Published online Apr 14, 2008. doi: 10.3748/wjg.14.2226
Revised: December 21, 2007
Published online: April 14, 2008
AIM: To investigate the mechanism of action of thermal cutaneous stimulation on the gastric motor inhibition.
METHODS: The gastric tone of 33 healthy volunteers (20 men, mean age 36.7 ± 8.4 years) was assessed by a barostat system consisting of a balloon-ended tube connected to a strain gauge and air-injection system. The tube was introduced into the stomach and the balloon was inflated with 300 mL of air. The skin temperature was elevated in increments of 3°C up to 49°C and the gastric tone was simultaneously assessed by recording the balloon volume variations expressed as the percentage change from the baseline volume. The test was repeated after separate anesthetization of the skin and stomach with lidocaine and after using normal saline instead of lidocaine.
RESULTS: Thermal cutaneous stimulation resulted in a significant decrease of gastric tone 61.2% ± 10.3% of the mean baseline volume. Mean latency was 25.6 ± 1.2 ms. After 20 min of individual anesthetization of the skin and stomach, thermal cutaneous stimulation produced no significant change in gastric tone.
CONCLUSION: Decrease in the gastric tone in response to thermal cutaneous stimulation suggests a reflex relationship which was absent on individual anesthetization of the 2 possible arms of the reflex arc: the skin and the stomach. We call this relationship the “cutaneo-gastric inhibitory reflex”. This reflex may have the potential to serve as an investigative tool in the diagnosis of gastric motor disorders, provided further studies are performed in this respect.
- Citation: Shafik A, Shafik AA, Sibai OE, Shafik IA. Effect of thermal cutaneous stimulation on the gastric motor activity: Study of the mechanism of action. World J Gastroenterol 2008; 14(14): 2226-2229
- URL: https://www.wjgnet.com/1007-9327/full/v14/i14/2226.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.2226
External stimuli have been shown to affect gastric motility. Centrally acting stressful stimuli produce gastrointestinal motility changes in rats[1–6], dogs[78], and humans[9–11]. These actions seem to be mediated through humoral pathways[12–14]. Thus α- and β-adrenergic blockers are claimed to abolish the inhibition of gastric motility induced by cold pain in humans[15]. Other investigators suggest that other humoral factors, such as acoustic stress, may be involved in the mediation of the gastrointestinal motor disturbances[16–19]. Gastric ulcer and acute pancreatitis may also be related to stress[1120].
It has been shown that various types of stressors cause a release of the corticotrophin-releasing factor and that intra-cerebroventricular administration of this factor mimics the motor, metabolic, and hemodynamic responses to such stimuli in animals[21–24]. However, in anesthetized rodents, an increased gastric motility was observed during restraint stress[2526], whereas pinching of the skin was accompanied by gastric motor inhibition[20].
Although skin pinching or stressful cutaneous stimuli have been demonstrated to be associated with gastric motor inhibition[2728], the mechanisms involved in this action have not been elucidated in the literature. Therefore, hypothesizing that skin stimulation induces its effect on the gastric motor activity through a reflex action, we conducted the current study.
Thirty-three subjects [20 men and 13 women; mean age 36.7 ± 8.4 (range 26-45) years] were enrolled in this study after they had given an informed consent. The results of physical examination including neurological assessment were normal. Laboratory work up including blood count, renal and hepatic function tests, as well as electrocardiography were normal. The study was approved by the Faculty of Medicine Review Board and Ethics Committee of Cairo University.
Thermal cutaneous stimulation (TCS) was performed by means of a thermal pad applied to the skin, and the gastric motor activity was recorded with a barostat. A 6F polyvinyl gastric tube, with multiple side holes 4 to 6 cm from its distal end, was used. A thin compliant polyethylene balloon (London Rubber Industries Ltd, London, UK) was fastened to the distal part of the tube that contained the side holes. The tube had a metallic clip applied to its distal end for fluoroscopic control. It was connected to a strain gauge and a computer-controlled air-injection system (G&J Electronics Inc, Toronto, Ontario). This barostat system keeps the pressure within the balloon constant. Thus, when the gastric tone increases, the air in the bag is withdrawn, and when the tone diminishes, the air rushes into the balloon; hence the pressure in the balloon is kept constant at all times. Using this technique, the gastric tone could be assessed by recording the balloon volume variations, expressed as the percentage change from the baseline volume.
The tube was introduced into the stomach through the nose. The tests were performed 20 min later so that the stomach would have adapted to the inserted catheter. The balloon was then inflated with 300 mL of air. Thermal stimulation of the skin was induced by a thermal pad applied to the skin of the upper arm and connected to a thermostat.
The skin temperature was recorded at rest. The pad temperature was then elevated in increments of 3°C above the resting skin temperature up to 49°C or the highest tolerable temperature. Throughout the period of successive skin temperature elevation, the gastric wall tone was simultaneously assessed by measuring the variations in the balloon volume, expressed as the percentage change from the baseline volume. We calculated the latency which is the period between the start of thermal skin stimulation and the beginning of the gastric tone response.
To define whether the effect of thermal cutaneous stimulation (TCS) on the stomach was a direct or a reflex action, the following test was performed.
The aforementioned test was repeated after individual anesthetization of the skin and stomach. The skin area, over which the thermal pad was applied, was anesthetized by injection of 3 mL of 2% lidocaine mixed with 3 mL of normal saline; the injection was performed at multiple points in the skin under the pad. The gastric tone response to TCS, as aforementioned, was recorded after 20 min and 3 h later when the anesthetic effect had waned. The stomach was then anesthetized by endoscopic injection of 30 mL of 2% lidocaine in 70 mL of normal saline. The injection was performed at multiple points in the stomach wall. The gastric tone response to TCS was then registered after 20 min and 3 h later. The aforementioned tests were repeated using normal saline instead of lidocaine.
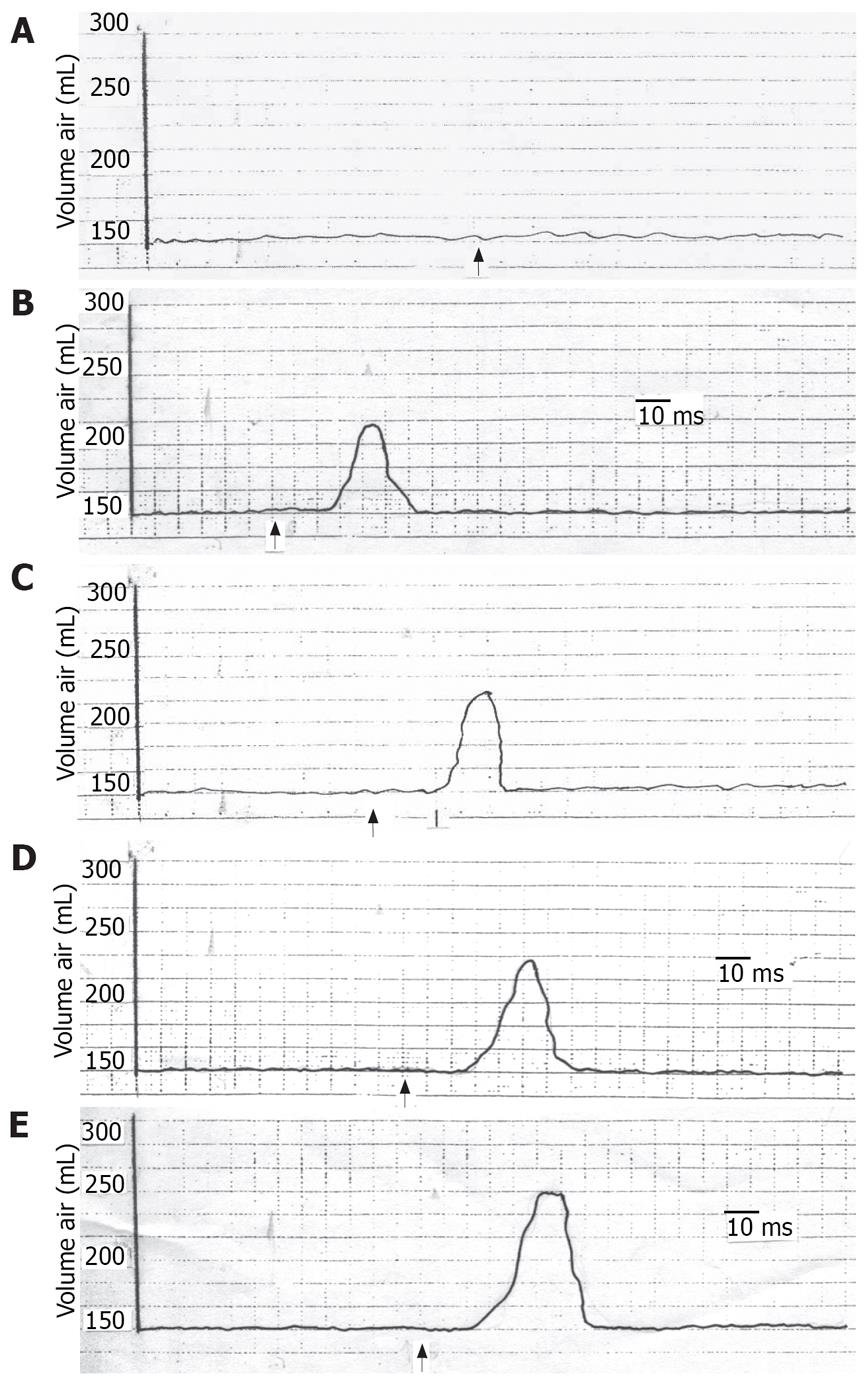
The study was completed without any adverse side effects during or after the tests. During TCS, all of the subjects showed a significant decrease in the gastric tone which varied from 40% to 83% (mean 61.2% ± 10.3%) of the baseline volume according to the degree of TCS (Figure 1, Table 1). There was a progressive decrease in the gastric tone with increasing TCS (Figure 1, Table 1). Gastric tone decline was greater in men than in women but the difference was not significant (P > 0.05). Also, there was no significant difference in the gastric tone decrease between the younger and older subjects. The latency varied from 20.6-28.8 ms (mean 25.6 ± 1.29). It decreased with increasing TCS. There was no significant difference in the latency when we compared men to women or younger to older subjects.
| Skin temperature (°C) | Basal tone (% of baseline volume) | |
| Mean | Range | |
| 37 (basal) | 0 | 0 |
| 40 | 48.2 ± 6.4 | 40-56 |
| 43 | 57.3 ± 5.1 | 52-63 |
| 46 | 69.7 ± 3.3 | 66-74 |
| 49 | 78.6 ± 4.1 | 76-83 |
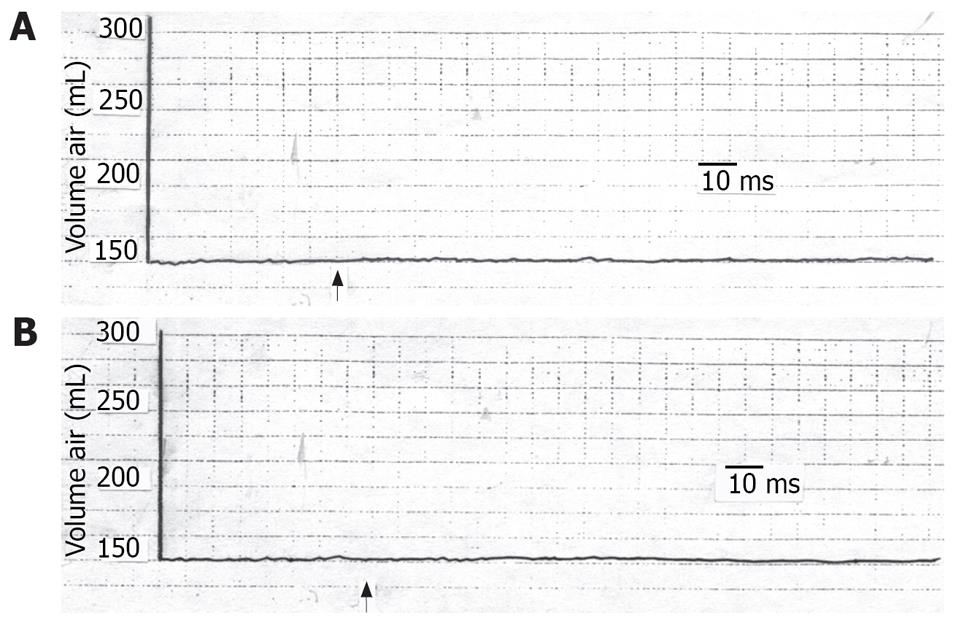
TCS performed 20 min after individual anesthetization of the skin or stomach produced no significant changes in the gastric tone (Figure 2). Three hours later, when the anesthetic effect had waned, the TCS caused a decrease in gastric tone similar to that before anesthetization (P > 0.05). When the above tests were repeated using saline instead of lidocaine, the gastric tone response was similar to that before saline application (P > 0.05).
Upon repetition of the aforementioned tests in the same subject, similar results were obtained with no significant differences.
It is established that centrally acting stressful stimuli induce changes in the gastrointestinal motility[1–11]. These changes are suggested to occur through humoral pathways as supported by the release of β-endorphin and catecholamines into the peripheral circulation during stress[12–15]. In agreement with such hypothesis, naloxone or a combination of α- and β-adrenergic blockers abolish gastric motility inhibition induced by cold pain. Yet, this hypothesis can be ruled out because the aforementioned drugs have no effect on the migrating duodenal activity induced by labyrinthine stimulation in humans[24–28] or on acoustic stress-induced inhibition of gastric motility in dogs[27]. This suggests that other factors may be involved in the mediation of gastrointestinal motor disturbances induced by centrally acting stimuli.
The current study demonstrated that TCS affected inhibition of gastric motor activity which progressively increased on incremental enhancement of TCS. This effect was abolished on anesthetization of either the stimulated cutaneous area or the stomach. The inhibited gastric motor activity presumably denotes gastric wall relaxation and gastric dilatation. It seems that the stomach dilates on stress to avoid gastric stimulation that might result in vomiting.
The current findings led to the assumption that the inhibited gastric motor activity in response to cutaneous stressful condition is mediated through a reflex pathway. This hypothesis is evidenced by the findings that, with individual anesthetization of the suggested 2 arms of the reflex arc, i.e. the skin and the stomach, the gastric response was absent. Saline on the other hand did not give rise to such effect. The response returned after the anesthetic condition had worn off. Furthermore, the reproducibility of the effect points to the constancy of the results. We call the suggested reflex response of the stomach to cutaneous stimulation, the “cutaneo-gastric inhibitory reflex (CGIR)”. It may be argued that this effect could be humoral as already mentioned by investigators[10–18]. However, if the effects of cutaneous stimulation on the stomach were humoral, it would not vanish with either gastric or cutaneous anesthetization as has been shown in the current findings. Meanwhile, the effect of centrally acting stressful stimuli on the stomach[1–6] cannot be ignored, albeit that this role alone does not seem to explain the non-response of the stomach to stimulation of the anesthetized skin.
It seems that TCS activates the cutaneous nerve endings which send impulses along the afferent fibers to the spinal cord. Impulses from the spinal cord are in turn transmitted along efferent fibers to the stomach, inhibiting its motor activity.
The point that needs to be discussed is: what could be the possible clinical significance of the CGIR? It is suggested that the CGIR might be of diagnostic significance in gastric motility disorders. Diminished gastric tone response to TCS would indicate a defect in the reflex pathway, such as gastric musculature or nerve damage resulting from a disease of the peripheral nerves, spinal nerve roots or spinal cord or from a central lesion. Significant prolongation of the latency of the CGIR on the other hand may indicate a disorder of the reflex arc. We believe that the CGIR may be incorporated as an investigative tool in the study of patients with gastric disorders after it has been further studied in various pathologic gastric lesions. The reflex assesses the integrity of the gastric motor activity.
In conclusion, TCS results in decrease of the gastric motor activity which apparently leads to gastric wall relaxation. The decrease in gastric tone upon TCS postulates a reflex relationship which was absent on individual anesthetization of the assumed two arms of the reflex are: the skin and the stomach. We call this relationship the CGIR. This reflex may prove to be of diagnostic significance in gastric motor disorders and have the potential to serve as an investigative tool, provided further studies are performed to validate the current results.
External stimuli have been shown to affect gastric motility. Centrally acting stressful stimuli produce gastrointestinal motility changes in rats, dogs, and humans. These actions seem to be mediated through humoral pathways. Thus α- and β- adrenergic blockers are claimed to abolish the inhibition of gastric motility induced by cold pain in humans. Other investigators suggest that other humoral factors such as acoustic stress may be involved in the mediation of the gastrointestinal motor disturbances. We hypothesized that skin stimulation induces its effect on the gastric motor activity through a reflex action. This hypothesis was investigated in the current study.
It is established that centrally acting stressful stimuli induce changes in the gastrointestinal motility. Other factors may be involved in the mediation of gastrointestinal motor disturbances induced by centrally acting stimuli.
The point that needs to be discussed is: what could be the possible clinical significance of the cutaneo-gastric inhibitory reflex (CGIR)? It is suggested that the CGIR might be of diagnostic significance in gastric motile disorders. Diminished gastric tone response to thermal cutaneous stimulation (TCS) would indicate a defect in the reflex pathway, such as gastric musculature or nerve damage resulting from a disease of the peripheral nerves, spinal nerve roots or spinal cord or from a central lesion. We believe that the CGIR may be incorporated as an investigative tool in the study of patients with gastric disorders after it has been further studied in various pathologic gastric lesions. The reflex assesses the integrity of the gastric motor activity.
The authors had asked a simple question, namely whether gastric tone responds to cutaneous stimulation with heat. The answer is straight forward: Heat application leads to gastric relaxation and this effect can be abolished by intracutaneous and intragastric injections of lidocaine.
| 1. | Brodie DA. Ulceration of the stomach produced by restraint in rats. Gastroenterology. 1962;43:107-109. |
| 2. | Fioramonti J, Bueno L. Gastrointestinal myoelectric activity disturbances in gastric ulcer disease in rats and dogs. Dig Dis Sci. 1980;25:575-580. |
| 3. | Koo MW, Ogle CW, Cho CH. The effect of cold-restraint stress on gastric emptying in rats. Pharmacol Biochem Behav. 1985;23:969-972. |
| 4. | Graves GM, Becht JL, Rawlings CA. Metoclopramide reversal of decreased gastrointestinal myoelectric and contractile activity in a model of canine postoperative ileus. Vet Surg. 1989;18:27-33. |
| 5. | Ruckebusch Y, Pairet M, Becht JL. Origin and characterization of migrating myoelectric complex in rabbits. Dig Dis Sci. 1985;30:742-748. |
| 6. | Koo MW, Cho CH, Ogle CW. Effects of cold-restraint stress on gastric ulceration and motility in rats. Pharmacol Biochem Behav. 1986;25:775-779. |
| 7. | Gue M, Fioramonti J, Frexinos J, Alvinerie M, Bueno L. Influence of acoustic stress by noise on gastrointestinal motility in dogs. Dig Dis Sci. 1987;32:1411-1417. |
| 8. | Kowalewski K, Kolodej A. Myoelectrical and mechanical activity of stomach and intestine in hypothyroid dogs. Am J Dig Dis. 1977;22:235-240. |
| 9. | Thompson DG, Richelson E, Malagelada JR. Perturbation of upper gastrointestinal function by cold stress. Gut. 1983;24:277-283. |
| 10. | Yin J, Levanon D, Chen JD. Inhibitory effects of stress on postprandial gastric myoelectrical activity and vagal tone in healthy subjects. Neurogastroenterol Motil. 2004;16:737-744. |
| 11. | Cosen-Binker LI, Binker MG, Negri G, Tiscornia O. Influence of stress in acute pancreatitis and correlation with stress-induced gastric ulcer. Pancreatology. 2004;4:470-484. |
| 12. | Bortz WM 2nd, Angwin P, Mefford IN, Boarder MR, Noyce N, Barchas JD. Catecholamines, dopamine, and endorphin levels during extreme exercise. N Engl J Med. 1981;305:466-467. |
| 13. | Cohen M, Pickard D, Dubois M, Roth YF, Naber D, Bunney WE Jr. Surgical stress and endorphins. Lancet. 1981;1:213-214. |
| 14. | Kalin NH. Behavioral effects of ovine corticotropin-releasing factor administered to rhesus monkeys. Fed Proc. 1985;44:249-253. |
| 15. | Kopin IJ, Lake RC, Ziegler M. Plasma levels of nore-pinephrine. Ann Intern Med. 1978;88:671-680. |
| 16. | Gue M, Bueno L. Diazepam and muscimol blockade of the gastrointestinal motor disturbances induced by acoustic stress in dogs. Eur J Pharmacol. 1986;131:123-127. |
| 17. | Thompson DG, Richelson E, Malagelada JR. Perturbation of gastric emptying and duodenal motility through the central nervous system. Gastroenterology. 1982;83:1200-1206. |
| 18. | Graeff FG, Viana MB, Mora PO. Dual role of 5-HT in defense and anxiety. Neurosci Biobehav Rev. 1997;21:791-799. |
| 19. | Graeff FG, Guimaraes FS, De Andrade TG, Deakin JF. Role of 5-HT in stress, anxiety, and depression. Pharmacol Biochem Behav. 1996;54:129-141. |
| 20. | Pilchman J, Lefton HB, Braden GL. Cytoprotection and stress ulceration. Med Clin North Am. 1991;75:853-863. |
| 21. | Brown MR, Fisher LA, Rivier J, Spiess J, Rivier C, Vale W. Corticotropin-releasing factor: effects on the sympathetic nervous system and oxygen consumption. Life Sci. 1982;30:207-210. |
| 22. | Rivier C, Rivier J, Vale W. Inhibition of adrenocorticotropic hormone secretion in the rat by immunoneutralization of corticotropin-releasing factor. Science. 1982;218:377-379. |
| 23. | Sutton RE, Koob GF, Le Moal M, Rivier J, Vale W. Cortico-tropin releasing factor produces behavioural activation in rats. Nature. 1982;297:331-333. |
| 24. | van den Elzen BD, van den Wijngaard RM, Tytgat GN, Boeckxstaens GE. Influence of corticotropin-releasing hormone on gastric sensitivity and motor function in healthy volunteers. Eur J Gastroenterol Hepatol. 2007;19:401-407. |
| 25. | Zacchi P, Mearin F, Malagelada JR. Effect of experimental cold pain stress on gastroesophageal junction. Dig Dis Sci. 1994;39:641-647. |
| 26. | Nakae Y, Kagaya M, Takagi R, Matsutani Y, Horibe H, Kondo T. Cold pain prolongs gastric emptying of liquid but not solid meal: an electrical impedance tomography (EIT) study. J Gastroenterol. 2000;35:593-597. |
| 27. | Sato Y, Terui N. Changes in duodenal motility produced by noxious mechanical stimulation of the skin in rats. Neurosci Lett. 1976;2:189-193. |
| 28. | Fone DR, Horowitz M, Maddox A, Akkermans LM, Read NW, Dent J. Gastroduodenal motility during the delayed gastric emptying induced by cold stress. Gastroenterology. 1990;98:1155-1161. |