacob J, Novelli M, Prentice G, Williamson J, Wright NA. Hepatocytes from non-hepatic adult stem cells. Nature. 2000;406:257. |
| 21. | Jang YY, Collector MI, Baylin SB, Diehl AM, Sharkis SJ. Hematopoietic stem cells convert into liver cells within days without fusion. Nat Cell Biol. 2004;6:532-539. |
| 22. | Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41-49. |
| 23. | Kanazawa Y, Verma IM. Little evidence of bone marrow-derived hepatocytes in the replacement of injured liver. Proc Natl Acad Sci USA. 2003;100 Suppl 1:11850-11853. |
| 24. | Wang X, Willenbring H, Akkari Y, Torimaru Y, Foster M, Al-Dhalimy M, Lagasse E, Finegold M, Olson S, Grompe M. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature. 2003;422:897-901. |
| 25. | Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, Dzau VJ. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9:1195-1201. |
| 26. | Kagawa M, Sano T, Ishibashi N, Hashimoto M, Okuno M, Moriwaki H, Suzuki R, Kohno H, Tanaka T. An acyclic retinoid, NIK-333, inhibits N-diethylnitrosamine-induced rat hepatocarcinogenesis through suppression of TGF-alpha expression and cell proliferation. Carcinogenesis. 2004;25:979-985. |
| 27. | Sakata K, Hara A, Hirose Y, Yamada Y, Kuno T, Katayama M, Yoshida K, Zheng Q, Murakami A, Ohigashi H. Dietary supplementation of the citrus antioxidant auraptene inhibits N,N-diethylnitrosamine-induced rat hepatocarcinogenesis. Oncology. 2004;66:244-252. |
| 28. | Choudhury S, Zhang R, Frenkel K, Kawamori T, Chung FL, Roy R. Evidence of alterations in base excision repair of oxidative DNA damage during spontaneous hepatocarcinogenesis in Long Evans Cinnamon rats. Cancer Res. 2003;63:7704-7707. |
| 29. | Wakasa T, Wakasa K, Shutou T, Hai S, Kubo S, Hirohashi K, Umeshita K, Monden M. A histopathological study on combined hepatocellular and cholangiocarcinoma: cholangiocarcinoma component is originated from hepatocellular carcinoma. Hepatogastroenterology. 2007;54:508-513. |