Published online May 21, 2008. doi: 10.3748/wjg.14.2995
Revised: February 19, 2008
Published online: May 21, 2008
Cholangiocarcinoma is a rare malignancy of the biliary tract. Key factors in determining therapeutic options include knowledge of tumor extent, anatomy and obtaining tissue diagnosis. Endoscopically, there are three modalities available to make the diagnosis of cholangiocarcinoma. These include endoscopic retrograde cholangiopancreatography, endoscopic ultrasound with fine needle aspiration and cholangioscopy. Management of cholangiocarcinoma endoscopically is typically confined to stent placement for palliative purposes or as a bridge to surgery. In this article, we will review the endoscopic techniques available for the diagnosis and management of cholangiocarcinoma.
- Citation: Nguyen K, Jr JTS. Review of endoscopic techniques in the diagnosis and management of cholangiocarcinoma. World J Gastroenterol 2008; 14(19): 2995-2999
- URL: https://www.wjgnet.com/1007-9327/full/v14/i19/2995.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.2995
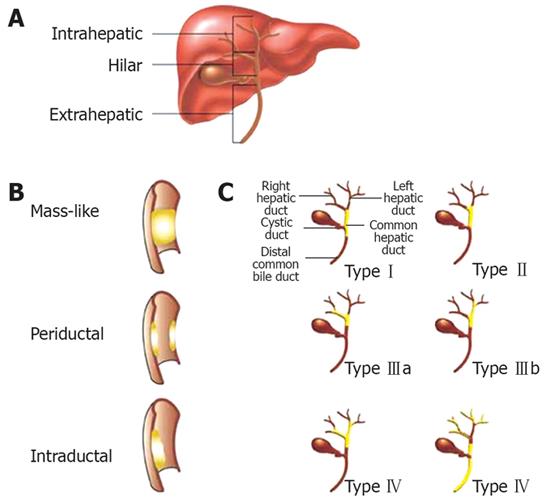
Cholangiocarcinomas are rare malignancies involving the biliary tract. They can be divided into three anatomic groups: intrahepatic, perihilar, and distal extrahepatic. Perihilar tumors, also known as Klastkin tumors, involve the hepatic duct bifurcation. They are the most common, accounting for about 60%-80% of cholangiocarcinomas. Intrahepatic tumors are least common[1]. The Bismuth classification is used to describe the biliary tract involvement and is helpful in planning surgical intervention. TypeItumors are found below the bifurcation of the left and right hepatic ducts. Type II tumors involve the bifurcation. Type IIIa and IIIb tumors occlude the common hepatic duct and either the right or left hepatic duct, respectively. Type IV tumors are multicentric, or they involve the bifurcation and both the right and left hepatic ducts (Figure 1). The incidence rates for cholangiocarcinomas vary depending on geographic location with the highest rates found in Southeast Asia. In the United States, between 4000 and 5000 cases are found annually. For unknown reason, the incidence of and mortality rates for intrahepatic cholangiocarcinomas have been increasing in recent years while the incidence of extrahepatic cholangiocarcinomas have been decreasing[2]. Accurate knowledge of tumor extent and anatomy as well as obtaining a tissue diagnosis is important in determining therapeutic options. In this article, we will review the endoscopic modalities available in the diagnosis and management of cholangiocarcinomas.
The etiology of biliary strictures can often be difficult to establish. The differential diagnosis of biliary strictures is extensive and includes, but is not limited to, primary sclerosing cholangitis, gallbladder carcinoma, pancreatic carcinoma, intraductal papillary mucinous tumor, or benign biliary strictures from causes such as pancreatitis.
Cholangiocarcinomas often pose a diagnostic challenge due to difficulties in obtaining an adequate specimen for cytology. Tissue diagnosis is important in certain subgroups of patients such as those who are borderline surgical candidates, those with indeterminate strictures (e.g. patients with primary sclerosing cholangitis), or before chemotherapy and radiation therapy.
Magnetic resonance cholangiopancreatography (MRCP) can be a useful noninvasive adjunct to current techniques. It has the ability to define the proximal and distal extent of strictures as well as to evaluate for any intrahepatic mass lesion. One series evaluated the role of MRCP in patients with bile duct obstruction. Of 126 patients, 14 had bile duct malignancy. Of those 14, 12 patients were diagnosed by MRCP, with a sensitivity of 86% and specificity of 98%[3]. Another study by Rosch et al[4] had lower specificity for malignant obstructions. This study compared endoscopic retrograde cholangiopancreatography (ERCP), MRCP, computed tomography (CT) and endoscopic ultrasound (EUS) in the evaluation of biliary strictures. The specificity and sensitivity for MRCP to detect malignancy was 77% and 63%, respectively. Although MRCP provides the same imaging information as ERCP, many times ERCP is still required to provide a tissue diagnosis.
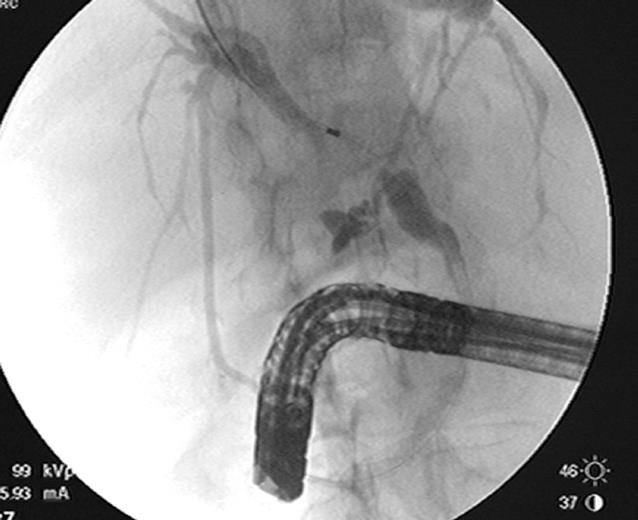
ERCP is useful in both the diagnosis and management of cholangiocarcinomas. It can delineate the anatomy of the biliary system and determine the extent of bile duct involvement which is important in determining resectability and surgical management (Figure 2). On cholangiography, the appearance of a stricture can suggest malignancy, but is not conclusive. Some characteristics suggestive of malignancy include a length greater than 10 mm, irregular margins, and an abrupt transition from normal duct to stricture, also known as shouldering[5]. Hilar strictures should also raise the suspicion for malignancy. Although, the appearance and location of a biliary stricture can suggest malignancy, tissue confirmation is usually needed in the majority of patients. Tissue for cytology may be obtained during ERCP by brushing, biopsy, bile aspiration or a combination of these. When necessary, therapeutic procedures can be performed, such as the placement of a biliary stent for treatment of obstructive jaundice.
Brush cytology has a high specificity of nearly 100%, but sensitivity is much lower, ranging from 18%-60% in most series[67]. The low sensitivity is likely related to low cellularity of these tumors and the desmoplastic reaction that is present.
Stricture manipulation by dilation theoretically should increase the availability of malignant cells for cytological examination. However, studies by deBellis et al[8] did not show a statistically significant difference in sensitivity before and after dilation. Patients underwent dilation with either a graduated dilating catheter or a dilating balloon. However, when the results of the pre- and post-dilation brushings were combined, the diagnostic yield increased from 35% to 44% (P = 0.001). This indicates that repeated brushing, not necessarily the stricture manipulation, should increase the diagnostic yield.
Further studies compared different brush lengths and stiffness[6]. A standard cytology brush, 1.5 cm long with soft bristles, was compared to the Cytolong brush, 5 cm long with rigid bristles. Detection rates were not increased with usage of the longer cytology brush.
Advances in diagnostic methods have increased the diagnostic yield of brush cytology. Digital image analysis (DIA) is useful in specimens with limited cellularity as it looks at the DNA content of individual cells. DIA uses spectrophotometric methods to quantify DNA content, chromatin distribution, and nuclear morphology. Aneuploidy, or the presence of increased amounts of DNA, is quantitated and, if present, suggests malignancy. DIA increases the sensitivity of routine brush cytology from 18%-40%, but decreased the specificity from 98%-77%[9].
Fluorescence in-situ hybridization (FISH) uses a com-mercial probe set to assess for polysomy of chromosomes 3, 7, 17, and 9p21. FISH increased the sensitivity from 15%-34%, and increased the specificity from 91%-98%[10].
Of special note, when performing ERCP in patients with biliary strictures, there is a risk of cholangitis due to the injection of contrast and possible bacteria into an obstructed biliary system. Therefore, it is important to obtain adequate drainage across the biliary obstruction with placement of a stent to decrease this risk. In patients with a stent that had been previously placed, the removed biliary stent can be sent for cytology in order to increase diagnostic yield.
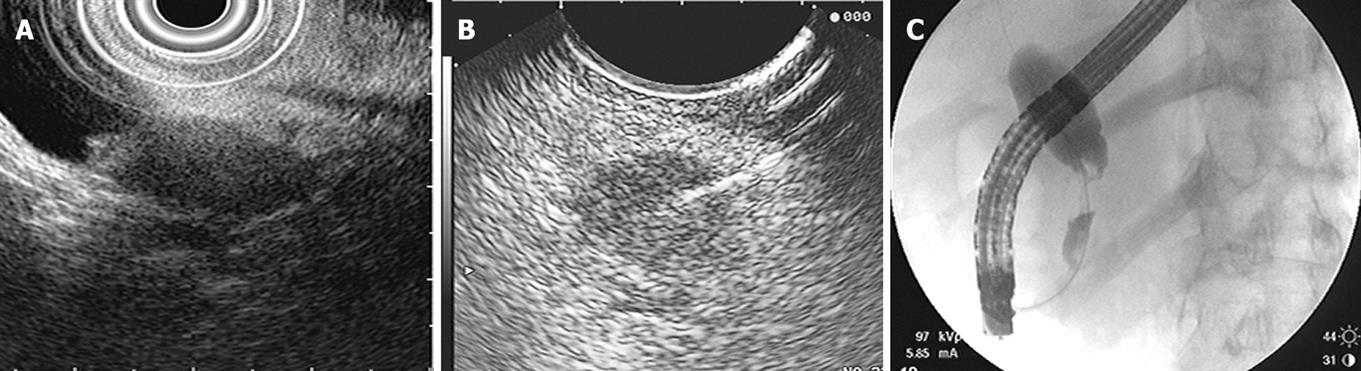
Another modality that has become useful in diagnosing hilar cholangiocarcinomas is EUS with fine needle aspiration (FNA). CT or percutaneous ultrasound guided fine needle aspiration are not routinely used because these tumors are small and isoechoic to the liver, making them more difficult to assess. EUS has high-resolution imaging and can visualize lesions of 3 mm or greater. Although ERCP is the conventional test for evaluating biliary strictures, as we have discussed, the sensitivity remains low. In patients with ERCPs that are indeterminate or non-diagnostic for malignancy, EUS with fine needle aspiration is a useful tool (Figure 3).
EUS images, alone without FNA, are not reliable in evaluating hilar lesions. Criteria such as echotexture, size of mass, contour abnormalities, and the shape and borders of the stenosis do not reliably differentiate malignant from benign lesions. EUS also provides visualization of hilar, celiax axis and para-aortic lymph nodes to determine local and distant metastasis. Fine needle aspiration of these lymph nodes is the most accurate way to diagnose cholangiocarcinoma and also allows for staging. In addition, EUS can evaluate the pancreas for causes of biliary strictures such as pancreatic masses or changes of chronic pancreatitis.
In a study by Fritscher-Ravens et al[11], patients with hilar strictures and inconclusive tissue diagnosis by ERCP, underwent EUS with fine needle aspiration. Of 44 patients, lesions at the hilum were noted in all the patients, and adequate material was obtained in 43 patients. Cytology revealed hilar cholangiocarcinoma in 59% of patients, with an accuracy of 91%, sensitivity of 89% and specificity of 100%. Accurate diagnosis changed the management in more than half of these patients that previously had non-diagnostic ERCPs.
In 2004, Eloubeidi et al[12] evaluated 28 patients in a prospective study to assess how EUS-FNA impacted patient management. Of the 28 patients, 3 were excluded because the lesion could not be identified by EUS. The sensitivity, specificity, and accuracy were 86%, 100%, and 88%, respectively, with numbers similar to the study by Fritscher-Ravens et al. A positive impact was made in 84% of patients. In 10 patients, surgery was prevented in patients with inoperable disease, 8 patients had surgery facilitated as they had unidentifiable cancer by other modalities, and 4 patients with benign disease avoided surgery. Prior studies have shown that 13%-24% of patients with suspected cholangiocarcinomas had benign disease at the time of surgery. By performing EUS with FNA in these patients with indeterminate strictures, surgical treatment could be tailored and appropriate management decisions be made.
A further modification of ultrasound technology allows the placement of a high frequency intraductal ultrasound probe (IDUS). Although several features such as irregular wall thickening can be highly suggestive of malignancy, IDUS as yet has no associated capability for tissue acquisition.
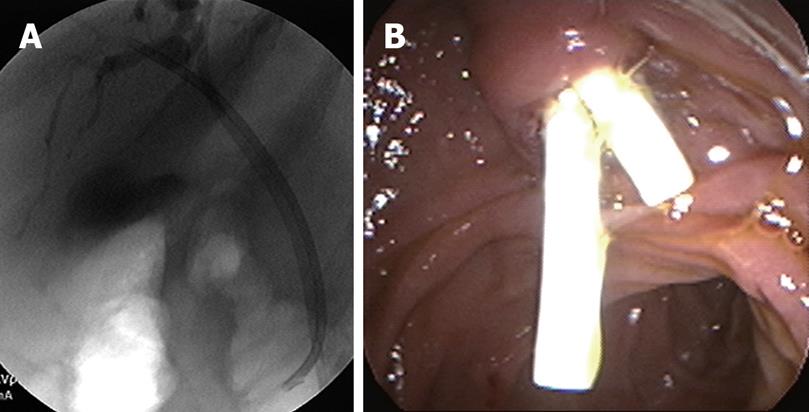
During ERCP, miniature cholangioscopes can be used to directly visualize the bile ducts and any strictures or filling defects seen during ERCP. Directed tissue biopsies can also be obtained with miniature cholangioscopic biopsy forceps. Shah et al[13] in 2006 evaluated 62 patients with suspected pancreatic or biliary malignancy that had prior nondiagnostic studies. Cholangioscopy with either cholangioscopic-directed or assisted biopsies performed when applicable. Sixty-two patients underwent 72 examinations and 53 lesions were seen on cholangioscopy. Twenty-nine patients had either cholangioscopy-directed or assisted biopsies and 24 had both. Cholangiocarcinoma was identified in 14 patients. Two patients with intrahepatic cholangiocarcinomas were missed by cholangioscopy. In this study, the sensitivity and specificity for cholangioscopy to detect malignancy was 89% and 96%, respectively.
More recently, a single-operator peroral cholangio-pancreatoscopy system known as Spyglass has been developed[1415]. Older cholangioscopes were fragile, had limited tip deflection, and had limited ability to clean the lens and visual field. In addition, they required two endoscopists, one to operate the duodenoscope and another to operate the cholangioscpe. With the Spyglass system, a single operator can control both scopes, there is 4-way deflected steering, and there are separate irrigation channels. A single operator system allows tight coordination of the duodenoscope and cholangioscpe. Mastering the use of the system does require experience and advanced skills. The increased maneuverability of the Spyglass system allows for 4 quadrant biopsies. In bench stimulations, the Spyglass system had 100% success rates in obtaining target quadrant biopsies compared to 50% in conventional choledochoduodenoscopes. A feasibility study was performed with 35 patients, 22 of whom had indeterminate strictures. The procedure was successful in 91% of patients. Spyglass-directed biopsies were performed in 20 patients, and 19 had adequate tissue for examination. The preliminary sensitivity and specificity of Spyglass to detect malignancy were 71% and 100%, respectively. In this study, 2 patients (6%) developed complications; one developed ascending cholangitis and the other intrahepatic abscess. Both patients recovered without sequelae. Currently, prospective multicenter clinical trials are ongoing.
Cholangiocarcinomas have a very poor prognosis with an average five-year survival of only 5%-10%. The only curative therapy for cholangiocarcinomas is surgical resection. If patients are not candidates for surgical resection, their median survival is 6.7-11.6 mo compared to 37.4-42.9 mo for patients who undergo surgical resection[1617]. Distal cholangiocarcinomas have the highest resectability rates of about 91% while perihilar tumors have the lowest at 56%. Based on the experience at Johns Hopkins Hospital over 23 years, distal, intrahepatic, perihilar cholangiocarcinomas after resection have five-year survival rates of 28%, 44%, and 11%, respectively[18].
Biliary decompression by placing a stent prior to surgery is a controversial issue. A biliary stent may make it difficult to assess the proximal extent of the tumor intraoperatively and may increase the risk of infections postoperatively. However, elevated bilirubin levels and liver dysfunction are factors that adversely affect postoperative morbidity. Indications for biliary stent placement preoperatively include cholangitis or prevention of cholangitis after a diagnostic ERCP is performed or if surgery is to be delayed for an extended amount of time[1920].
Only about 10%-20% of patients are candidates for surgery at the time of diagnosis secondary to advanced disease or overall poor medical health. In these patients with unresectable disease, the survival is very poor and there is rapid progression with biliary obstruction. Biliary decompression for palliative purposes can be accomplished surgically, radiologically or endoscopically.
In order to provide palliation and relieve jaundice, only 25% of the liver needs to be adequately drained. Therefore, unilateral stents of either the left or the right system are typically sufficient. In a randomized controlled prospective trial, De Palma et al[21] evaluated 157 patients with malignant hilar biliary obstruction due to cholangiocarcinoma, gallbladder cancer or periportal metastatic lymphadenopathy. In patients with unilateral stenting, there was a higher success rate for stent insertion (89% vs 77%) and drainage (81% vs 73%) and, therefore, a lower early complication rate (19% vs 27%) when compared to bilateral stenting of both hepatic lobes. Early complications included cholangitis and stent occlusion. No differences were found in survival or procedure-related mortality.
In order to decrease the risk of cholangitis during an ERCP, it is important not to inject contrast above the level of a stricture unless adequate drainage can be ensured. Selective cannulization with a guidewire above the level of the stricture should be performed. Following that, the catheter should be passed above the stricture before injecting contrast. With the guidewire in place, a stent can be placed in the proper position ensuring that the contaminated segment will be properly drained (Figure 4).
Both plastic and metal biliary stents are available. Numerous studies have compared plastic versus metal stents with regards to cost, complication rates, and survival[22–24]. There are no differences in survival with the use of either stents. Plastic stents have a higher risk of occlusion, with 30% occlusion rates after 3 mo and 70% after 6 mo[23]. In order to prevent problems with occlusion and cholangitis, they need to be exchanged every 3 mo. Metal stents have a longer patency of approximately 12 mo due to the fact that they have larger diameters compared to plastic stents (10 mm vs 3.8 mm). However, once placed they are very difficult to manipulate or remove. As far as cost effectiveness, the initial cost of a metal biliary stent is higher. However, with plastic stents, there are subsequent costs due to the need for repeat procedures for stent exchange and hospitalization for complications. Overall, there is no significant difference in the cost between metal and plastic stents. The decision to place a plastic versus metal stent should take into consideration the patients’ overall health, expected length of survival, quality of life and local expertise. Often, a plastic stent is placed initially while further diagnostic workup is underway. Once the diagnosis is made and the patient has unresectable disease and a life expectancy of more than 6 mo, then the plastic stent can be replaced with a metal stent. Placement of a metal stent eliminates the need for repeated procedures and their associated risks.
The diagnosis of cholangiocarcinomas is often challenging. Multiple endoscopic modalities are available to evaluate strictures or masses of indeterminate origin. ERCP with brush cytology using FISH or DIA technology along with EUS with FNA and cholangioscopy are available. Oftentimes, repeated procedures and a combination of these different techniques are necessary to achieve a tissue diagnosis. Having a cytologic diagnosis as well as knowing the stage of the disease plays an important role in decisions regarding management. Surgery is curative if the disease is detected at an early stage. When there is metastatic or advanced disease, endoscopic drainage plays a central role in providing palliation and improving quality of life. Placement of a unilateral stent is sufficient in providing adequate drainage and has lower morbidity than bilateral stents. In patients who require short-term drainage, plastic stents are a good option. Because long term survival is so poor, metal stents should be considered if patients are not surgical candidates.
| 1. | Ahrendt SA, Nakeeb A, Pitt HA. Cholangiocarcinoma. Clin Liver Dis. 2001;5:191-218. |
| 2. | Patel T. Cholangiocarcinoma. Nat Clin Pract Gastroenterol Hepatol. 2006;3:33-42. |
| 3. | Guibaud L, Bret PM, Reinhold C, Atri M, Barkun AN. Bile duct obstruction and choledocholithiasis: diagnosis with MR cholangiography. Radiology. 1995;197:109-115. |
| 4. | Rosch T, Meining A, Fruhmorgen S, Zillinger C, Schusdziarra V, Hellerhoff K, Classen M, Helmberger H. A prospective comparison of the diagnostic accuracy of ERCP, MRCP, CT, and EUS in biliary strictures. Gastrointest Endosc. 2002;55:870-876. |
| 5. | Park MS, Kim TK, Kim KW, Park SW, Lee JK, Kim JS, Lee JH, Kim KA, Kim AY, Kim PN. Differentiation of extrahepatic bile duct cholangiocarcinoma from benign stricture: findings at MRCP versus ERCP. Radiology. 2004;233:234-240. |
| 6. | Fogel EL, deBellis M, McHenry L, Watkins JL, Chappo J, Cramer H, Schmidt S, Lazzell-Pannell L, Sherman S, Lehman GA. Effectiveness of a new long cytology brush in the evaluation of malignant biliary obstruction: a prospective study. Gastrointest Endosc. 2006;63:71-77. |
| 7. | de Bellis M, Sherman S, Fogel EL, Cramer H, Chappo J, McHenry L Jr, Watkins JL, Lehman GA. Tissue sampling at ERCP in suspected malignant biliary strictures (Part 2). Gastrointest Endosc. 2002;56:720-730. |
| 8. | de Bellis M, Fogel EL, Sherman S, Watkins JL, Chappo J, Younger C, Cramer H, Lehman GA. Influence of stricture dilation and repeat brushing on the cancer detection rate of brush cytology in the evaluation of malignant biliary obstruction. Gastrointest Endosc. 2003;58:176-182. |
| 9. | Baron TH, Harewood GC, Rumalla A, Pochron NL, Stadheim LM, Gores GJ, Therneau TM, De Groen PC, Sebo TJ, Salomao DR. A prospective comparison of digital image analysis and routine cytology for the identification of malignancy in biliary tract strictures. Clin Gastroenterol Hepatol. 2004;2:214-219. |
| 10. | Patel T, Singh P. Cholangiocarcinoma: emerging approaches to a challenging cancer. Curr Opin Gastroenterol. 2007;23:317-323. |
| 11. | Fritscher-Ravens A, Broering DC, Knoefel WT, Rogiers X, Swain P, Thonke F, Bobrowski C, Topalidis T, Soehendra N. EUS-guided fine-needle aspiration of suspected hilar cholangiocarcinoma in potentially operable patients with negative brush cytology. Am J Gastroenterol. 2004;99:45-51. |
| 12. | Eloubeidi MA, Chen VK, Jhala NC, Eltoum IE, Jhala D, Chhieng DC, Syed SA, Vickers SM, Mel Wilcox C. Endoscopic ultrasound-guided fine needle aspiration biopsy of suspected cholangiocarcinoma. Clin Gastroenterol Hepatol. 2004;2:209-213. |
| 13. | Shah RJ, Langer DA, Antillon MR, Chen YK. Cholangioscopy and cholangioscopic forceps biopsy in patients with indeterminate pancreaticobiliary pathology. Clin Gastroenterol Hepatol. 2006;4:219-225. |
| 14. | Chen YK. Preclinical characterization of the Spyglass peroral cholangiopancreatoscopy system for direct access, visualization, and biopsy. Gastrointest Endosc. 2007;65:303-311. |
| 15. | Chen YK, Pleskow DK. SpyGlass single-operator peroral cholangiopancreatoscopy system for the diagnosis and therapy of bile-duct disorders: a clinical feasibility study (with video). Gastrointest Endosc. 2007;65:832-841. |
| 16. | Roayaie S, Guarrera JV, Ye MQ, Thung SN, Emre S, Fishbein TM, Guy SR, Sheiner PA, Miller CM, Schwartz ME. Aggressive surgical treatment of intrahepatic cholangiocarcinoma: predictors of outcomes. J Am Coll Surg. 1998;187:365-372. |
| 17. | Weber SM, Jarnagin WR, Klimstra D, DeMatteo RP, Fong Y, Blumgart LH. Intrahepatic cholangiocarcinoma: resectability, recurrence pattern, and outcomes. J Am Coll Surg. 2001;193:384-391. |
| 18. | Nakeeb A, Pitt HA, Sohn TA, Coleman J, Abrams RA, Piantadosi S, Hruban RH, Lillemoe KD, Yeo CJ, Cameron JL. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg. 1996;224:463-473; discussion 473-475. |
| 19. | Strasberg SM. ERCP and surgical intervention in pancreatic and biliary malignancies. Gastrointest Endosc. 2002;56:S213-S217. |
| 20. | Freeman ML, Sielaff TD. A modern approach to malignant hilar biliary obstruction. Rev Gastroenterol Disord. 2003;3:187-201. |
| 21. | De Palma GD, Galloro G, Siciliano S, Iovino P, Catanzano C. Unilateral versus bilateral endoscopic hepatic duct drainage in patients with malignant hilar biliary obstruction: results of a prospective, randomized, and controlled study. Gastrointest Endosc. 2001;53:547-553. |
| 22. | Soderlund C, Linder S. Covered metal versus plastic stents for malignant common bile duct stenosis: a prospective, randomized, controlled trial. Gastrointest Endosc. 2006;63:986-995. |
| 23. | Prat F, Chapat O, Ducot B, Ponchon T, Pelletier G, Fritsch J, Choury AD, Buffet C. A randomized trial of endoscopic drainage methods for inoperable malignant strictures of the common bile duct. Gastrointest Endosc. 1998;47:1-7. |
| 24. | Kaassis M, Boyer J, Dumas R, Ponchon T, Coumaros D, Delcenserie R, Canard JM, Fritsch J, Rey JF, Burtin P. Plastic or metal stents for malignant stricture of the common bile duct? Results of a randomized prospective study. Gastrointest Endosc. 2003;57:178-182. |