Published online Apr 14, 2008. doi: 10.3748/wjg.14.2246
Revised: December 31, 2007
Published online: April 14, 2008
AIM: To investigate dynamic changes of serum IL-2, IL-10, IL-2/IL-10 and sFas in rats with acute necrotizing pancreatitis. To explore the expression of Fas in intestinal mucosa of rats with acute necrotizing pancreatitis (ANP).
METHODS: A total of 64 Sprague-Dawley (SD) rats were randomly divided into two groups: normal control group (C group), ANP group (P group). An ANP model was induced by injection of 50 g/L sodium taurocholate under the pancreatic membrane. Normal control group received isovolumetric injection of 9 g/L physiological saline solution using the same method. The blood samples of the rats in each group were obtained via superior mesenteric vein to measure levels of IL-2, IL-10, sFas and calculate the value of IL-2/IL-10. The levels of IL-2, IL-10 and sFas were determined by ELISA. The severity of intestinal mucosal injury was evaluated by pathologic score. The expression of Fas in intestinal mucosal tissue was determined by immunohistochemistry staining.
RESULTS: Levels of serum IL-2 were significantly higher in P group than those of C group (2.79 ± 0.51 vs 3.53 ± 0.62, 2.93 ± 0.89 vs 4.35 ± 1.11, 4.81 ± 1.23 vs 6.94 ± 1.55 and 3.41 ± 0.72 vs 4.80 ± 1.10, respectively, P < 0.01, for all) and its reached peak at 6 h. Levels of serum IL-10 were significantly higher in P group than those of C group at 6 h and 12 h (54.61 ± 15.81 vs 47.34 ± 14.62, 141.15 ± 40.21 vs 156.12 ± 43.10, 89.18 ± 32.52 vs 494.98 ± 11.23 and 77.15 ± 22.60 vs 93.28 ± 25.81, respectively, P < 0.01, for all). The values of IL-2/IL-10 were higher significantly in P group than those of C group at 0.5 h and 2 h (0.05 ± 0.01 vs 0.07 ± 0.02 and 0.02 ± 0.01 vs 0.03 ± 0.01, respectively, P < 0.01, for all), and it were significantly lower than those of C group at 6 h (0.05 ± 0.02 vs 0.01 ± 0.01, P < 0.01) and returned to the control level at 12 h (0.04 ± 0.01 vs 0.05 ± 0.02, P > 0.05). In sFas assay, there was no significant difference between P group and C group (3.16 ± 0.75 vs 3.31 ± 0.80, 4.05 ± 1.08 vs 4.32 ± 1.11, 5.93 ± 1.52 vs 5.41 ± 1.47 and 4.62 ± 1.23 vs 4.44 ± 1.16, respectively, P > 0.05, for all). Comparison of P group and C group, the pathological changes were aggravated significantly in P group. Immunohistochemistry staining show the expression of Fas was absent in normal intestinal tissues, however, it gradually increased after induction of pancreatitis in intestinal tissue, then reached their peaks at 12 h.
CONCLUSION: Fas were involved in the pathogenesis of pancreatitis associated intestinal injury. The mechanisms of Fas may be associated to Fas mediated T helper cell apoptosis.
- Citation: Dang SC, Zhang JX, Qu JG, Mao ZF, Wang XQ, Zhu B. Dynamic changes of IL-2/IL-10, sFas and expression of Fas in intestinal mucosa in rats with acute necrotizing pancreatitis. World J Gastroenterol 2008; 14(14): 2246-2250
- URL: https://www.wjgnet.com/1007-9327/full/v14/i14/2246.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.2246
Acute pancreatitis (AP) is sudden inflammation of the pancreas that may be mild or life threatening but that usually subsides. Although usually self-limiting, 10% to 20% of afflicted patients will progress to acute necrotizing pancreatitis (ANP)[12]. The mortality rate among patients with ANP may approach 30% when they progress to multiple organ failure (MOF)[3]. It is generally accepted that AP is often complicated by intestinal injury. Failure of intestinal barrier function often occurs in this condition, resulting in the increased intestinal permeability. It is clear that increased intestinal permeability and bacteria with or without endotoxin translocation plays a key role in the development of severe complications such as systemic inflammatory response syndrome (SIRS), sepsis, multiple organ dysfunction syndrome (MODS) and MOF[4–7]. However, its pathogenesis remains unclear.
The Fas system was originally characterized as a key mechanism for inducing apoptosis in immune cells, but later it transpired to be very common in various tissues such as liver, ovary, kidney, and testis, especially under I/R conditions. Apoptosis is a teleologically beneficial form of cell death in AP. However, little is known about how the induction of apoptosis reduces the severity of AP[8]. Recent research has demonstrated that a Th1 to Th2 immune deviation is beneficial to ANP. The antigen-induced deletion of Th is often accompanied by an imbalance in Th1 and Th2. The two most polarized patterns of cytokine production, Th1 (characterized by production of IL-2, IFN-γ, TNF-α, and TNF-β) and Th2 (characterized by production of IL-4, 5, 6, 10, and -13) were reported[9].
In the present study, we performed immunohisto-chemistry staining of apoptosis-related protein Fas and investigated dynamic changes of serum IL-2, IL-10, IL-2/ IL-10 and sFas in rats with ANP.
Adult Sprague-Dawley rats of both sexes weighing 250-300 g were provided by the Laboratory Animal Center of Jiangsu University. The animals were fed with standard rat chow and water ad libitum. The rats were allowed to acclimatize to our laboratory conditions for 1 wk and then subjected to mesh stainless-steel cages at a constant temperature (21 ± 1°) in a 12 h day/night cycle. The animals were fasted for 12 h before the experiments but had free access to water. Animal care and experimental procedure were performed in accordance with the guidelines for Animal Experimentation of Jiangsu University with the approval of the Institutional Animal Care and Use Committee.
The animals were randomly divided into: control group (C group), ANP group (P group) with 32 rats in each group. Each group was further divided into 0.5, 2, 6 and 12 h subgroups, respectively. The mortality in the present series was not calculated. The rats were anesthetized with an intraperitoneal injection of sodium pentobarbital (50 mg/kg body weight). The rats were infused with sodium taurocholate (4 mL/kg, Na-Tc, Sigma) the pancreatic membrane to induce ANP model as previously described[10]. After 30 min, pancreatic edema and dotted bleeding occurred. Normal control group received isovolumetric injection of 9 g/L physiological saline solution using the same method. Animals in each group were sacrificed at 0.5, 2, 6 and 12 h after infusion for further examination. Part of distal ileum and pancreas were removed immediately and fixed in paraformaldehyde solution for 12-24 h and paraffin-embedded for routine histopathologic analysis. The histopathologists were blinded to routine histopathologic analysis.
The blood of rats in each group was obtained via superior mesenteric vein for determination of serum IL-2, L-10 and sFas levels at 0.5, 2, 6 and 12 h after infusion. Serum levels of IL-2, L-10 and sFas were measured by double antibody sandwich ELISA according to the manufacturer’s protocol (Shanghai Senxiong Technology Enterprise Co., Ltd.). The optical density of each well was determined within 30 min using a microplate reader (492 nm).
The whole pancreas and parts of distal ileum were obtained and promptly fixed in 40 g/L phosphate-buffered formaldehyde for further studies. Paraffin-embedded tissue sections (5 &mgr;m thick) were stained with hematoxylin and eosin. Mucosal damage was assessed according to the standard scale of Chiu et al[11]. Grading was performed and classified as 0 = normal mucosa; 1 = development of subepithelial space at the tip of the villus; 2 = extension of the space with epithelial lifting; 3 = massive epithelial lifting; 4 = denuded villi; 5 = disintegration of the lamina propria.
After embedding in paraffin, sections 5 &mgr;m in thickness were immersed twice into xylene for 5 min each, followed by immersion twice for 3 min each in 100% ethanol and then 95% ethanol. Slides were rinsed for 30 sec using deionized water and then immersed twice in deionized water for 5 min. To detect Fas expression, heat-induced Ag retrieval was performed using 0.01 mol/L citrate buffer (pH 6.2) and 10 min slide immersion into 95°C waterbath. Immunoenzyme double staining of intestinal tissue was performed using DAKO EnVision Doublestain System. The sections were then counterstained using hematoxylin before study.
All data were analyzed with the SPSS 11.0 software. The results were expressed in mean ± SD except for date on the grading of intestinal mucosal lesions. Differences of grading of intestinal mucosal lesions were determined using the non-parametric Mann-Whitney test. Statistical analysis was performed with post-hoc test. P < 0.05 was considered statistically significant.
At 0.5 h after injection of 50 g/L sodium taurocholate, serum IL-2 level in the samples from mesentery vein in P group were higher than those in C group. From 0.5 h, there was a significant difference between P group and C group (P < 0.01, Table 1).
Upon stimulation, serum IL-10 level was significantly increased in the P group as compared with that of C group at 6 and 12 h (P < 0.01, Table 1). There was no significant difference between P group and C group at 0.5 and 2 h.
As shown in Table 2, the values of IL-2/IL-10 were higher significantly in P group than those of C group at 0.5 h and 2 h, and it were significantly lower than those of C group at 6 h (P < 0.01) and returned to the control level at 12 h (P > 0.05) (Table 2) .
In serum sFas assay as illustrated Table 2, there was no significant difference between P group and C group. sFas levels were moderate at 0.5 h, peaked at 6 h and decreased at 12 h.
After induction of ANP model, pancreas showed mild edema and congestion. 2 h after introduction of the model, typical pathologic changes were found in P group, such as a large number of inflammatory cells, necrosis of adjacent fat tissues, interstitial edema, parenchyma hemorrhage and necrosis, large amount of ascites. The degree of intestinal pathological injury is shown in Table 3. The grades of P group were significantly higher those of control group (P < 0.01).
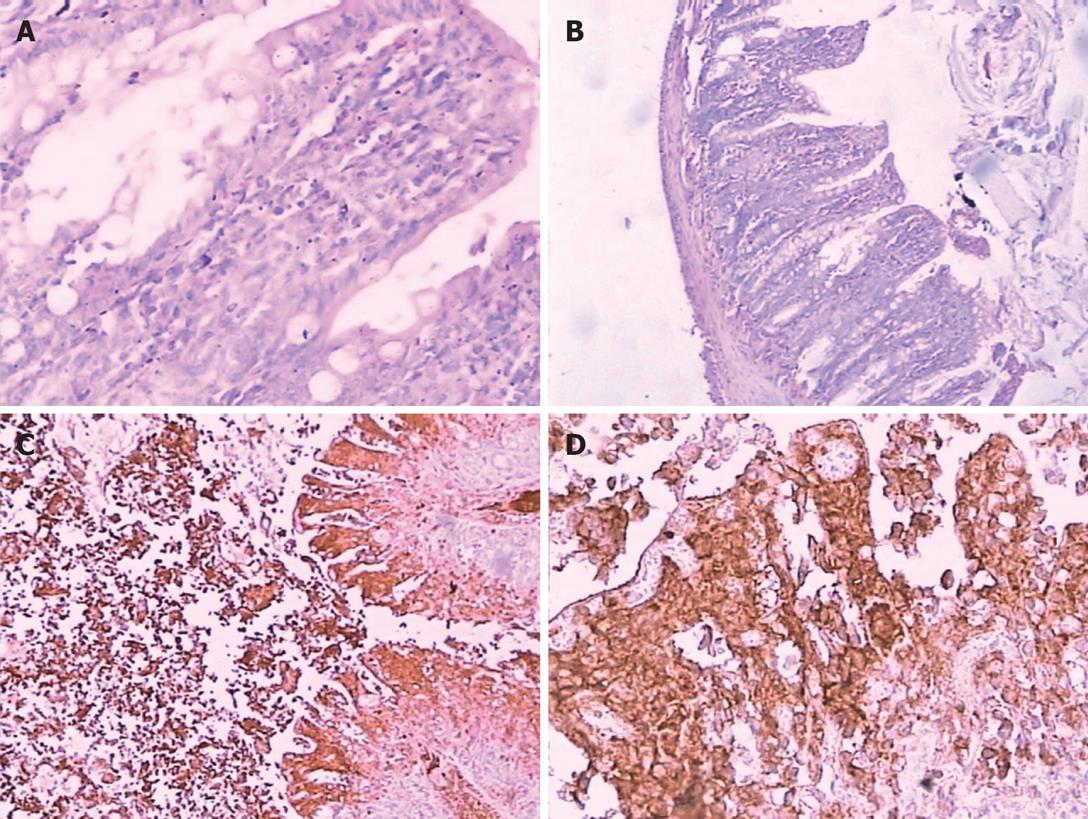
We detected the expression of Fas on intestinal tissue with immunocytochemical technique. Immunohistochemistry staining showed Fas expression in intestinal tissue was absent in normal intestinal tissue, Fas expression in intestinal tissue gradually increased 0.5 h after induction of pancreatitis, and then reached a peak at 12 h (Figure 1).
The Fas system is a widely recognized apoptotic signal transduction pathway in which a ligand-receptor interaction triggers the cell death pathway[12]. The Fas system has been implicated in the control of the immune response and inflammation, the response to infection and death of parenchymal cells in several organs[13–15], which is involved in maintaining homeostasis in various systems, including maintenance of peripheral T cell[16]. Recent research has demonstrated that a Th1 to Th2 immune shift is beneficial to mucosal immunity[1718]. A key component of the mucosal immune defense against pathogens is mediated by CD4+ T lymphocytes that can differentiate into functionally distinct subsets[19]. Whereas T-helper 1 (Th1) cells secrete the cytokines IL-2, IFN-γ, TNF-α, and TNF-β, Th2 cells secrete IL-4, 5, 6, 10, and 13. In the current study, we used IL-2 levels as a marker of Th1 response and IL-10 as a marker of Th2 response. In this study, the role of the Fas-mediated cell death pathway in intestinal mucosal injury models was assessed and dynamic changes of serum IL-2, IL-10, IL-2/IL-10 and sFas in rats with ANP were also investigated.
According to the result of immunohistochemistry, Fas had a lower expression in intestinal tissue in the P group at 0.5 h and higher expression after 2, 6 and 12 h. In the C groups, Fas was not detected in any part of intestinal tissue. Previous study has shown that IL-10 is a kind of important anti-inflammatory cytokine and plays a role of self-defense mechanism, limiting the intensity of inflammatory process[20–23]. However, the effect of IL-10 level in the course of acute pancreatitis is still not clear[24–26]. IL-10 is a powerful Th2 cell cytokine produced by lymphoid cells. A marked activation of immune system may be observed in patients with AP, being balanced between pro- and anti-inflammatory cytokines in patients with mild but not severe AP. A reduced functional reserve for the synthesis of IL-10 may be observed in patients with severe AP, which might lead to a worst prognosis[27–30].
From the results of this study, it was found that the release of IL-10 was significantly increased in the P group as compared with that of C group at 6 and 12 h. Serum IL-2 level in the P group were higher than those in C group. From 0.5 h, there was a significant difference between P group and C group. The IL-2/IL-10 ratio was significantly increased in the P group as compared with that of C group at 0.5 and 2 h, suggesting that a pro-inflammatory response was predominant in these rats and significantly lower in P group at 6 h suggesting that an anti-inflammatory response was predominant. In the sFas assay, there was no significant difference between P group and C group. Interestingly, IL-2, IL-10 and sFas levels were moderate at 0.5 h, peaked at 6 h and decreased at 12 h. In our model, the intestinal tissue injury which was assessed according to the standard scale of pathological examination was closely paralleled by Fas expression and dynamic changes of IL-2/IL-10.
In conclusion, the abnormal apoptosis of Fas can significantly affect the cytokine. Fas were involved in the pathogenesis of intestinal injury in ANP. The mechanisms of Fas may have been related to Fas mediated T helper cell apoptosis.
Acute pancreatitis (AP) is sudden inflammation of the pancreas that may be mild or life threatening but that usually subsides. It is generally accepted that AP is often complicated by intestinal injury. The Fas system was originally characterized as a key mechanism for inducing apoptosis in immune cells. Apoptosis is a teleologically beneficial form of cell death in AP. However, little is known about how the induction of apoptosis reduces the severity of AP. Recent research has demonstrated that a Th1 to Th2 immune deviation is beneficial to mucosal immunity.
Recent research has demonstrated that a Th1 to Th2 immune deviation is beneficial to mucosal immunity. The antigen-induced deletion of Th is often accompanied by an imbalance in Th1 and Th2.
In the present study, we performed immunohistochemistry staining of apoptosis-related protein Fas and investigated dynamic changes of serum IL-2, IL-10, IL-2/ IL-10 and sFas in rats with ANP.
To provide the experimental basis by expression of Fas in intestinal mucosa in rats with ANP and provide experimental evidence for the immune treatment of patients with ANP.
Th1 and Th2 response: Th1-type cytokines tend to produce the proinflammatory responses responsible for killing intracellular parasites and for perpetuating autoimmune responses. Interferon gamma is the main Th1 cytokine. Excessive proinflammatory responses can lead to uncontrolled tissue damage, so there needs to be a mechanism to counteract this. The Th2-type cytokines include interleukins 4, 5, 13 and interleukin-10, which has more of an anti-inflammatory response.
This is a potentially interesting study to understand the dynamic changes of several cytokines and sFas in rats with ANP and provide experimental evidence for the immune treatment of ANP patients.
| 1. | Bodnar Z. Changes in the management of acute pancreatitis as related to its pathogenesis. Orv Hetil. 2005;146:499-505. |
| 2. | Hartwig W, Werner J, Uhl W, Buchler MW. Management of infection in acute pancreatitis. J Hepatobiliary Pancreat Surg. 2002;9:423-428. |
| 3. | Granger J, Remick D. Acute pancreatitis: models, markers, and mediators. Shock. 2005;24 Suppl 1:45-51. |
| 4. | Foitzik T, Eibl G, Hotz B, Hotz H, Kahrau S, Kasten C, Schneider P, Buhr HJ. Persistent multiple organ microcirculatory disorders in severe acute pancreatitis: experimental findings and clinical implications. Dig Dis Sci. 2002;47:130-138. |
| 5. | Senthil M, Brown M, Xu DZ, Lu Q, Feketeova E, Deitch EA. Gut-lymph hypothesis of systemic inflammatory response syndrome/multiple-organ dysfunction syndrome: validating studies in a porcine model. J Trauma. 2006;60:958-965; discussion 965-967. |
| 6. | Zhang XP, Ye Q, Jiang XG, Ma ML, Zhu FB, Zhang RP, Cheng QH. Preparation method of an ideal model of multiple organ injury of rat with severe acute pancreatitis. World J Gastroenterol. 2007;13:4566-4573. |
| 7. | Ammori BJ, Leeder PC, King RF, Barclay GR, Martin IG, Larvin M, McMahon MJ. Early increase in intestinal permeability in patients with severe acute pancreatitis: correlation with endotoxemia, organ failure, and mortality. J Gastrointest Surg. 1999;3:252-262. |
| 8. | Cao Y, Adhikari S, Clement MV, Wallig M, Bhatia M. Induction of apoptosis by crambene protects mice against acute pancreatitis via anti-inflammatory pathways. Am J Pathol. 2007;170:1521-1534. |
| 9. | Coffman RL, Seymour BW, Lebman DA, Hiraki DD, Christiansen JA, Shrader B, Cherwinski HM, Savelkoul HF, Finkelman FD, Bond MW. The role of helper T cell products in mouse B cell differentiation and isotype regulation. Immunol Rev. 1988;102:5-28. |
| 10. | Zhang JX, Dang SC, Qu JG, Wang XQ, Chen GZ. Changes of gastric and intestinal blood flow, serum phospholipase A2 and interleukin-1beta in rats with acute necrotizing pancreatitis. World J Gastroenterol. 2005;11:3578-3581. |
| 11. | Chiu CJ, McArdle AH, Brown R, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg. 1970;101:478-483. |
| 12. | Meggiolaro D, Porcelli F, Carnevali A, Crepaldi P, Savarese E, Ferrandi B. A possible role of Fas antigen in ejaculated spermatozoa of fertile bulls: an immunocytochemical quantitative approach. Acta Histochem. 2006;107:463-468. |
| 14. | Seino K, Iwabuchi K, Kayagaki N, Miyata R, Nagaoka I, Matsuzawa A, Fukao K, Yagita H, Okumura K. Chemotactic activity of soluble Fas ligand against phagocytes. J Immunol. 1998;161:4484-4488. |
| 15. | Biancone L, Martino AD, Orlandi V, Conaldi PG, Toniolo A, Camussi G. Development of inflammatory angiogenesis by local stimulation of Fas in vivo. J Exp Med. 1997;186:147-152. |
| 16. | Mogil RJ, Radvanyi L, Gonzalez-Quintial R, Miller R, Mills G, Theofilopoulos AN, Green DR. Fas (CD95) participates in peripheral T cell deletion and associated apoptosis in vivo. Int Immunol. 1995;7:1451-1458. |
| 17. | Lin MT, Hsu CS, Yeh SL, Yeh CL, Chang KJ, Lee PH, Chen WJ. Effects of omega-3 fatty acids on leukocyte Th1/Th2 cytokine and integrin expression in rats with gut-derived sepsis. Nutrition. 2007;23:179-186. |
| 18. | Gremy O, Benderitter M, Linard C. Caffeic acid phenethyl ester modifies the Th1/Th2 balance in ileal mucosa after gamma-irradiation in the rat by modulating the cytokine pattern. World J Gastroenterol. 2006;12:4996-5004. |
| 19. | Neurath MF, Finotto S, Glimcher LH. The role of Th1/Th2 polarization in mucosal immunity. Nat Med. 2002;8:567-573. |
| 20. | Ramudo L, Manso MA, Vicente S, De Dios I. Pro- and anti-inflammatory response of acinar cells during acute pancreatitis. Effect of N-acetyl cysteine. Cytokine. 2005;32:125-131. |
| 21. | Ohmoto K, Yamamoto S. Serum interleukin-6 and interleukin-10 in patients with acute pancreatitis: clinical implications. Hepatogastroenterology. 2005;52:990-994. |
| 22. | Keceli M, Kucuk C, Sozuer E, Kerek M, Ince O, Arar M. The effect of interleukin-10 on acute pancreatitis induced by cerulein in a rat experimental model. J Invest Surg. 2005;18:7-12. |
| 23. | Chen X, Wu H, Huang X, Wu X. The alteration of inflammatory cytokine during acute pancreatitis. Huaxi Yike Daxue Xuebao. 2002;33:238-240, 243. |
| 24. | Gallagher SF, Peng Y, Haines K, Baksh K, Epling-Burnette PK, Yang J, Murr MM. Fas/FasL play a central role in pancreatitis-induced hepatocyte apoptosis. J Gastrointest Surg. 2005;9:467-474; discussion 474-475. |
| 25. | Deviere J, Le Moine O, Van Laethem JL, Eisendrath P, Ghilain A, Severs N, Cohard M. Interleukin 10 reduces the incidence of pancreatitis after therapeutic endoscopic retrograde cholangiopancreatography. Gastroenterology. 2001;120:498-505. |
| 26. | Dembiński A, Warzecha Z, Ceranowicz P, Dembiński M, Cieszkowski J, Pawlik WW, Tomaszewska R, Konturek SJ, Konturek PC. Effect of ischemic preconditioning on pancreatic regeneration and pancreatic expression of vascular endothelial growth factor and platelet-derived growth factor-A in ischemia/reperfusion-induced pancreatitis. J Physiol Pharmacol. 2006;57:39-58. |
| 27. | Van Laethem JL, Eskinazi R, Louis H, Rickaert F, Robberecht P, Deviere J. Multisystemic production of interleukin 10 limits the severity of acute pancreatitis in mice. Gut. 1998;43:408-413. |
| 28. | Rongione AJ, Kusske AM, Kwan K, Ashley SW, Reber HA, McFadden DW. Interleukin 10 reduces the severity of acute pancreatitis in rats. Gastroenterology. 1997;112:960-967. |
| 29. | Chen ZQ, Tang YQ, Zhang Y, Jiang ZH, Mao EQ, Zou WG, Lei RQ, Han TQ, Zhang SD. Adenoviral transfer of human interleukin-10 gene in lethal pancreatitis. World J Gastroenterol. 2004;10:3021-3025. |
| 30. | Fantini L, Tomassetti P, Pezzilli R. Management of acute pancreatitis: current knowledge and future perspectives. World J Emerg Surg. 2006;1:16. |