Published online Apr 28, 2008. doi: 10.3748/wjg.14.2487
Revised: January 14, 2008
Published online: April 28, 2008
AIM: To study the effect of the transfected Twist gene on invasion and metastasis of gastric carcinoma cells and the possible mechanisms involved.
METHODS: Human gastric carcinoma MKN28 cells were stably transfected with Twist sense plasmid, and MKN45 cells were stably transfected with Twist antisense plasmid using the lipofectamine transfection technique. RT-PCR, Western blotting, EMSA, gelatin zymography assay, and in vitro invasion and migration assays were performed. Nude mice metastasis models were established by the abdominal cavity transfer method.
RESULTS: Cell models (TwistS-MKN28) that steadily expressed high Twist protein were obtained. Compared with MKN28 and pcDNA3-MKN28 cells, adherence, migration and invasion ability of TwistS-MKN28 cells were clearly raised. The number of cancer nodules was increased significantly in the abdominal cavity and liver of nude mice inoculated with TwistS-MKN28 cells. Overexpression of Twist in MKN28 cells increased Tcf-4/Lef DNA binding activity, and promoted expression of Tcf-4’s downstream target genes cyclin D1 and MMP-2. However, suppression of Twist (TwistAS-MKN45) inhibited MKN45 cell invasion and the expression of cyclin D1 was reduced. The activity of MMP-2 was also decreased.
CONCLUSION: These results indicate that Twist promotes gastric cancer cell migration, invasion and metastasis, and Twist may play an important role in Wnt/Tcf-4 signaling.
-
Citation: Luo GQ, Li JH, Wen JF, Zhou YH, Hu YB, Zhou JH. Effect and mechanism of the
Twist gene on invasion and metastasis of gastric carcinoma cells. World J Gastroenterol 2008; 14(16): 2487-2493 - URL: https://www.wjgnet.com/1007-9327/full/v14/i16/2487.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.2487
Gastric cancer is one of the most common cancers in the world. Several lines of evidence implicate the Wnt signaling pathway as a contributor to gastric carcinogenesis[1]. In the presence of certain Wnt proteins, or due to the loss of tumor suppressors such as APC, glycogen synthase kinase-3 (GSK-3) activity is inhibited, which results in inhibition of β-catenin phosphorylation and inhibition of degradation. The resulting accumulation of β-catenin leads to the activation of the Tcf-4/Lef transcription factor, which up-regulates the expression of downstream target genes. People with a germ-line mutation of the APC tumor suppressor gene have a 10-fold increased risk of developing gastric cancer as compared with normal individuals[2]. Mutations in the APC gene have been found in sporadic gastric cancer[3]. β-catenin mutations have also been detected in intestinal-type gastric carcinoma tissues and gastric cancer cell lines[4]. Based on these studies, we conclude that the Wnt/Tcf-4 signaling pathway is very important in gastric cancer cells.
Besides transforming growth factor β and receptor tyrosine kinase/Ras signaling, autocrine factors and Wnt-dependent pathways are reported to contribute to epithelial mesenchymal transition (EMT). EMT is a process whereby epithelial cells lose polarity and cell-to-cell adhesion, and undergo dramatic remodeling of the cytoskeleton. Concurrent with loss of epithelial cell adhesion and cytoskeletal components, cells undergoing EMT acquire expression of mesenchymal components and a migratory phenotype. EMT was first recognized in embryogenesis in the early 1980s. Today, evidence is growing that carcinoma cells activate the dormant EMT program in promoting cell migration, invasion and metastasis[5–7]. However, its pathogenesis in human carcinoma is obscure.
Several key inducers of EMT are transcription factors that repress E-cadherin expression, such as Snail, Slug, SIP1 and Twist. Recent studies have shown that Twist, a highly conserved basic helix-loop-helix protein that is essential for early embryogenesis, promotes EMT and plays an essential role in metastasis in a breast tumor model[8]. Twist has also been suggested to have oncogenic properties. Overexpression of Twist in rhabdomyosarcoma inhibits myc-induced apoptosis and interferes with p53 tumor suppression[9]. Up-regulation of Twist is associated with malignant transformation in T-cell lymphoma[10]. Forced expression of Twist triggers resistance of human cancer cells to drugs that inhibit microtubule formation, such as taxol and vincristine[11]. Furthermore, expression of Twist has been implicated in promotion of metastasis and invasive pathological subtypes in several types of carcinoma[1213].
However, the effect and mechanism of Twist gene on invasion and metastasis of gastric carcinoma remain enigmatic. Therefore, in the present work, two gastric cancer cell lines with different differentiation were steadily transfected with sense and antisense Twist vectors. The effect of Twist gene on cell migration, invasion and metastasis was investigated.
Human gastric carcinoma cell line MKN28 was kindly provided by Dr. JI Shuyu (Kunming Medical college, Kunming, China). Gastric carcinoma cell line MKN45 was purchased from Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). Cells were cultured in RPMI 1640 medium (Gibco Biocult, Paisley, UK) that contained 10% fetal bovine serum at 37°C in a humidified 5% CO2 atmosphere.
The Twist sense and antisense expression vectors (pcDNA3/TwistS, pcDNA3/TwistAS) were kindly provided by Dr. Glackin C[14]. The identity of Twist was confirmed by gene sequencing and routine agarose gel electrophoresis. Primary antibodies (anti-Twist, anti-MMP-2 and anti-β-actin) were from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and secondary antibody (HRP-linked IgG) was from Cell Signaling Technology (Beverly, MA, USA).
Cell transfection was carried out using Lipofectamine 2000 according to the manufacturer’s instructions. Briefly, cells were grown to 80%-90% confluence, without antibiotics. Vectors that contained the different constructs (10 &mgr;g) were diluted in RPMI 1640 (100 &mgr;L) and mixed with the transfection solution for 15 min. After washing, the cells were incubated with the transfection mixture at 37°C for 10 h, and then allowed to grow in fresh medium. Stable transfectants were isolated by selection with 600 mg/mL G418 (Geniticin; Amresco, Solan, OH, USA) for 2 wk. Pools of geneticin-resistant clones were passaged and expanded for Western blot analysis. Cells transfected with the pcDNA3 vector were used as controls.
Cell total proteins were prepared in SDS sample buffer and boiled for 3 min. Equal amounts of cell protein, quantified by the BCA Protein Assay kit (Pierce Biotechnology, Rockford, IL, USA), were loaded onto 10% SDS-PAGE. After electrophoresis, the separated proteins were transferred to nitrocellulose membranes. The membranes were stained with ponceau (Amresco) and blocked with 5% non-fat milk for 1.5 h, and then incubated with antibody for 18 h at room temperature. The blots were subsequently incubated with an HRP-conjugated secondary antibody. Proteins were visualized using 3,3’-diaminobenzidine, with β-actin as a control.
Cells were lysed in 400 &mgr;L ice-cold buffer A (10 mmol/L HEPES, pH 7.9, 10 mmol/L KCl, 0.1 mmol/L EDTA, 0.1 mmol/L EGTA, 1 mmol/L DTT, 0.5 mmol/L PMSF) by gentle pipetting. The cells were allowed to swell on ice for 15 min, then 40 &mgr;L of a 10% solution of Nonidet P-40 was added, and the tube was vigorously vortexed for 10 s. The homogenate was centrifuged for 30 s in a microfuge. The supernatant was transferred and the nuclear pellet was lysed with 50 &mgr;L buffer C (20 mmol/L HEPES, pH 7.9, 0.42 mol/L NaCl, 1 mmol/L EDTA, 1 mmol/L EGTA, 1 mmol/L DTT, 1 mmol/L PMSF), and the tube was vigorously rocked at 4°C for 15 min on a shaking platform. The nuclear extract was centrifuged for 5 min in a microfuge at 4°C. The supernatant containing nuclear protein was quantified by the BCA Protein Assay kit (Pierce Biotechnology) and stored at -70°C.
EMSA was performed with the LightShift Chemiluminescent kit (Pierce Biotechnology). Specific oligonucleotides for binding of TCF-4 (S: 5’-CCCTTTGATCTTACC-3’; A: 3’-GGTAAGATCAAAGGG-5’) were prepared by end labeling of the 5’ terminus with biotin (synthesized by Bioasia Biotech, Shanghai, China). Briefly, nuclear extract protein (10 &mgr;g) was incubated with reaction mixture [10 × binding buffer, 1 &mgr;g/L poly (dI.dC), 1% NP-40, labeled probe] for 20 min at room temperature. Each sample was electrophoresed in 6% non-denaturing polyacrylamide gel at 100 V for 1.5 h. The gel was then electrophoretically transferred to a nylon membrane. Finally, the membrane was detected by chemiluminescence. In competitive studies, a 100-fold excess of unlabeled probe was included in the reaction mixture.
Cells were lysed in TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and total RNA was prepared according to the manufacturer’s instructions. cDNA was synthesized by the SuperscriptTM First Strand Synthesis System (Life Technologies, Gaithersburg, MD, USA). The cDNA was then amplified by PCR with specific primers, cycline D1 sense: 5’-ACGGCCGAG-AAGCTGTGCAT; antisense: 5’-TTCCAATCCGCCCTCCATGGA. The cDNA of GAPDH was amplified as a control for the amount of cDNA present in each sample (sense: 5’-ACGGATTTGGTCGTATTGGG antisense: 5’-TGATTTTGGAGGG-ATCTCGC). The relative expression levels were generated by comparing the density to the controls and indicated underneath each gel.
Fifty milligrams per liter Matrigel solution diluted with sterilized double distilled water (1:8) was prepared, added to 96-well plates (50 &mgr;L/well), and incubated for 12 h at 4°C. Ten grams per liter BSA were used as a control. After abandoning remnant liquid, no-serum culture solution that contained 10 g/L BSA (50 &mgr;L/well) was added and incubated at 37°C for 30 min. Tumor cells were digested with 2.5 g/L pancreatic enzyme and modulated to a density of 1 × 105 cells/mL. One hundred microliters of cell suspension was seeded in invested Matrigel per well. Every group had four samples at equal pace. The cells were cultivated with RPMI 1640 medium that contained 10 g/L BSA and 10% fetal bovine serum for 1 h at 37°C, then the absorbance (A) was determined by the MTT colorimetric method. Cell adhesion rate of the Matrigel group was calculated by the following formula:
Adhesion rate = (Aexp/ABSA - 1) × 100% equation 1
Invasion assays were done in a Boyden chamber with polyethylene terephthalate filter inserts for 24-well plates containing 8-&mgr;m pores (Becton Dickinson Labware, NY, USA). Briefly, after coating the filter with 100 &mgr;L 1:3 diluted Matrigel (Becton Dickinson) overnight at 4°C, cells were seeded in the upper chamber at a final concentration of 1.0 × 105/mL in serum-free medium with 0.1% BSA. Eight hundred microliters medium conditioned with 10 &mgr;g/mL fibronectin was placed in the lower compartment of the chamber as a chemoattractant. After 24 h incubation, the remaining tumor cells on the upper surface of the filters were removed by wiping with cotton swabs, and the invading cells on the lower surface were stained with hematoxylin-eosin. The invading cells on the underside of the membrane were photographed and counted under a microscope at a magnification of × 200. We performed four individual experiments using the invasion assay in triplicate. In vitro migration assays were done under the same conditions as the invasion assays, but in non-Matrigel-coated chambers.
Cells were digested with 2.5 g/L pancreatic enzymes, washed with no-serum culture solution, and centrifuged at 1800 r/min. The cell sediment was washed with serum-free culture solution, centrifuged twice and floated in sterile PBS solution. Two hundred microliters of cell suspension that contained 1 × 107 MKN28, pcDNA3-MKN28 and TwistS-MKN28 cells was seeded into the abdominal cavity of nude mice by syringe. The vim, appetite and defecation of nude mice were observed regularly, and their weight was recorded. After 9 wk, nude mice were killed and examined.
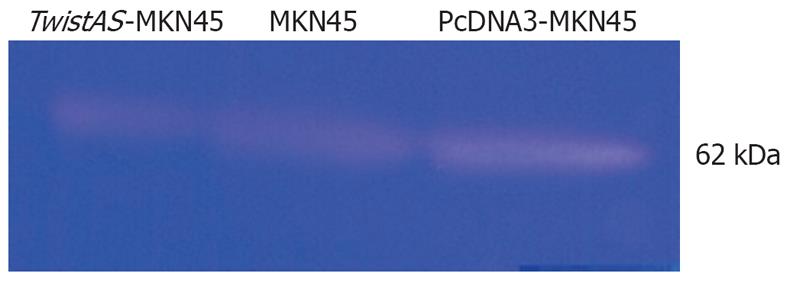
MKN45, pcDNA3-MKN45 and TwistAS-MKN45 cells were cultured. Three days later, cells were washed by D-Hanks’ solution, and then seeded into serum-free culture solution. Twenty-four hours later, the supernatant was collected and concentrated with Amicon filters (Millipore) to a 10% initial volume. Each sample was guaranteed to contain the same amount of total protein. Gelatin zymography was performed in 10% (w/v) polyacrylamide that contained 0.1% (w/v) gelatin. The identification of transparent bands at 62 kDa on the Coomassie blue background of the slab gel was considered positive for the presence of enzymatic activity.
Results were expressed as mean ± SD for experiments with triplicate measurements. Differences between groups were tested with Student’s t test and P < 0.05 was considered significant.
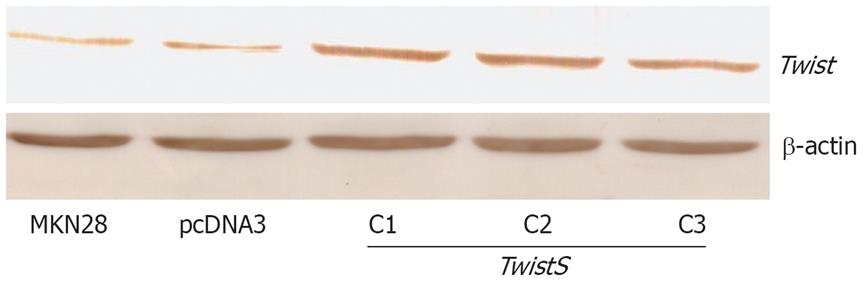
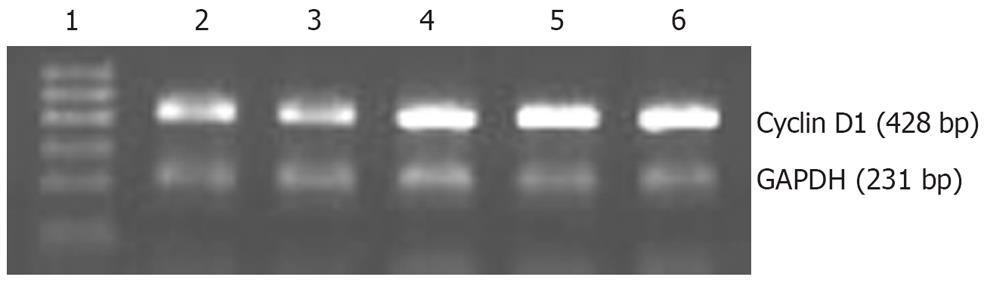
MKN28 cell clones transfected with pcDNA3 or pcDNA3-TwistS were obtained after gene transfection. Expression of Twist protein was increased in the three positive cell clones that were transfected with pcDNA3-TwistS (Figure 1). To avoid the deviation brought about by a single cell clone, three positive cell clones were used simultaneously.
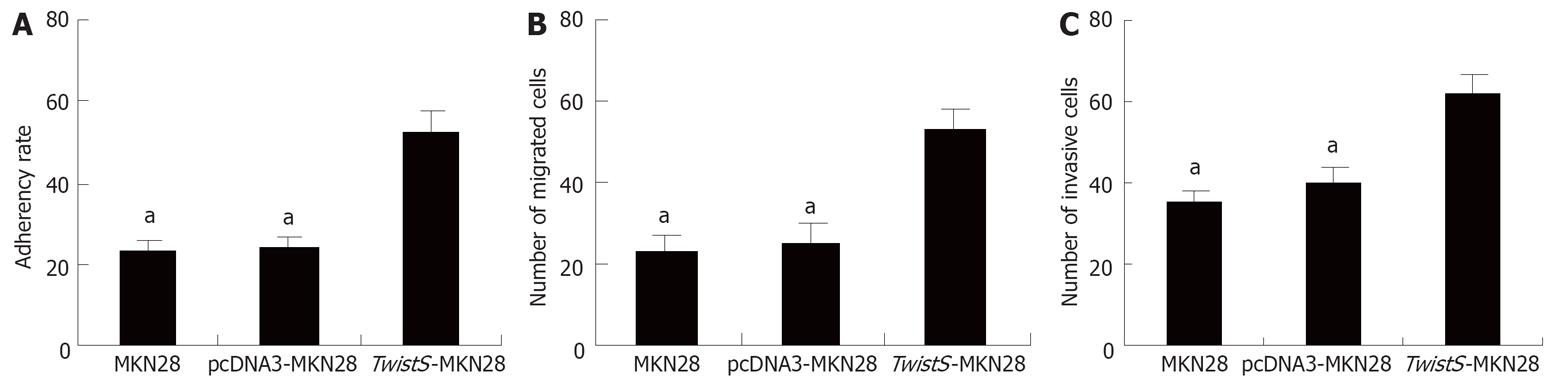
A value was decided by the MTT colorimetric method and cell adhesion rate was calculated by equation 1. Compared with that of MKN28 (23.5%) and pcDNA3-MKN28 cells (24.2%), the adherence rate of TwistS-MKN28 cells (52.8%) was obviously increased (P < 0.05) (Figure 2A). Compared with that of MKN28 (22) and pcDNA3-MKN28 cells (25), the migration rate of TwistS-MKN28 cells (54) clearly increased (P < 0.05) (Figure 2B). Compared with that of MKN28 (40) and pcDNA3-MKN28 cells (36), the invasion rate of TwistS-MKN28 cells (62) also clearly increased (P < 0.05) (Figure 2C). Every sample was counted for five different visual fields.
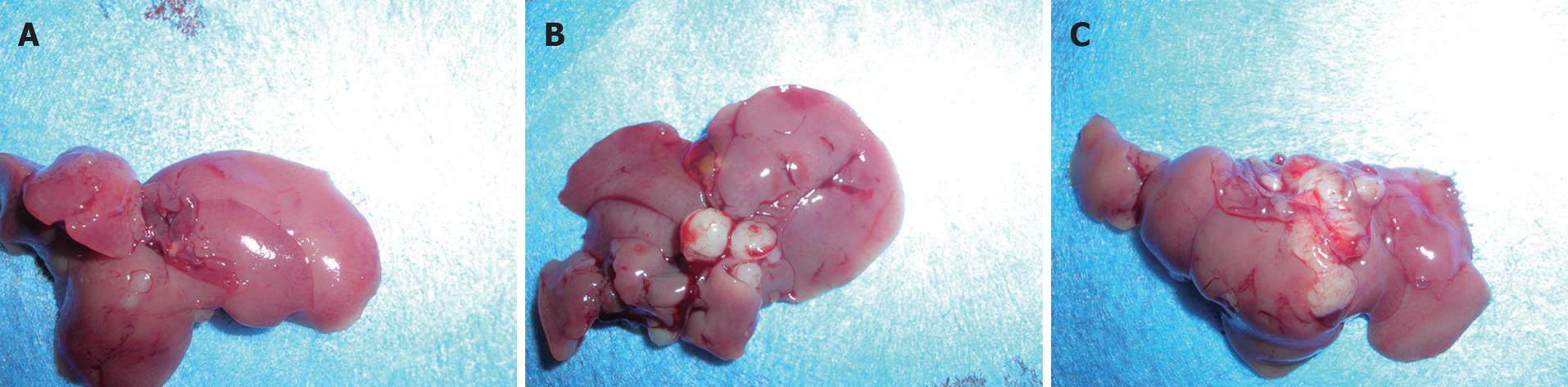
The metastasis models of gastric carcinoma cells were established in fifteen nude mice. Eight weeks later, the mice were killed and underwent exploratory laparotomy. Number and size of metastatic nodules were calculated and measured. There were lots of bigger cancer nodules in the abdominal cavity and liver of the nude mice inoculated with TwistS-MKN28 cells, while fewer nodules were present in the nude mice inoculated with MKN28 and pcDNA3-MKN28 cells (Figure 3).
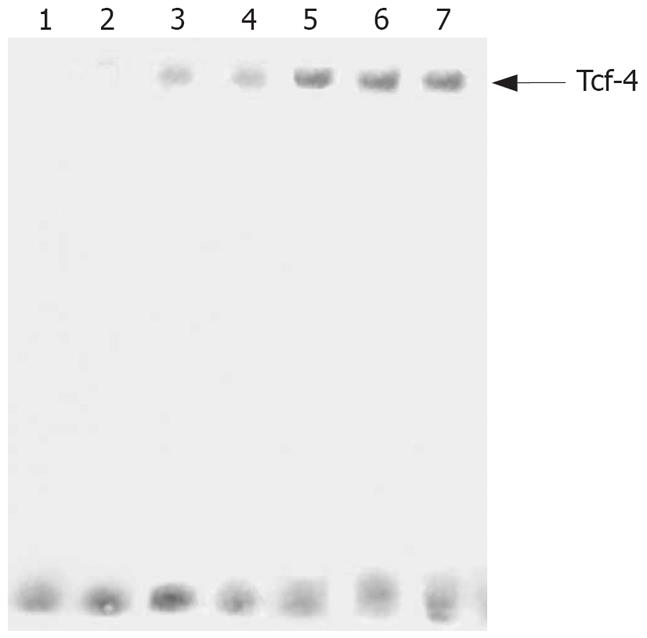
Consistent with the increasing expression levels of Twist, induction of Tcf-4/Lef binding activity was observed in MKN28 and pcDNA3 control cells, Moreover, Tcf-4/Lef DNA binding was significantly increased in TwistS transfectants (Figure 4).
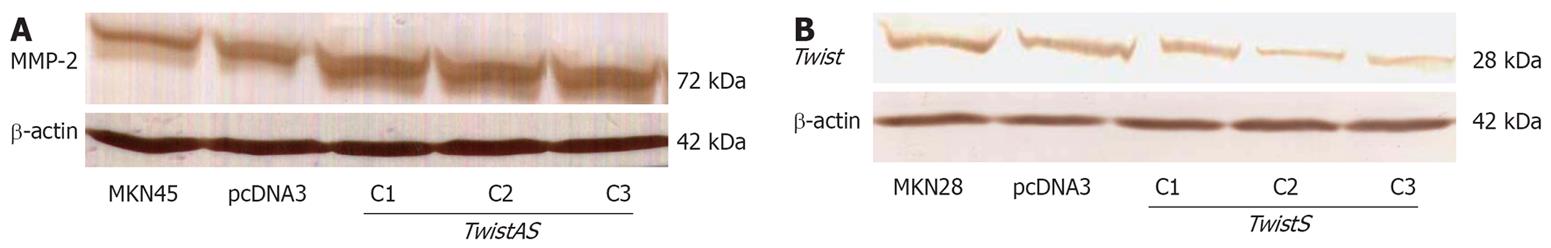
We explored whether overexpression of Twist affected expression of cyclin D1 and mmp-2. By RT-PCR, cyclin D1 RNA levels were markedly higher in TwistS cells than in MKN28 and pcDNA3 control cells (Figure 5). Western blot analysis showed that TwistS cells exhibited increased expression levels of MMP-2 compared with those in MKN28 cells and pcDNA3 control cells (Figure 6A).
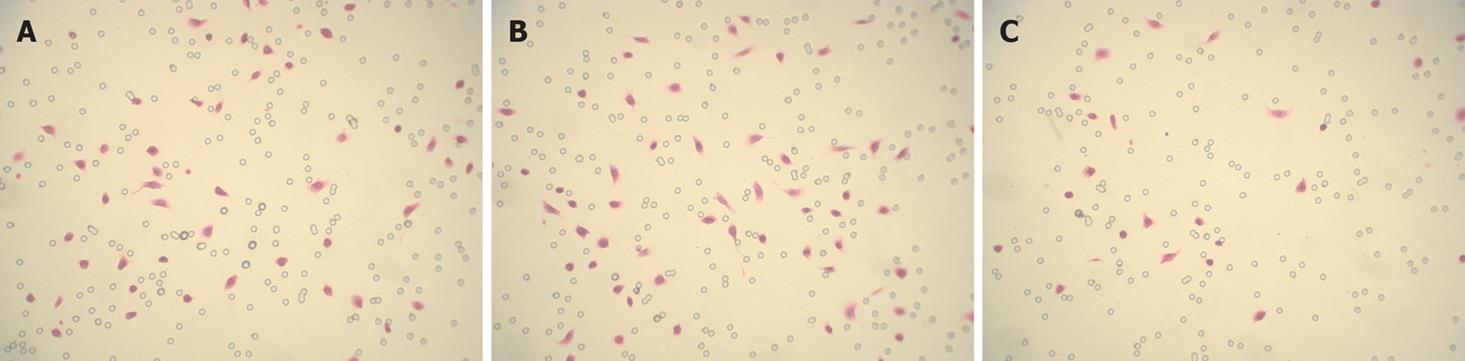
To observe the invasion-promoting effect of Twist in gastric cancer cells, a pcDNA3/-TwistAS vector was transfected into MKN45 cells to generate stable transfectants. As shown in Figure 6B, Twist expression was inhibited by TwistAS transfectants at the protein level. We assessed the effect of down-regulation of Twist on cell invasion. Representative photos showed that invasion in TwistAS transfectants was markedly reduced compared with that of MKN45 or pcDNA3 –MKN45 cells (Figure 7).
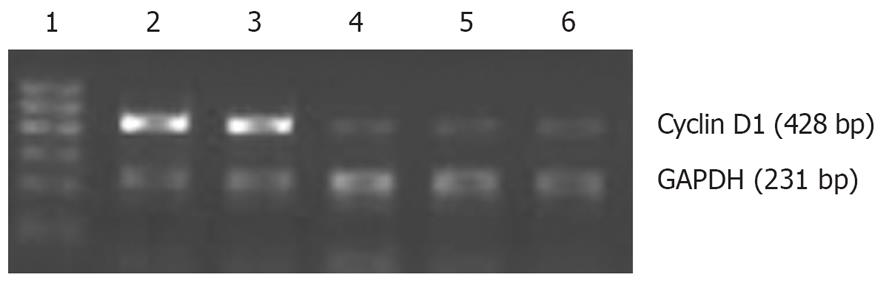
We investigated whether suppression of Twist affects the expression of cyclin D1. Cyclin D1 RNA levels were markedly lower in TwistAS cells than in MKN45 and pcDNA3 control cells (Figure 8).
Gelatin zymography revealed prominent 62 kDa bands (Figure 9), and there was activity of MMP-2 in MKN45, pcDNA3-MKN45 and TwistAS-MKN45 cell supernatants. Compared with that of MKN45 and pcDNA3-MKN45 cells, the activity of MMP-2 in TwistAS-MKN45 cells dropped markedly.
It is commonly believed that Twist expression is correlated with potent invasiveness as well as poor prognosis in epithelial cancer[8111319]. In gastric cancer, a recent report has shown overexpression of the Twist gene is more frequently found in diffuse-type carcinoma tissues with high N-cadherin gene expression[15]. However, no definitive results have indicated Twist promotes the invasion and metastasis of gastric cancer. We have previously demonstrated endogenous Twist is expressed abundantly in MKN45 cells, but at much lower levels in MKN28 cells. Therefore, we transfected MKN28 cells with the Twist sense plasmid. Our findings suggested Twist probably promoted adherence, migration, invasion and metastasis of gastric cancer cells, using the MTT and Boyden chamber methods, and metastasis models of gastric carcinoma in nude mice. However, when we transfected MKN45 cells with the Twist antisense vector, results indicated suppression of Twist inhibited cell invasion. Therefore, we think Twist may play an important role in invasion and metastasis of gastric carcinoma.
A large number of genes relevant for tumor formation and progression have been found to be transcriptionally activated by the β-catenin/Tcf complex. Some of these are implicated in growth control and cell cycling (c-myc, cyclin D1), Some are relevant for cell survival (Id2, MDR1), one is the EMT marker vimentin, and others are implicated in tumor invasion and metastasis (matrilysin, VEGF, cd44)[16–18]. Tcf-4/Lef is the transcription factor for Wnt signaling. Our results indicated that overexpression of Twist in MKN28 cells increased Tcf-4/Lef DNA binding activity, and promoted expression of Tcf-4’ downstream gene cyclin D1. The reason may be that overexpression of Twist redistributes β-catenin to the nucleus, in which it forms a functional transacting complex by associating with the Tcf4/Lef-1 transcription factor, and enhances the transactivation of a number of genes including cyclin D1, VEGF and EMT marker vimentin. Cyclin D1 plays an important pole in cell proliferation[20]. Our results agreed with previous studies that showed that Twist promotes growth of breast cancer cells. In breast cancer MCF-7 cells, colocalization of β-catenin and E-cadherin was prominent at the plasma membrane, whereas in MCF-7 cells with overexpression of Twist, these proteins were distributed within the cytoplasm and to a lesser extent within the nucleus. In addition, the total amount of epithelial marker protein β-catenin and E-cadherin was lower in MCF-7 cells[21]. This can partly explain the phenomenon of EMT by Twist.
Metastatic potential requires proteolytic degradation of the extracellular matrix, and MMPs are thought to play an important role in tumor invasion and metastasis. We demonstrated that overexpression of Twist in MKN28 cells promoted expression of mmp-2, while suppression of Twist in MKN45 cells inhibited the activity of mmp-2. However, to the best of our knowledge, the E-box site, which is the binding site for Twist, was not observed in the promoter region of the MMP gene family. One possible explanation is that MTI-MMP expression is induced through β-catenin/Tcf4 expression[2223], followed by increased activation of MMP-2. Another explanation is that MMP-2 activity is induced through the up-regulation of MT1-MMP expression, by inhibition of zonula occludens 1 tight junction complex expression that is changed by EMT[24].
In conclusion, Twist may contribute to the invasion and metastasis of gastric carcinoma cells, mainly through EMT after regulation of Wnt signaling, or through an effect on MMP-2. At the same time, recent evidence suggests Twist is a major factor that participates in tumor development and progression[25–28]. As a novel player in the invasion and metastatic program, Twist is gaining rapid attention[2930]. Our findings of a functional link between Twist and Tcf4/Lef-1 suggest targeting Twist may provide novel therapeutic cocktails for gastric cancer intervention.
Invasion and metastasis are the most common cause of death and is a major obstacle to successful treatment of gastric cancer. It is necessary to develop effective new strategies for the prediction, diagnosis and treatment of gastric cancer invasion and metastasis.
Recent investigations have shown Twist is elevated in prostate and breast cancer. High expression of Twist is positively related to cancer invasion and metastasis, but few studies have investigated Twist expression in gastric cancer, and the impact of Twist on prognosis.
Twist promotes gastric caner cells migration, invasion and metastasis, and Twist may play an important role in Wnt/Tcf-4 signaling.
Our finding of a functional link between Twist and Tcf4/Lef-1 suggests that targeting Twist may provide novel therapeutic cocktails for gastric cancer intervention.
EMT is a process whereby epithelial cells lose polarity and cell-to-cell adhesion, and undergo dramatic remodeling of the cytoskeleton. Concurrent with loss of epithelial cell adhesion and cytoskeletal components, cells undergoing EMT acquire expression of mesenchymal components and a migratory phenotype.
This study was well designed and may be important for providing additional evidence to support the role of Twist expression in promoting tumor metastasis, including in gastric cancer.
| 1. | Clements WM, Wang J, Sarnaik A, Kim OJ, MacDonald J, Fenoglio-Preiser C, Groden J, Lowy AM. beta-Catenin mutation is a frequent cause of Wnt pathway activation in gastric cancer. Cancer Res. 2002;62:3503-3506. |
| 2. | Offerhaus GJ, Giardiello FM, Krush AJ, Booker SV, Tersmette AC, Kelley NC, Hamilton SR. The risk of upper gastrointestinal cancer in familial adenomatous polyposis. Gastroenterology. 1992;102:1980-1982. |
| 3. | Nakatsuru S, Yanagisawa A, Ichii S, Tahara E, Kato Y, Nakamura Y, Horii A. Somatic mutation of the APC gene in gastric cancer: frequent mutations in very well differentiated adenocarcinoma and signet-ring cell carcinoma. Hum Mol Genet. 1992;1:559-563. |
| 4. | Park WS, Oh RR, Park JY, Lee SH, Shin MS, Kim YS, Kim SY, Lee HK, Kim PJ, Oh ST. Frequent somatic mutations of the beta-catenin gene in intestinal-type gastric cancer. Cancer Res. 1999;59:4257-4260. |
| 5. | Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442-454. |
| 6. | Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;17:548-558. |
| 7. | Kang Y, Massague J. Epithelial-mesenchymal transitions: twist in development and metastasis. Cell. 2004;118:277-279. |
| 8. | Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927-939. |
| 9. | Maestro R, Dei Tos AP, Hamamori Y, Krasnokutsky S, Sartorelli V, Kedes L, Doglioni C, Beach DH, Hannon GJ. Twist is a potential oncogene that inhibits apoptosis. Genes Dev. 1999;13:2207-2217. |
| 10. | van Doorn R, Dijkman R, Vermeer MH, Out-Luiting JJ, van der Raaij-Helmer EM, Willemze R, Tensen CP. Aberrant expression of the tyrosine kinase receptor EphA4 and the transcription factor twist in Sezary syndrome identified by gene expression analysis. Cancer Res. 2004;64:5578-5586. |
| 11. | Kwok WK, Ling MT, Lee TW, Lau TC, Zhou C, Zhang X, Chua CW, Chan KW, Chan FL, Glackin C. Up-regulation of TWIST in prostate cancer and its implication as a therapeutic target. Cancer Res. 2005;65:5153-5162. |
| 12. | Elias MC, Tozer KR, Silber JR, Mikheeva S, Deng M, Morrison RS, Manning TC, Silbergeld DL, Glackin CA, Reh TA. TWIST is expressed in human gliomas and promotes invasion. Neoplasia. 2005;7:824-837. |
| 13. | Lee TK, Poon RT, Yuen AP, Ling MT, Kwok WK, Wang XH, Wong YC, Guan XY, Man K, Chau KL. Twist overexpression correlates with hepatocellular carcinoma metastasis through induction of epithelial-mesenchymal transition. Clin Cancer Res. 2006;12:5369-5376. |
| 14. | Lee MS, Lowe GN, Strong DD, Wergedal JE, Glackin CA. TWIST, a basic helix-loop-helix transcription factor, can regulate the human osteogenic lineage. J Cell Biochem. 1999;75:566-577. |
| 15. | Rosivatz E, Becker I, Specht K, Fricke E, Luber B, Busch R, Hofler H, Becker KF. Differential expression of the epithelial-mesenchymal transition regulators snail, SIP1, and twist in gastric cancer. Am J Pathol. 2002;161:1881-1891. |
| 16. | Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422-426. |
| 17. | Yamada T, Takaoka AS, Naishiro Y, Hayashi R, Maruyama K, Maesawa C, Ochiai A, Hirohashi S. Transactivation of the multidrug resistance 1 gene by T-cell factor 4/beta-catenin complex in early colorectal carcinogenesis. Cancer Res. 2000;60:4761-4766. |
| 18. | Mann B, Gelos M, Siedow A, Hanski ML, Gratchev A, Ilyas M, Bodmer WF, Moyer MP, Riecken EO, Buhr HJ. Target genes of beta-catenin-T cell-factor/lymphoid-enhancer-factor signaling in human colorectal carcinomas. Proc Natl Acad Sci USA. 1999;96:1603-1608. |
| 19. | Kyo S, Sakaguchi J, Ohno S, Mizumoto Y, Maida Y, Hashimoto M, Nakamura M, Takakura M, Nakajima M, Masutomi K. High Twist expression is involved in infiltrative endometrial cancer and affects patient survival. Hum Pathol. 2006;37:431-438. |
| 20. | Liu Y, Xi L, Liao G, Wang W, Tian X, Wang B, Chen G, Han Z, Wu M, Wang S. Inhibition of PC cell-derived growth factor (PCDGF)/granulin-epithelin precursor (GEP) decreased cell proliferation and invasion through downregulation of cyclin D and CDK4 and inactivation of MMP-2. BMC Cancer. 2007;7:22. |
| 21. | Mironchik Y, Winnard PT Jr, Vesuna F, Kato Y, Wildes F, Pathak AP, Kominsky S, Artemov D, Bhujwalla Z, Van Diest P. Twist overexpression induces in vivo angiogenesis and correlates with chromosomal instability in breast cancer. Cancer Res. 2005;65:10801-10809. |
| 22. | Wang H, Keiser JA. Hepatocyte growth factor enhances MMP activity in human endothelial cells. Biochem Biophys Res Commun. 2000;272:900-905. |
| 23. | Takahashi M, Tsunoda T, Seiki M, Nakamura Y, Furukawa Y. Identification of membrane-type matrix metalloproteinase-1 as a target of the beta-catenin/Tcf4 complex in human colorectal cancers. Oncogene. 2002;21:5861-5867. |
| 24. | Polette M, Gilles C, Nawrocki-Raby B, Lohi J, Hunziker W, Foidart JM, Birembaut P. Membrane-type 1 matrix metalloproteinase expression is regulated by zonula occludens-1 in human breast cancer cells. Cancer Res. 2005;65:7691-7698. |
| 25. | Song LB, Liao WT, Mai HQ, Zhang HZ, Zhang L, Li MZ, Hou JH, Fu LW, Huang WL, Zeng YX. The clinical significance of twist expression in nasopharyngeal carcinoma. Cancer Lett. 2006;242:258-265. |
| 26. | Horikawa T, Yang J, Kondo S, Yoshizaki T, Joab I, Furukawa M, Pagano JS. Twist and epithelial-mesenchymal transition are induced by the EBV oncoprotein latent membrane protein 1 and are associated with metastatic nasopharyngeal carcinoma. Cancer Res. 2007;67:1970-1978. |
| 27. | Puisieux A, Valsesia-Wittmann S, Ansieau S. A twist for survival and cancer progression. Br J Cancer. 2006;94:13-17. |
| 28. | Cheng GZ, Chan J, Wang Q, Zhang W, Sun CD, Wang LH. Twist transcriptionally up-regulates AKT2 in breast cancer cells leading to increased migration, invasion, and resistance to paclitaxel. Cancer Res. 2007;67:1979-1987. |
| 29. | Terauchi M, Kajiyama H, Yamashita M, Kato M, Tsukamoto H, Umezu T, Hosono S, Yamamoto E, Shibata K, Ino K. Possible involvement of TWIST in enhanced peritoneal metastasis of epithelial ovarian carcinoma. Clin Exp Metastasis. 2007;24:329-339. |
| 30. | Puisieux A, Valsesia-Wittmann S, Ansieau S. A twist for survival and cancer progression. Br J Cancer. 2006;94:13-17. |