Published online May 14, 2008. doi: 10.3748/wjg.14.2838
Revised: April 8, 2008
Published online: May 14, 2008
AIM: To compare the efficacy of a 7-d vs 10-d triple therapy regarding H pylori eradication, endoscopic findings and histological gastric inflammatory inactivation in the Ecuadorian population.
METHODS: 136 patients with dyspepsia and H pylori infection were randomized in 2 groups (68 per group): group 1, 7-d therapy; group 2, 10-d therapy. Both groups received the same medication and daily dosage: omeprazole 20 mg bid, clarithromycin 500 mg bid and amoxicillin 1 g bid. Endoscopy was performed for histological assessment and H pylori infection status before and 8 wk after treatment.
RESULTS: H pylori was eradicated in 68% of group 1 vs 83.8% of group 2 for the intention-to-treat analysis (ITT) (P = 0.03; OR = 2.48; 95% CI, 1.1-5.8), and 68% in group 1 vs 88% in group 2 for the per-protocol analysis (PP) (P = 0.008; OR = 3.66; 95% CI, 1.4-10). Endoscopic gastric mucosa normalization was observed in 56.9% in group 1 vs 61.2% in group 2 for ITT, with similar results for the PP, the difference being statistically not significant. The rate of inflammatory inactivation was 69% in group 1 vs 88.7% in group 2 for ITT (P = 0.007; OR = 3.00; 95% CI, 1.2-7.5), and 69% in group 1 vs 96% in group 2 for PP (P = 0.0002; OR = 7.25; 95% CI, 2-26).
CONCLUSION: In this Ecuadorian population, the 10-d therapy was more effective than the 7-d therapy for H pylori eradication as well as for gastric mucosa inflammatory inactivation.
-
Citation: Robles-Jara C, Robles-Medranda C, Moncayo M, Landivar B, Parrales J. Is a 7-day
Helicobater pylori treatment enough for eradication and inactivation of gastric inflammatory activity? World J Gastroenterol 2008; 14(18): 2838-2843 - URL: https://www.wjgnet.com/1007-9327/full/v14/i18/2838.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.2838
H pylori bacterial infection is worldwide in humans. It is the main etiologic factor in chronic gastritis and gastroduodenal ulcer disease[1]. It is also closely related to gastric adenocarcinoma and low grade gastric lymphoma of mucosa-associated lymphoid tissue (MALT). Forty percent to 50% of the world population is estimated to be infected, with the highest incidence occurring in older people and in those living in areas with low standards of sanitation[2]. In South American countries, the prevalence of H pylori infection ranges from 70% to 90%[3], being 89.5% in Ecuador[4].
A proton pump inhibitor (PPI) associated with two antibiotics is considered the standard therapy for H pylori eradication[5]. Clinical trials show H pylori eradication rates varying from 45% to 92.3% using PPI associated with amoxicillin and clarithromycin. However, duration of eradication therapy continues to be controversial[6]. In many developed countries, a 7-d therapy is considered enough for bacterial eradication. On the other hand, there is still an argument for increasing the duration of treatment to 10 d or even 14 d[6].
In Europe, a 7-d triple therapy is still recommended because 14-d therapy had an insignificant advantage in terms of treatment success rate[7]. On the other hand, guidelines from North America recommend 10-d to 14-d therapy, as some studies have reported superior cure rates with prolonged therapy using triple regimens[8]. In Asia, an Indian study reported that prolonged triple therapy with lansoprazole, amoxicillin and tinidazole achieved a significant increase in eradication rates: 47.6% vs 80% vs 91.3% for 1 wk, 2 wk and 3 wk of therapy, respectively[9].
However, in a recent large multicenter randomized trial based on a Korean population, no differences were observed between 7 d vs 14 d of triple therapy with omeprazole associated with amoxicillin and clarithromycin[10].
Two studies from Turkey using PPI-based triple therapy with amoxicillin and clarithromycin have shown low eradication rates[1112]. The first study with a 7-d triple therapy showed an eradication rate of 63.6%[11]. The second one using a 14-d therapy showed a 45% eradication rate[12].
In the Mexican population, triple therapy with rabeprazole, amoxicillin and ofloxacin for 14 d achieved significantly superior eradication rates when compared with 7-d therapy (92.3% vs 62.2%)[13].
In South America, it is difficult to identify the best regimen, due to the absence of large multicenter controlled trials. Some recommendations have been published based on consensus reports. The last Brazilian consensus, in 2005, recommended a 7-d triple therapy[14].
The aim of H pylori eradication is to stop the chronic inflammatory activity that leads to histological changes in the gastric mucosa. This inflammatory inactivation has also shown to improve patients’ symptoms[1516].
The histopathology associated with H pylori infection includes a specific structural disorganization of the epithelial cells and inflammatory infiltration (neutrophils, lymphocytes)[17]. Epithelial nonspecific changes include: presence of microcrypts, reduction of foveolar mucus secretion, atrophy, hyperplasia, metaplasia, atypia, or dysplasia[17].
Many studies have well demonstrated the histological resolution of inflammatory activity after H pylori eradication using PPI associated with two antibiotics (amoxicillin, clarithromycin or metronidazole) for 7 d, 10 d or 14 d[1518–20]. Only two studies, however, correlated bacterial eradication, gastric inflammatory inactivation and duration of treatment, comparing 7-d vs 14-d and 10-d vs 14-d therapy[1621].
This is the first study evaluating the best treatment approach for the Ecuadorian population. Our objective was to compare the efficacy of 7-d vs 10-d triple therapy regarding H pylori eradication rates, the endoscopic findings and the histological gastric inflammatory inactivation.
This was a prospective, randomized, open-label study performed at the Ecuadorian Institute of Digestive Diseases (IECED), in Portoviejo, Ecuador. The study population consisted of patients with dyspepsia who were referred for upper endoscopy. Dyspepsia was defined as pain or discomfort localized in the upper abdomen. Patients were enrolled if they were infected with H pylori.
The exclusion criteria were: (1) Age < 18 years; (2) Use of proton pump inhibitors, antibiotics, H2-receptor antagonists or bismuth compounds in the last 4 wk preceding the inclusion of the patient in the study; (3) Chronic use of non-steroidal anti-inflammatory drugs (NSAIDs); (4) Patients with well-known antibiotic allergy; (5) Pregnant women; (6) Patients with chronic hepatic or renal disease; (7) Patients with Zollinger-Ellison syndrome; (8) Previous gastric surgery; (9) Previous failed H pylori therapy.
Written informed consent was obtained from all participants prior to enrollment.
During endoscopy, 5 biopsies were obtained for histological assessment in accordance with the updated Sydney System (2 from the antrum, 1 from the incisura angularis and 2 from the corpus)[22]. Biopsies were fixed, paraffin embedded and stained with hematoxylin-eosin/Giemsa. A pre-pyloric biopsy was obtained for the rapid urease test. Patients were considered to be infected if histological and rapid urease tests were positive. Eight weeks after treatment, bacterial eradication was checked by using the same endoscopy procedure for histology. At endoscopy, gastritis was defined as the presence of visible alterations of the mucosal appearance, presumably caused by vascular or infiltrative changes (Sydney system definition)[23]. Histological diagnosis was coded in two classes according to the Sydney System classification as follows: (1) non-atrophic chronic gastritis, when inflammatory activity of any grade or antral atrophy grade I were present without intestinal metaplasia lesion; and (2) atrophic chronic gastritis, when grade 2 or 3 atrophic mucosa lesions were observed with or without intestinal metaplasia.
The gastric mucosa inflammation activity was also graded in accordance with the updated Sydney System and was considered as the presence of mononuclear cells infiltrate and neutrophilic polymorphonuclear in a background of chronic inflammation[22].
Patients were randomized in a 1:1 ratio to receive omeprazole 20 mg bid, clarithromycin 500 mg bid and amoxicillin 1 g bid for 7 d (group 1) or 10 d (group 2). Treatment compliance was assessed by patient report and pill count at the follow-up visit. Good compliance was defined as consumption of more than 90% of the prescribed drugs. After completing treatment, no patients received PPI or antisecretory drugs during the follow-up period.
Statistical analysis was performed using StatView for Windows (version 5.0). The 95% confidence intervals were determined with the chi-square test or Fisher’s exact test for small sample sizes, and comparisons were made with Student’s test. P value of < 0.05 were considered as statistically significant. Per protocol and intention to treat approach was performed for statistical analysis in each group.
As there is no data regarding H pylori eradication rates in the Ecuadorian population, the sample size was calculated a priori, based on available data in the literature. By hypothesizing an 89% eradication rate for the 10-d triple therapy[30] and less than 70% (65%) for 7-d triple therapy[13], the estimated sample size was 54 subjects per group, with a power of 0.85 and a significance level of 0.05. Due to the probability of loss from follow-up, the risk of data loss was estimated at around 25%; therefore the final size was considered 68 patients in each group.
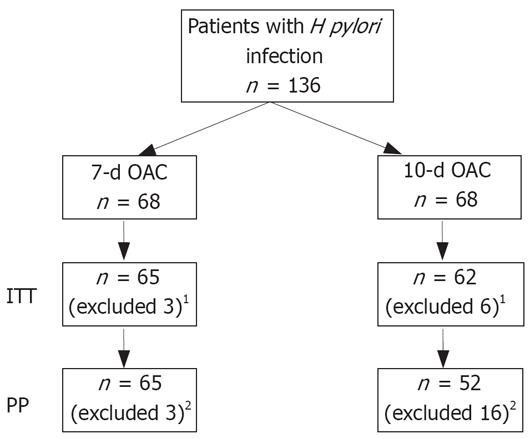
One hundred and thirty-six patients (68 patients by group) were enrolled in the study (Figure 1). At endoscopy, the initial diagnoses were: gastritis in 69/136 (50.7%), duodenal ulcer in 26/136 (19.11%), and gastric ulcer in 22/136 (16.17%). Endoscopy was normal in 19/136 (13.9%).
At histology non-atrophic chronic active gastritis was present in 88.2% of patients for group 1 (60/68) and 82.3% (56/68) for group 2. In group 1, eight patients had atrophic chronic active gastritis (11.7%) in which the presence of intestinal metaplasia was identified in 5/8. In group 2, the presence of atrophic chronic active gastritis was observed in 12 patients (17.6%), all of them with intestinal metaplasia. There were no significant differences between the treatment groups in any of the baseline characteristics. Demographic, endoscopic and histological characteristics are summarized in Table 1.
| Group 1 (n = 68) OAC 7-d | Group 2 (n = 68) OAC 10-d | P | |
| Age (yr, mean ± SD) | 42 ± 11 | 44 ± 12 | NS |
| Sex (M/F) | 23/45 | 27/41 | NS |
| Endoscopic findings: | |||
| Normal | 9 | 10 | NS |
| Gastritis (IM) | 31 (4) | 38 (6) | NS |
| Gastric ulcer (IM) | 13 (1) | 9 (2) | NS |
| Duodenal ulcer (IM) | 15 (0) | 11 (4) | NS |
| Histological findings: | |||
| Nonatrophic chronic active gastritis (IM) | 60 | 56 | NS |
| Atrophic chronic active gastritis (IM) | 8 (5) | 12 (12) | NS |
Three patients (4.4%) in group 1 and six patients (8.8%) in group 2 did not return for follow-up and were excluded from the study. Additionally, ten patients (14.7%) in group 2 were not compliant with the treatment: therapy was stopped at d 8 in 7 patients, at d 3 in 2 patients and at d 4 in 1 patient. In 6 patients (8.8%), the non-compliance was correlated with treatment-adverse events (4 with moderate abdominal pain, 1 with nausea, and 1 with diarrhea).
One hundred and seventeen patients underwent a PP analysis (Table 2): 65 patients in group 1 and 52 patients in group 2. Eight weeks after treatment the endoscopy showed a normal gastric mucosa in 56.9% (37/65) of patients in group 1 and in 63.5% (32/52) in group 2. Ulcer healing was observed in 93.7% (45/48) of patients (both groups). In group 1, gastritis was observed in 25 patients (intestinal metaplasia in 5 patients at histology without presence of gastric mucosa inflammation) and an active ulcer in 3 patients (2 with gastric ulcer and 1 with duodenal ulcer). In group 2, gastritis was present in 20 patients (12 patients with intestinal metaplasia at histology and without gastric mucosa inflammation).
| Group 1 (n = 65) OAC 7-d | Group 2 (n = 52) OAC 10-d | P | |
| H pylori eradication | 44/65 (68) | 46/52 (88) | 0.008 |
| Normal endoscopy | 37/65 (56.9) | 32/52 (63.5) | NS |
| Inflammatory inactivation of gastric mucosa | 45/65 (69) | 49/52 (96) | 0.0002 |
H pylori eradication was obtained in 68% (44/65) of patients for group 1 and 88% (46/52) for group 2 (P = 0.008; OR = 3.66; 95% CI, 1.4-10).
The total average rate of inflammatory inactivation of gastric mucosa was 80% for both groups. In group 1, 45 patients (69%) showed an inactivation of the inflammation compared with 49 patients (96%) in group 2 (P = 0.0002; OR = 7.25; 95% CI, 2-26).
One hundred and twenty-seven patients underwent an ITT assessment (Table 3): 65 patients in group 1 and 62 patients in group 2. After treatment, the endoscopy showed a normal gastric mucosa in 56.9% (37/65) of patients for group 1 and 61.2% (37/62) in group 2. Gastritis was present in 25 patients in group 2 (12 patients with intestinal metaplasia at histology and without presence of gastric mucosa inflammation).
| Group 1 (n = 65) OAC 7-d | Group 2 (n = 62) OAC 10-d | P | |
| H pylori eradication | 44/65 (68) | 52/62 (83.8) | 0.03 |
| Normal endoscopy | 37/65 (56.9) | 37/62 (61.2) | NS |
| Inflammatory inactivation of gastric mucosa | 45/65 (69) | 54/62 (88.7) | 0.007 |
H pylori eradication was obtained in 68% (44/65) of patients for group 1 and 83.8% (52/62) for group 2 (P = 0.03; OR = 2.48; 95% CI, 1.1-5.8).
The total average rate of gastric mucosa inflammatory inactivation was 79.5% for both groups. In group 1, 45/65 patients (69%) showed an inactivation of the inflammation compared with 54/62 patients (88.7%) in group 2 (P = 0.007; OR = 3.00; 95% CI, 1.2-7.5).
After the discovery of H pylori many studies were reported for its treatment, including various meta-analyses. Nowadays there is no doubt that triple therapies, that is, PPI & two antibiotics (amoxicillin associated with clarithromycin or metronidazole) are currently the most preferred first-line therapy regimens in clinical practice.
Therapy regimens and their duration could not be standardized because results of clinical data are different between countries. In effect, the results for efficacy of therapy are varied worldwide. This variation of the results could be a consequence of many factors such as bacterial virulence, environmental factors and antibacterial resistance, which are peculiar for each country. In addition, individual factors such as: patient’s age less than 60, presence of duodenal ulcer and treatment duration have been also linked to therapy efficacy[24].
Until now, no data or consensus about the duration of treatment for H pylori in Ecuador was available. We therefore evaluated the twice-daily triple therapy of omeprazole 20 mg bid, amoxicillin 1 g bid and clarithromycin 500 mg bid.
Metronidazole resistance in H pylori strains varies geographically, and influences negatively the effectiveness of therapies containing this antibiotic[25]. In Latin-American countries, more than 62% of the population is resistant to metronidazole treatment[326]. This can be explained by the indiscriminate use of these agents for many other diseases (such as parasitosis).
The rates of metronidazole resistance are as high as 81% in Ecuador. In contrast, clarithromycin resistance is still small: < 10%[27]. This is the reason for using clarithromycin in our study.
Two clinical trials from South America reported eradication rates of more than 85% with 7-d triple therapy using PPI, amoxicillin and clarithromycin. Coelho et al[28], in the Brazilian population showed 87% of H pylori eradication with a 7-d treatment (using pantoprazole, amoxicillin and clarithromycin) in 71 patients. In Peru, a randomized study with 72 patients showed 86.1% of H pylori eradication with no differences between 7-d vs 10-d triple therapy[29].
In the Ecuadorian population our study showed a 68% and an 86% eradication rate for the 7-d and 10-d triple therapy, respectively, this difference being statistically significant.
Many clinical trials have demonstrated similar eradication rates using 10-d triple therapy with PPI, amoxicillin and clarithromycin[15163034]. A large multicenter double-blind trial in the United States, demonstrated an eradication rate of 84%[30]. Fennerty et al, also found comparable results (84% eradication rate) in 284 patients, without difference when compared to 14-d therapy[16]. In the Czech Republic, the rate of H pylori eradication obtained was 87%[15].
Moreover, a recent meta-analysis has shown that prolonging triple therapies from 7 to 10 d or 14 d improves treatment cure rates[31]. Other meta-analysis suggested that 10-d therapy was associated with less failure than 7-d therapy[24]. Our study also indicates that a 10-d regimen was statistically more effective than the 7-d regimen for inactivating the gastric mucosa inflammation.
Acid suppression with PPI in H pylori gastritis only decreases the gastric inflammatory activity, but doesn’t produce its inactivation[32]. Histological changes after H pylori eradication show a rapid improvement of neutrophils infiltration in the first 2 mo, progressive declination of mononuclear cell infiltration during the first 2 years, and no significant changes in the 12 first mo regarding atrophy or intestinal metaplasia[17–2033]. The presence of an inflammatory activity is seen in fewer cases, even 12 mo after the eradication[3233].
In our patients, 8 wk after treatment, the endoscopic assessment revealed the persistence of gastric lesions in 43.1% of patients (28/65) in group 1 and 38.5% of patients (20/52) in group 2. However, an inflammatory inactivation was obtained in 69% and 94% of patients in groups 1 and 2 respectively. All cases with intestinal metaplasia had H pylori eradication and gastric inflammatory inactivation. In patients with abnormal control endoscopy, persistence of H pylori was observed in 21/28 patients in group 1 and in 6/20 patients in group 2, being associated with the persistence of an inflammatory activity.
Discordance between endoscopic and histological findings after H pylori eradication with 7-d triple therapy (PPI, amoxicillin, clarithromycin) was previously reported[1620]. Dajani et al, observed a persistence of lesions at the control endoscopy in 46.4% of patients (7.4% of duodenal ulcer and 39% of gastritis lesions), in spite of 93% eradication rates with a remarkable resolution of the inflammatory activity[20]. Another study comparing 10 vs 14 d of treatment shows the persistence of an active ulcer in 28% of patients and gastritis in 9% after an eradication rate of 84%[16].
Prolonging therapy duration has been associated with an increased risk of non-compliance due to side-effects[3035]. This was also observed in our study, which shows an increased non-compliance rate in the 10-d therapy group (group 2), being correlated in 6 patients with treatment side-effects.
Finally, although our data points to the superior benefits of the 10-d therapy, we should be careful before establishing definitive recommendations for the Ecuadorian population.
On a day-to-day basis, every medical treatment must consider the cost-benefit analysis, mainly in developing countries, where it is difficult for patients to afford their medications in function of its price. The cost of H pylori eradication therapy in South America is very high[3]. Some recommended eradication therapies have 7-d duration mainly because of improved compliance and decreased medical cost[13]. In our study, 32% of patients in the 7-d vs 12% in 10-d therapy did not eradicate H pylori. Probably, the re-treatment of this 32% of patients in group 1 with second or third line therapies would be more expensive and less effective than directly treating them for 10 d with the first line approach. This evaluation however was not the objective of our paper, and should be the subject of a future study.
In conclusion, this randomized open study showed that in Ecuador, a 10-d triple therapy using PPI associated with amoxicillin and clarithromycin was significantly more effective than a 7-d triple therapy. The cost-benefit of this treatment in our population should be evaluated in a future study.
The aim of H pylori eradication is to stop chronic inflammatory activity that leads to histological changes in the gastric mucosa. A proton pump inhibitor (PPI) associated with two antibiotics is considered the standard therapy for H pylori eradication. However, duration of eradication therapy continues to be controversial, due to the variable results from all over the world. There is no data about H pylori treatment in the Ecuadorian population and to our knowledge there are no studies correlating the bacterial eradication, gastric inflammatory inactivation and duration of treatment comparing 7-d vs 10-d.
This study determined the best valid H pylori eradication treatment in a population with higher rates of infection correlating the bacterial eradication rate, the endoscopic and histological results with the treatment duration.
Many studies have well demonstrated the histological resolution of inflammatory activity after H pylori eradication using PPI associated with two antibiotics (amoxicillin, clarithromycin or metronidazole) for 7 d, 10 d or 14 d. Only two studies, however, correlated bacterial eradication, gastric inflammatory inactivation and duration of treatment, comparing 7-d vs 14-d and 10-d vs 14-d therapy. This is the first study evaluating the best treatment approach for the Ecuadorian population. We compared the efficacy of 7-d vs 10-d triple therapy regarding H pylori eradication rates, the endoscopic findings and the histological gastric inflammatory inactivation.
In this Ecuadorian population, 10-d triple therapy using PPI associated with amoxicillin and clarithromycin was significantly more effective than 7-d triple therapy. The cost-benefit of this treatment in our population should be evaluated in a future study.
This is a reasonably well done clinical trial comparing 7-d and 10-d PPI-based triple therapy for H pylori infection. Ten-day treatment was significantly superior. The trial is well described.
| 1. | Gisbert JP, Gonzalez L, Calvet X. Systematic review and meta-analysis: proton pump inhibitor vs. ranitidine bismuth citrate plus two antibiotics in Helicobacter pylori eradication. Helicobacter. 2005;10:157-171. |
| 2. | Pounder RE, Ng D. The prevalence of Helicobacter pylori infection in different countries. Aliment Pharmacol Ther. 1995;9 Suppl 2:33-39. |
| 3. | Castro Lde P, Coelho LG. Helicobacter pylori in South America. Can J Gastroenterol. 1998;12:509-512. |
| 4. | Gomez NA, Alvarez LR, Zapatier JA, Vargas PE. [Efficacy of stool antigen and serologic tests in the diagnosis of Helicobacter pylori in Ecuadorian population]. Rev Gastroenterol Mex. 2005;70:146-150. |
| 5. | Malfertheiner P, Megraud F, O'Morain C, Hungin AP, Jones R, Axon A, Graham DY, Tytgat G. Current concepts in the management of Helicobacter pylori infection--the Maastricht 2-2000 Consensus Report. Aliment Pharmacol Ther. 2002;16:167-180. |
| 6. | Bytzer P, O'Morain C. Treatment of Helicobacter pylori. Helicobacter. 2005;10 Suppl 1:40-46. |
| 7. | Malfertheiner P, Megraud F, O'Morain C, Bazzoli F, El-Omar E, Graham D, Hunt R, Rokkas T, Vakil N, Kuipers EJ. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 2007;56:772-781. |
| 8. | Chey WD, Wong BC. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007;102:1808-1825. |
| 9. | Chaudhary A, Ahuja V, Bal CS, Das B, Pandey RM, Sharma MP. Rank order of success favors longer duration of imidazole-based therapy for Helicobacter pylori in duodenal ulcer disease: a randomized pilot study. Helicobacter. 2004;9:124-129. |
| 10. | Kim BG, Lee DH, Ye BD, Lee KH, Kim BW, Kim SG, Kim SW, Kim SK, Kim JJ, Kim HY. Comparison of 7-day and 14-day proton pump inhibitor-containing triple therapy for Helicobacter pylori eradication: neither treatment duration provides acceptable eradication rate in Korea. Helicobacter. 2007;12:31-35. |
| 11. | Sivri B, Simsek I, Hulagu S, Kadayifci A, Tozun N, Akarsu M, Uraz S, Savas MC, Koruk M, Bozbas A. The efficacy, safety and tolerability of pantoprazole-based one-week triple therapy in H. pylori eradication and duodenal ulcer healing. Curr Med Res Opin. 2004;20:1301-1307. |
| 12. | Altintas E, Sezgin O, Ulu O, Aydin O, Camdeviren H. Maastricht II treatment scheme and efficacy of different proton pump inhibitors in eradicating Helicobacter pylori. World J Gastroenterol. 2004;10:1656-1658. |
| 13. | Bosques-Padilla FJ, Garza-Gonzalez E, Calderon-Lozano IE, Reed-SanRoman G, de Arino Suarez M, Valdovinos-Diaz MA, Orozco-Gamiz A, Blancas-Valencia JM, Tamayo-de la Cuesta JL. Open, randomized multicenter comparative trial of rabeprazole, ofloxacin and amoxicillin therapy for Helicobacter pylori eradication: 7 vs. 14 day treatment. Helicobacter. 2004;9:417-421. |
| 14. | Coelho LG, Zaterka S. [Second Brazilian Consensus Conference on Helicobacter pylori infection]. Arq Gastroenterol. 2005;42:128-132. |
| 15. | Kyzekove J, Arlt J, Arltová M. Is there any relationship between functional dyspepsia and chronic gastritis associated with Helicobacter pylori infection? Hepatogastroenterology. 2001;48:594-602. |
| 16. | Fennerty MB, Kovacs TO, Krause R, Haber M, Weissfeld A, Siepman N, Rose P. A comparison of 10 and 14 days of lansoprazole triple therapy for eradication of Helicobacter pylori. Arch Intern Med. 1998;158:1651-1656. |
| 17. | Warren JR. Gastric pathology associated with Helicobacter pylori. Gastroenterol Clin North Am. 2000;29:705-751. |
| 18. | Abdul Aal GM, Dajani AI, Nounou M, Awad S, Abdul Rasheed Z, Gautam S, Ukabam S, Nayal S. Resolution of gastritis induced by Helicobacter pylori 4-5 weeks after successful eradication of infection using a triple therapy regimen of pantoprazole, amoxycillin and clarithromycin for one week. Digestion. 1999;60:286-297. |
| 19. | Jakic-Razumovic J, Tentor D, Kusec V, Cuzic S, Brkic T. Histopathological features of gastritis before and after treatment for Helicobacter pylori. Croat Med J. 2000;41:159-162. |
| 20. | Dajani AI, Awad S, Ukabam S, Nounou MA, Abdul Rasheed Z, Gautam S, Abdul Aal G, Nayal S. One-week triple regime therapy consisting of pantoprazole, amoxicillin and clarithromycin for cure of Helicobacter pylori-associated upper gastrointestinal diseases. Digestion. 1999;60:298-304. |
| 21. | Paoluzi P, Iacopini F, Crispino P, Nardi F, Bella A, Rivera M, Rossi P, Gurnari M, Caracciolo F, Zippi M. 2-week triple therapy for Helicobacter pylori infection is better than 1-week in clinical practice: a large prospective single-center randomized study. Helicobacter. 2006;11:562-568. |
| 22. | Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161-1181. |
| 23. | Tytgat GN. The Sydney System: endoscopic division. Endoscopic appearances in gastritis/duodenitis. J Gastroenterol Hepatol. 1991;6:223-234. |
| 24. | Broutet N, Tchamgoue S, Pereira E, Lamouliatte H, Salamon R, Megraud F. Risk factors for failure of Helicobacter pylori therapy--results of an individual data analysis of 2751 patients. Aliment Pharmacol Ther. 2003;17:99-109. |
| 25. | Chey WD. Helicobacter Pylori. Curr Treat Options Gastroenterol. 1999;2:171-182. |
| 26. | NA. World Gastroenterology Organisation (WGO). WGO Practice Guideline Helicobacter Pylori in developing countries. November 2006. Accessed Feb 14, 2007. Available at URL address: http://www.omge.org/globalguidelines/guide15/guideline15.htm. |
| 27. | Debets-Ossenkopp YJ, Reyes G, Mulder J, aan de Stegge BM, Peters JT, Savelkoul PH, Tanca J, Pena AS, Vandenbroucke-Grauls CM. Characteristics of clinical Helicobacter pylori strains from Ecuador. J Antimicrob Chemother. 2003;51:141-145. |
| 28. | Coelho LG, Mattos AA, Francisconi CF, Castro Lde P, Andre SB. [Efficacy of the dosing regimen of pantoprazole 40 mg, amoxicillin 1000 mg and clarithromycin 500 mg, twice daily for 7 days, in the eradication of Helicobacter pylori in patients with peptic ulcer]. Arq Gastroenterol. 2004;41:71-76. |
| 29. | Rodriguez W, Pareja Cruz A, Yushimito L, Ramírez Ramos A, Gilman RH, Watanabe Yamamoto J, Rodríguez Ulloa C, Mendoza Requena D, Guerra Valencia J, Leey Casella J. Omeprazole, amoxicillin and clarithromycin in the treatment of helicobacter pylori, in 7 and 10-day regimens. Rev Gastroenterol Peru. 2003;23:177-183. |
| 30. | Laine L, Suchower L, Frantz J, Connors A, Neil G. Twice-daily, 10-day triple therapy with omeprazole, amoxicillin, and clarithromycin for Helicobacter pylori eradication in duodenal ulcer disease: results of three multicenter, double-blind, United States trials. Am J Gastroenterol. 1998;93:2106-2112. |
| 31. | Calvet X, Garcia N, Lopez T, Gisbert JP, Gene E, Roque M. A meta-analysis of short versus long therapy with a proton pump inhibitor, clarithromycin and either metronidazole or amoxicillin for treating Helicobacter pylori infection. Aliment Pharmacol Ther. 2000;14:603-609. |
| 32. | Moayyedi P, Wason C, Peacock R, Walan A, Bardhan K, Axon AT, Dixon MF. Changing patterns of Helicobacter pylori gastritis in long-standing acid suppression. Helicobacter. 2000;5:206-214. |
| 33. | Salih BA, Abasiyanik MF, Saribasak H, Huten O, Sander E. A follow-up study on the effect of Helicobacter pylori eradication on the severity of gastric histology. Dig Dis Sci. 2005;50:1517-1522. |
| 34. | Laine L, Estrada R, Trujillo M, Fukanaga K, Neil G. Randomized comparison of differing periods of twice-a-day triple therapy for the eradication of Helicobacter pylori. Aliment Pharmacol Ther. 1996;10:1029-1033. |
| 35. | Vakil N, Connor J. Helicobacter pylori eradication: equivalence trials and the optimal duration of therapy. Am J Gastroenterol. 2005;100:1702-1703. |