Published online Apr 14, 2008. doi: 10.3748/wjg.14.2162
Revised: February 14, 2008
Published online: April 14, 2008
AIM: To study the effect of type 1 Na+/H+ exchanger (NHE1) antisense human gene transfection on the biological behavior of gastric carcinoma cell line SGC-7901.
METHODS: Antisense NHE1 eukaryotic expression on vector pcDNA3.1 was constructed by recombinant DNA technique and transfected into gastric carcinoma cell line SGC-7901 with DOTAP liposome transfection method. Morphological changes of cells were observed with optic and electron microscopes. Changes in cell proliferative capacity, apoptosis, intracellular pH (pHi), cell cycle, clone formation in two-layer soft agar, and tumorigenicity in nude mice were examined.
RESULTS: Antisense eukaryotic expressing vectors were successfully constructed and transfected into SGC-7901. The transfectant obtained named 7901-antisense (7901-AS) stablely produced antisense NHE1. There was a significant difference between the pHi of 7901-AS cells (6.77 ± 0.05) and that of 7901-zeo cells and SGC-7901 cells (7.24 ± 0.03 and 7.26 ± 0.03, P < 0.01). Compared with SGC-7901 and 7901-zeo cells, 7901-AS cells mostly showed cell proliferation inhibition, G1/G0 phase arrest, increased cell apoptotic rate, recovery of contact inhibition, and density contact. The tumorigenicity in nude mice and cloning efficiency in the two-layer soft agar were clearly inhibited.
CONCLUSION: NHE1 antisense gene significantly restrains the malignant behavior of human gastric carcinoma cells, suppresses cell growth and induces cell apoptosis, and partially reverses the malignant phenotypes of SGC-7901. These results suggest a potential role for human tumor gene therapy.
-
Citation: Liu HF, Teng XC, Zheng JC, Chen G, Wang XW. Effect of
NHE1 antisense gene transfection on the biological behavior ofSGC-7901 human gastric carcinoma cells. World J Gastroenterol 2008; 14(14): 2162-2167 - URL: https://www.wjgnet.com/1007-9327/full/v14/i14/2162.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.2162
The type 1 Na+/H+ exchanger (NHE1) is a transmembrane protein found in all eukaryotic cells. One of its functions is to remove excess H+ in the cytoplasm by Na+-H+ exchange system, resulting in a stable intracellular pH level[12]. The process of glycolysis induces the tumor cells to produce large quantities of lactic acid and H+. There is vigorous Na+-H+ exchange in the tumor cells, dependent upon the enhanced expression of NHE1 membrane protein. Most of the H+ pumped out of the cells prevents the intracellular acidification of tumor cells, resulting in the protection of tumor cells from apoptosis[34]. Previous studies have shown that NHE1 protein expression in gastric carcinoma tissues was significantly higher compared to that in normal gastric mucosa and precancerous lesions[5]. Therefore, inhibition of up-regulation of NHE-1 gene expression in human gastric carcinoma cells may induce intracellular acidification, resulting in apoptosis of tumor cells, which is helpful in the treatment of such tumors. In the present study, we constructed the antisense NHE-1 eukaryotic expression vector and transfected it into gastric carcinoma cell line SGC-7901 in order to investigate the effects of antisense NHE1 on malignant biological behavior of the gastric carcinoma cell line SGC-7901.
The human gastric carcinoma cell line SGC-7901 was used in this study. The cells were grown in RPMI1640 medium (Sigma, St Louis, MO, USA), supplemented with 10% FBS (fetal bovine serum) and antibiotics(100 &mgr;g/mL streptomycin and 100 U/mL penicillin ) in an atmosphere consisting of 5% CO2 in air at 37°C in an humidified incubator.
pEAP cloning vector of human NHE1 cDNA was kindly provided by Dr. Josset Noel (Montreal University, Canada). Eukaryotic expression vector pcDNA3.1 (-)/Zeo and Zeocin were obtained from Invitrogen Co. The experimental procedures of gene transfection were carried out according to the directions of DOTAPTM liposome transfection kit (Roche Diagnostics, Mannheim, Germany). The cells were plated at a density of 1.5-3 × 105 cells/35 mm dish and were grown for 24 h. The cells were transfected for 6 h with 2.5 &mgr;g plasmid DNA and 15 &mgr;L DOTAP in 2 mL of RPMI1640 medium without FBS and antibiotics. The cells were recovered for 48 h in the medium with 10% FBS. The stable transfectant was maintained in 100 &mgr;g/mL Zeocin (Invitrogen) in the medium for at least 20 d. The antisense NHE1 and the control plasmid transfectant were named 7901-AS and 7901-zeo, respectively.
In order to identify exogenous integration in the nucleic DNA of SGC-7901 cells, the Zeomycin-resistant antibiotic-selecting gene (ZeoR) was amplified by polymerase chain reaction (PCR) assays with a ZeoR-specific primer set (5’-GGCCAAGTTGACCAGTGC-3’ as forward and 5’-GTCAGTCCTGCTCCTCGG-3’ as backward). DNA was extracted from SGC-7901 and the transfected cells using standard techniques. The total volume of the PCR reaction system was 25 &mgr;L, containing 0.2 mmol/L dNTP, 1 mmol/L of each primer, 2U Taq polymerase, 1 × reaction buffer and 100 ng DNA template. After predegeneration at 94°C for 5 min in a PE cycle, 30 PCR cycles were performed at 94°C for 30 s, 53°C for 30 s, and 72°C for 30 s, then extended at 72°C for 5 min in a PCR thermocycler (Pekin-Elmer Cetus, Norwolk, CA, USA). Electrophoresis was performed on 1% agarose gel, and the findings were observed and pictures taken under the Burdick lamp.
High-concentration potassium-buffer containing the following chemicals (in mmol/L): 90-130 KCl, 5 NaCl, 1 MgSO4, 1 CaCl2, and 10 HEPES was infused into 6 tubes (5 mL in each), with pH values adjusted to 5.5, 6.0, 6.5, 7.0, 7.5, and 8.0, respectively. Nigericin (30 &mgr;mol/L) and BCECF (0.25 &mgr;mol/L) were added to the 6 tubes. At the logarithm growth phase, the cells were digested with pancreatin to prepare a single-cell suspension. The cells were washed twice with PBS, and an identical number of cells were added to the 6 tubes for inoculation at 37°C for 12 min. The cells were stimulated with argon ion laser at 488 nm. The emitted fluorescence was recorded at 530 nm and 640 nm and the ratio (FIR) was calculated. The ratio curve and the standard curve of pH were drawn.
Cells at the logarithm growth phase were digested with pancreatin, and centrifugated for 5 min at 1000 r/min. After removal of the supernatant, the cells were washed once using saline. BCECF/AM stock solution (2 mg/mL), prepared with anhydrous DMSO, was added to the serum-free and phenolsulfonphthalein-free medium until the BCECF concentration reached 0.25 &mgr;mol/L. Cells in the stock solution were incubated for 12 min for 1 h at room temperature. The cells were stimulated with argon ion laser at 488 nm and the emitted fluorescence at 530 and 640 was recorded, and the ratio (FIR) was calculated. Intracellular pH (pHi) was calculated according to the standard curve.
The shape, size and growth features (such as contact inhibition, density inhibition and anchorage-dependence) of 7901-AS cells were observed using invert microscopy and common microscopy after hematoxylin and eosin staining. Cell proliferation speed was assayed by the 3-(4,5-dimethyl-2 thiazoyl)-2,5-dipheny-2H-tetrazolium bromide method.
Exponentially growing cells were collected and fixed with 75% cold ethanol for at least 24 h and were analyzed for cell cycle distribution and apoptosis rate by DNA content analysis using propidium staining under flow cytometry (FACS420).
The cells were planted in 24-hole plastic plates spread with low melting point agar (0.35% in upper layer and 0.6% in low layer). Each type of cell was planted in five holes, with 1000 cells in each hole. The cells were cultivated at 37°C, with 5% CO2 and under saturation humidity for 2-3 wk. Cells that were larger than 75 &mgr;m in diameter or clones with more than 50 cells were counted under an invert microscope. Clone form-rate was calculated according to the clone form number/inoculation cell number × 100%.
Nine Balb/c athymic 4-5 wk old female nude mice, bred in specific pathogen free conditions (purchased from the Experimental Animal Center of the Third Military Medical University, Chongqing, China) were randomly divided into three groups. Three different cell lines (SGC-7901, 7901-zeo, 7901-AS), suspended in 0.1 mL serum-free RMPI 1640, were injected subcutaneously in a dose of 4-8 × 106 cells at two different sites of the mice. The tumor diameter was measured every 7 d. The animals were monitored regularly for tumor occurrence and size for at least 2 mo.
The findings are depicted as mean ± SD. Variance analysis and t-test for non-matched data were performed using a professional SPSS statistical program.
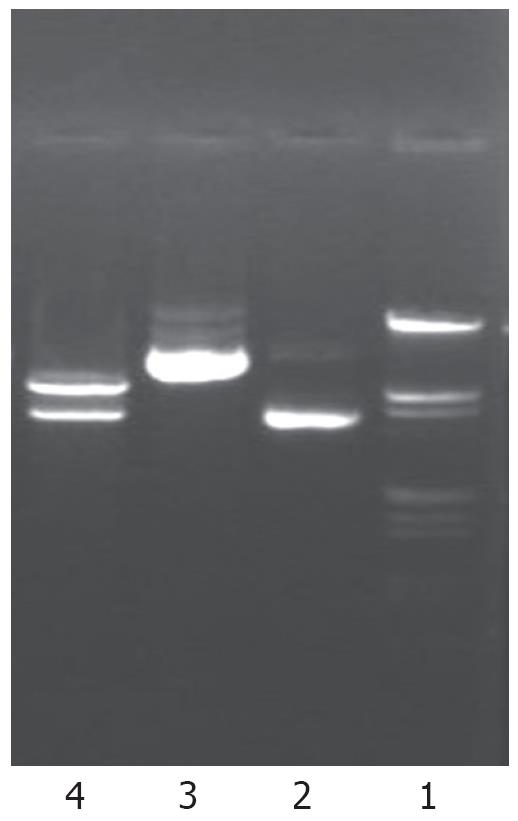
Human NHE1 cDNA (3.6 Kb) digested from plasmid pEAP with EcoRI and HindIII restriction endonuclease was inserted into the eukaryotic expression vector in antisense orientation. The recombinant DNA was further shown to be the same as designed by restriction analysis (Figure 1). A fragment of 3.6 Kb was released after digestion of the recombinant plasmid with EcoRI and HindIII, suggesting that the target fragment was successfully inserted into the expression vector.
The antisense NHE1 eukaryotic expression vector was named pcDNA3.1-NHE1.
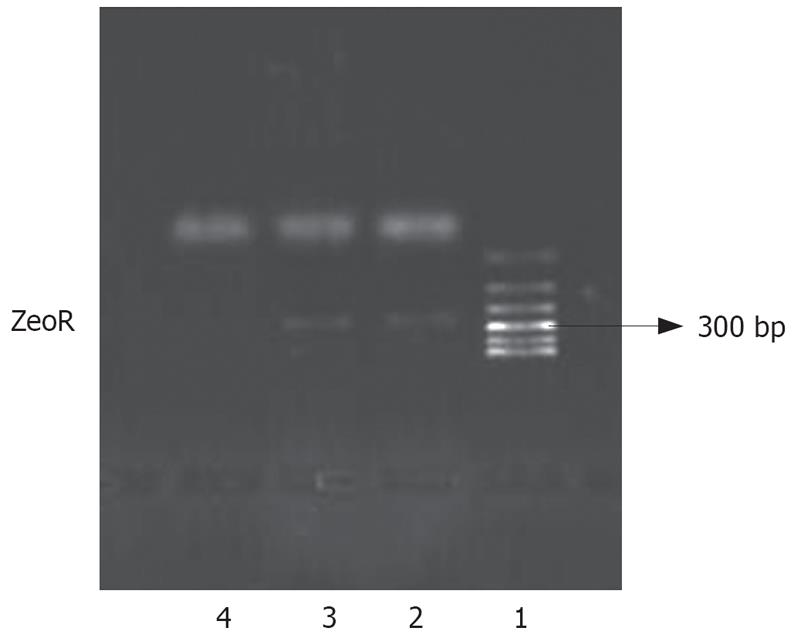
We introduced an antisense NHE1 cDNA sequence into the SGC-7901 cell line. Following Zeocin selection, drug-resistant individual clones were randomly collected from cultures infected with pcDNA3.1-NHE1 (7901-AS). For controls, drug-resistant clones were selected from the cultures infected with an empty vector pcDNA3.1 (-)/Zeo (7901-zeo). The expression of zeocin resistant gene (ZeoR) could be amplified in 7901-AS or 7901-zeo cells, but not in untransfected cells. This result suggested that exogenous genes had been integrated into the nucleic DNA of SGC-7901 cells (Figure 2).
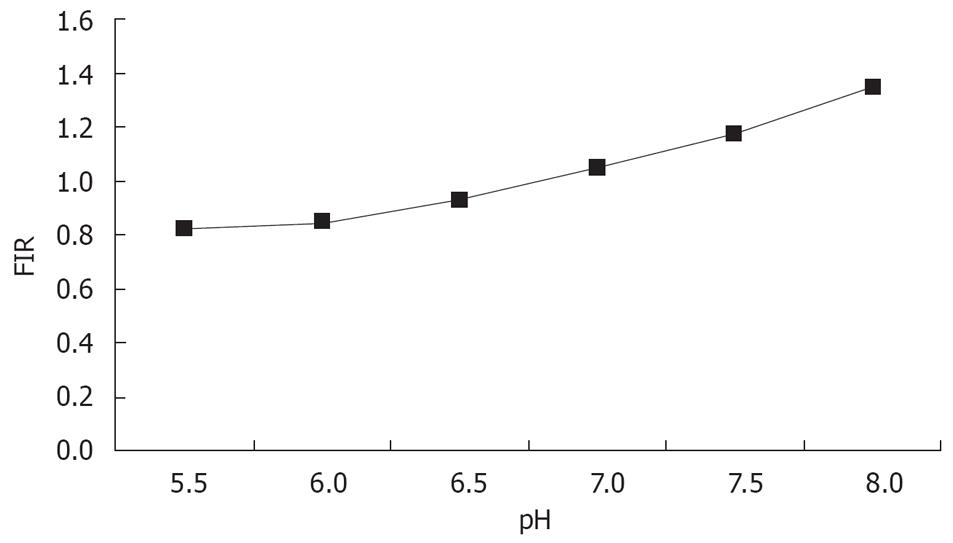
The standard curve drawn according to the buffer is shown in Figure 3. The regression equation was y = 0.2144χ - 0.4248 (r = 0.96).
The cells were stimulated with argon ion laser at 488 nm and the emitted fluorescence at 530 nm and 640 nm was recorded and the ratio of 530/640 was calculated. The intracellular pH (pHi) was calculated according to the standard curve. The pHi of 7901-AS cells was 6.77 ± 0.05, whereas the pHi of 7901-zeo and SGC-7901 cells were 7.24 ± 0.03 and 7.26 ± 0.03, respectively. There was a significant difference between the pHi of 7901-AS cells and that of 7901-zeo cells and SGC-7901 cells (P < 0.01). The reduced pHi of 7901-AS cells suggested that the transfected antisense NHE1 gene successfully inhibited the expression of NHE1 gene and blocked excessive exchange of Na+-H+, resulting in intracellular acidification. There was no significant difference in the pHi of SGC-7901 and 7901-zeo cells (Table 1).
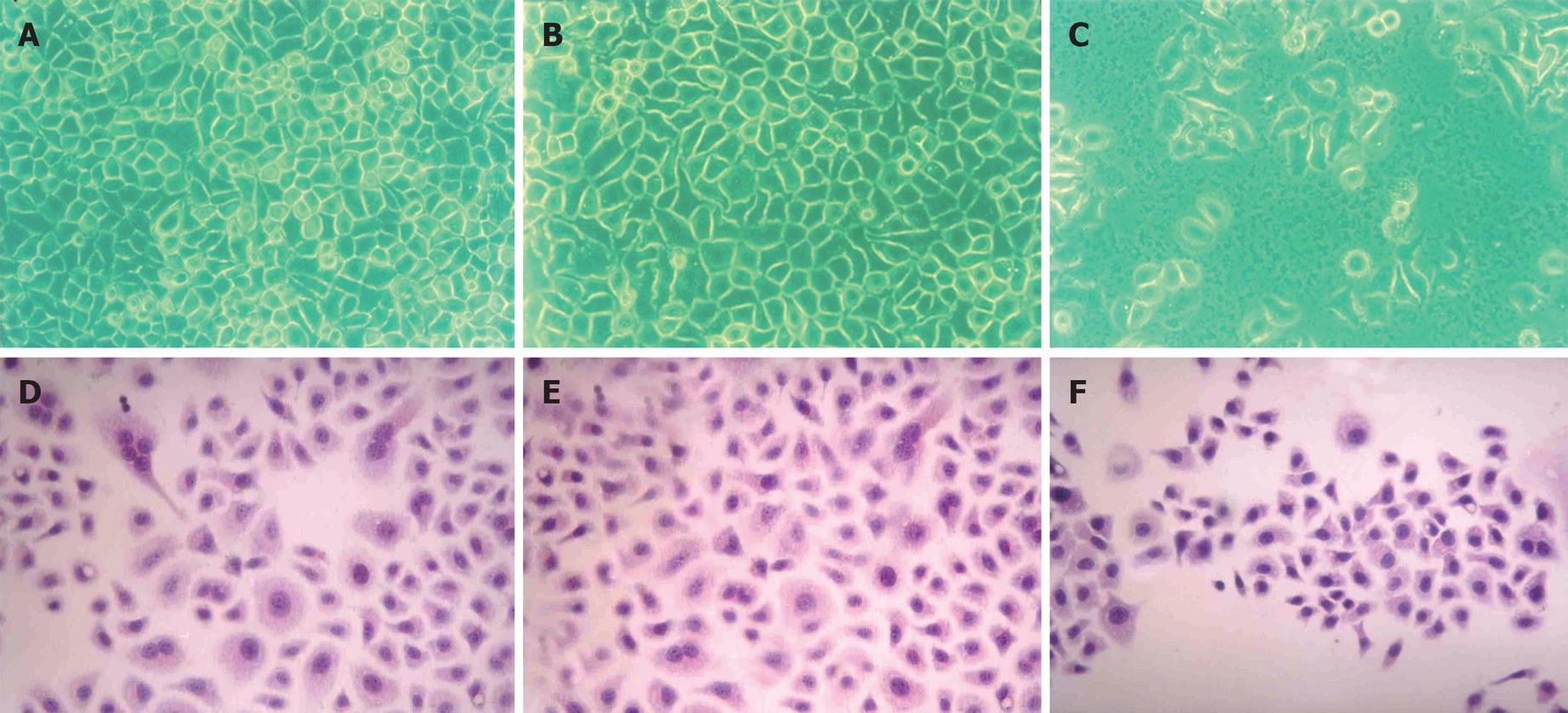
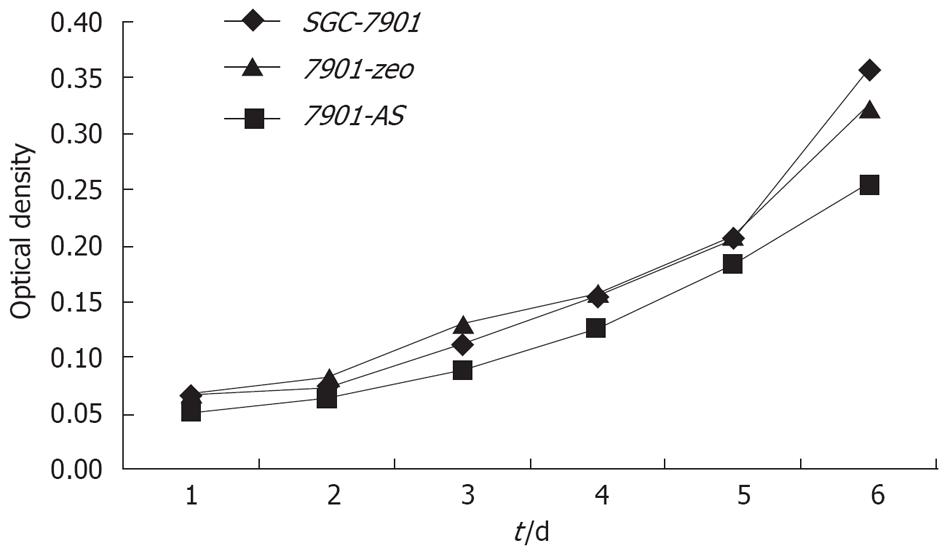
Compared with their parental cells and 7901-zeo cells, antisense NHE1-transfected 7901 cells displayed several morphological changes under light microscopy, such as decreased mitotic figures, multinucleate giant cells, giant nucleate cell numbers, and nucleus:cytoplasma ratio. The 7901-AS cells only grew in a monolayer and at low cell density. The cell proliferation slowed down on the sixth day when 7901-AS cells grew into complete confluence (Figures 4 and 5). Further study showed that the parent cells and 7901-zeo cells grew in clusters with large numbers of big clones. The formation rate was 11% for SGC-7901 cells and 9.5% for 7901-zeo cells after 2 wk culture in soft agar. By contrast, the 7901-AS cells were scattered in soft agar with less number of clones and the formation rate was only 2%.
The 7901-AS cells showed increased apoptotic cells and G0/G1 cells, and decreased S and G2M cells, and reduced proliferative index compared to SGC-7901 and 7901-zeo cells, suggesting that transfection of SGC-7901 cells with antisense NHE1 gene resulted in leftward shift of the cell cycles and reduced capacity for differentiation and proliferation. Flow cytometry showed apoptotic peak of 7901-AS cells with apoptotic rate of 26.1%, which was significantly higher than that of SGC-7901 cells (4.5%) and 7901-zeo cells (5.1%), suggesting that transfection with antisense NHE1 induced apoptosis of SGC-7901 cells (Table 2).
After inoculation with 4 × 106SGC-7901 cells or 7901-zeo cells, all mice (n = 3) grew palpable tumors on the sixteenth-seventeenth day. Subsequently, the tumors grew progressively. The size and speed of growth of the two different tumors showed no apparent difference at 2 mo. After inoculation with 4 × 106 and 8 × 106 dose of 7901-AS cells, none of the mice (n = 3, respectively) grew palpable tumors within 2 mo. This result indicates that antisense NHE1-transfected SGC-7901 cells completely loose tumorigenecity in nude mice.
Since the successful cloning of human NHE1cDNA in 1989 by the Sardet Laboratory[6], the 8 known subtypes of NHE, named as NHE1-8 respectively, have been shown to be identical in structure and are related in function. These form the gene family of membrane exchange protein[7–10]. The different subtypes have different number and distribution, different pharmacological properties, and are regulated by different factors. NHE1, a house-keeping gene, located at 1p[1] with mRNA of 3.8 Kb, is found in nearly all human tissues, and serves to remove excessive H+ in the cytoplasm by the Na+-H+ exchange system, resulting in the maintenance of a stable pHi[11–14]. Glycolysis of tumor cells may result in the production of large quantities of lactic acid and H+. Researchers have long believed that the pHi of tumor cells is more acidic than that of normal cells. However, 31P-NMR spectroscopic studies of tumor cells have shown that the pHi measured in situ is more alkaline (pH 7.0-7.2) than that of the normal cells (pH 6.5-7.0). This phenomenon of high pHi with low extracellular pH (pHe) is caused by the activation of NHE1 in the cell membrane with increased mRNA expression, resulting in strong Na+-H+ exchange in the tumor cells. Most of the H+ pumped out of the cells helps to create an acidic environment in the interstitial fluid of the tumor cells and maintains the pHi in the tumor cells as neutral or more basic[15–18]. Our previous studies have shown that the significantly greater expression level of NHE1 protein in gastric carcinoma tissues compared to that in normal gastric tissues is closely associated with the genesis and progression of tumors[5], suggesting that NHE1 can be used as the target site in the treatment of such tumors. However, further studies are needed to determine whether or not the intervention of antisense NHE1 gene can decrease type 1 Na+/H+ exchanger of membranous ion exchange protein in gastric carcinoma cells and reverse the malignant phenotypes. Therefore, in the present study, the purpose of transfection of antisense NHE1 gene into human gastric carcinoma cell line SGC-7901 was to investigate the effects of antisense therapy targeting NHE1 gene in the malignant phenotypes of gastric carcinoma cells. It was observed that reduced pHi partially reversed the malignant phenotypes of SGC-7901 cells transfected with antisense NHE1. Compared with SGC-7901 and 7901-zeo cells, the 7901-AS cells showed cell proliferation inhibition, G1/G0 phase arrest, increased cell apoptotic rate, recovery of contact inhibition and density contact, decreased invasive capacity, and loss of cloning efficiency in soft agar, and tumorigenecity in nude mice. These results indicate that the NHE1 gene is important in maintaining the phenotypes of the SGC-7901 cell line. The NHE1 gene may be closely associated with the malignant biological behavior of the tumor cells, and as a result, the phenotype of the tumor is restrained when the NHE1 gene is inhibited.
Overexpression of NHE1 gene plays an important role in the regulation of pHi in tumor cells[19–26]. The enhancement of Na+-H+ exchange by tumor cells, caused by increasing the quantitative distribution of NHE1 on the cell membrane, is the major molecular mechanism in the regulation of pHi in tumor cells. This step is of important biological significance in the maintenance of stable pHi and malignant growth of the tumor cells. The energy consumption process of Na+-H+ exchange which is dependent on the energy supplied by Na+-K+-ATPase, stimulates glucose absorption and glycolysis by tumor cells and produces more intracellular H+, leading to strong Na+-H+ exchange in the tumor cells[27–30]. Most of the H+ pumped out of the cells helps to create the acidic environment in the interstitial fluid of the tumor cells and keeps the pHi neutral or more alkaline in tumor cells, resulting in the protection of the tumor cells from apoptosis[1823]. In the present study, transfection of antisense NHE1 gene inhibited the expression of NHE1 gene in SGC-7901 gastric carcinoma cells, resulting in intracellular acidification and induced apoptosis of SGC-7901 cells. The inhibited proliferation, increased apoptotic rate, and decreased malignancy in tumor cells resulted in significantly reduced tumorigenicity in nude mice in vivo. These results indicate that transfected human NHE1 antisense gene successfully inhibited Na+-H+ exchange and destroyed the energy metabolism pattern of gastric carcinoma cells. Our findings point to the feasibility of treatment of gastric carcinoma by induction of cell apoptosis through the process of intracellular acidification, which may provide a new method for gene therapy of gastric carcinoma.
In conclusion, we transfected NHE1 antisense into SGC-7901 cells and observed the morphological and biological changes in the tumor cells. Our results reveal that NHE1 antisense gene transfection can partly reverse the malignant behavior, resulting in intracellular acidification and induction of apoptosis of SGC-7901 cells. These finding may provide a potential method for gastric carcinoma gene therapy in the future.
Gastric cancer is one of the most common malignant tumors in China, but the pathogenesis is unclear. Recent investigations have demonstrated that type 1 Na+/H+ exchanger (NHE1) mRNA expression is significantly increased in the carcinoma, relative to the occurrence and growth of tumors. Our previous studies have shown that significantly higher expression level of NHE1 protein in gastric carcinoma tissue compared to normal gastric tissue is closely associated with the genesis and progression of tumors. Therefore, the NHE1 gene may be a good target for antisense gene therapy for gastric cancer. However, further studies are needed to determine whether antisense NHE1 gene intervention can reduce type 1 Na+/H+ exchanger of the membranous ion exchange protein, and influence the biological behavior of the gastric carcinoma cells.
Over expression of NHE1 gene plays an important role in the regulation of pHi in tumor cells, which is of important biological significance in the malignant growth of tumor cells. However, the role of NHE1 in the regulation of tumorigenic and metastatic properties of tumor cells remains unclear and it is important to determine the precise role of NHE1.
Our results reveal that NHE1 antisense gene may significantly restrain the malignant behavior of human gastric carcinoma cells, result in intracellular acidification, suppress cell growth, induce cell apoptosis, and partially reverse the malignant phenotypes of SGC-7901. These findings suggest a potential role for human tumor gene therapy.
The present study may be helpful in determining a potential role for gastric carcinoma gene therapy in the future.
The term pHi means intracellular pH. The pHi of tumor cells measured in situ is more alkaline than that of normal cells. This phenomenon of high pHi with low extracellular pH (pHe) is caused by the activation of NHE1 in cell membrane and increased mRNA expression. NHE1, a type 1 Na+/H+ exchanger, is a transmembrane protein found in all eukaryotic cells. One of its functions is to reduce excess H+ in the cytoplasm by means of Na+-H+ exchange, resulting in stable intracellular pH levels.
This is an interesting article which examines the effect of inhibiting NHE1 on tumor survival. The manuscript is of interest and the data is good. It would be useful if NHE1 can be explained in more detail in the abstract.
| 1. | Slepkov E, Fliegel L. Structure and function of the NHE1 isoform of the Na+/H+ exchanger. Biochem Cell Biol. 2002;80:499-508. |
| 2. | Malo ME, Fliegel L. Physiological role and regulation of the Na+/H+ exchanger. Can J Physiol Pharmacol. 2006;84:1081-1095. |
| 3. | Pedersen SF. The Na+/H+ exchanger NHE1 in stress-induced signal transduction: implications for cell proliferation and cell death. Pflugers Arch. 2006;452:249-259. |
| 4. | Reshkin SJ, Bellizzi A, Cardone RA, Tommasino M, Casavola V, Paradiso A. Paclitaxel induces apoptosis via protein kinase A- and p38 mitogen-activated protein-dependent inhibition of the Na+/H+ exchanger (NHE) NHE isoform 1 in human breast cancer cells. Clin Cancer Res. 2003;9:2366-2373. |
| 5. | Teng XC, Liu HF, Liu YS, Duan L, Wang GA, He JT. Expression of Na+/H+ exchanger 1 in gastric carcinoma and precancerous lesions and its clinical significance. Acta Academiae Med. Militaris Tertiae. 2004;26:402-404. |
| 6. | Sardet C, Counillon L, Franchi A, Pouyssegur J. Growth factors induce phosphorylation of the Na+/H+ antiporter, glycoprotein of 110 kD. Science. 1990;247:723-726. |
| 7. | Ford P, Rivarola V, Kierbel A, Chara O, Blot-Chabaud M, Farman N, Parisi M, Capurro C. Differential role of Na+/H+ exchange isoforms NHE-1 and NHE-2 in a rat cortical collecting duct cell line. J Membr Biol. 2002;190:117-125. |
| 8. | Szaszi K, Paulsen A, Szabo EZ, Numata M, Grinstein S, Orlowski J. Clathrin-mediated endocytosis and recycling of the neuron-specific Na+/H+ exchanger NHE5 isoform. Regulation by phosphatidylinositol 3'-kinase and the actin cytoskeleton. J Biol Chem. 2002;277:42623-42632. |
| 9. | Brett CL, Wei Y, Donowitz M, Rao R. Human Na(+)/H(+) exchanger isoform 6 is found in recycling endosomes of cells, not in mitochondria. Am J Physiol Cell Physiol. 2002;282:C1031-C1041. |
| 10. | Goyal S, Vanden Heuvel G, Aronson PS. Renal expression of novel Na+/H+ exchanger isoform NHE8. Am J Physiol Renal Physiol. 2003;284:F467-F473. |
| 11. | De Vito P. The sodium/hydrogen exchanger: a possible mediator of immunity. Cell Immunol. 2006;240:69-85. |
| 12. | Putney LK, Denker SP, Barber DL. The changing face of the Na+/H+ exchanger, NHE1: structure, regulation, and cellular actions. Annu Rev Pharmacol Toxicol. 2002;42:527-552. |
| 13. | Loh SH, Chen WH, Chiang CH, Tsai CS, Lee GC, Jin JS, Cheng TH, Chen JJ. Intracellular pH regulatory mechanism in human atrial myocardium: functional evidence for Na(+)/H(+) exchanger and Na(+)/HCO(3)(-) symporter. J Biomed Sci. 2002;9:198-205. |
| 14. | Lin CY, Varma MG, Joubel A, Madabushi S, Lichtarge O, Barber DL. Conserved motifs in somatostatin, D2-dopamine, and alpha 2B-adrenergic receptors for inhibiting the Na-H exchanger, NHE1. J Biol Chem. 2003;278:15128-15135. |
| 15. | Lagana A, Vadnais J, Le PU, Nguyen TN, Laprade R, Nabi IR, Noel J. Regulation of the formation of tumor cell pseudopodia by the Na(+)/H(+) exchanger NHE1. J Cell Sci. 2000;113:3649-3662. |
| 16. | Maidorn RP, Cragoe EJ Jr, Tannock IF. Therapeutic potential of analogues of amiloride: inhibition of the regulation of intracellular pH as a possible mechanism of tumour selective therapy. Br J Cancer. 1993;67:297-303. |
| 17. | Beltrrn AR, Ramirez MA, Carraro-Lacroix LR, Hiraki Y, Reboucas NA, Malnic G. NHE1, NHE2, and NHE4 contribute to regulation of cell pH in T84 colon cancer cells. Pflugers Arch. 2008;455:799-810. |
| 18. | Goossens JF, Henichart JP, Dassonneville L, Facompre M, Bailly C. Relation between intracellular acidification and camptothecin-induced apoptosis in leukemia cells. Eur J Pharm Sci. 2000;10:125-131. |
| 19. | Shen MR, Wilkins RJ, Chou CY, Ellory JC. Anion exchanger isoform 2 operates in parallel with Na(+)/H(+) exchanger isoform 1 during regulatory volume decrease of human cervical cancer cells. FEBS Lett. 2002;512:52-58. |
| 20. | Pouyssegur J, Franchi A, Pages G. pHi, aerobic glycolysis and vascular endothelial growth factor in tumour growth. Novartis Found Symp. 2001;240:186-196; discussion 196-198. |
| 21. | McLean LA, Roscoe J, Jorgensen NK, Gorin FA, Cala PM. Malignant gliomas display altered pH regulation by NHE1 compared with nontransformed astrocytes. Am J Physiol Cell Physiol. 2000;278:C676-C688. |
| 22. | Cardone RA, Bellizzi A, Busco G, Weinman EJ, Dell'Aquila ME, Casavola V, Azzariti A, Mangia A, Paradiso A, Reshkin SJ. The NHERF1 PDZ2 domain regulates PKA-RhoA-p38-mediated NHE1 activation and invasion in breast tumor cells. Mol Biol Cell. 2007;18:1768-1780. |
| 23. | Huc L, Tekpli X, Holme JA, Rissel M, Solhaug A, Gardyn C, Le Moigne G, Gorria M, Dimanche-Boitrel MT, Lagadic-Gossmann D. c-Jun NH2-terminal kinase-related Na+/H+ exchanger isoform 1 activation controls hexokinase II expression in benzo(a)pyrene-induced apoptosis. Cancer Res. 2007;67:1696-1705. |
| 24. | Chiang Y, Chou CY, Hsu KF, Huang YF, Shen MR. EGF upregulates Na+/H+ exchanger NHE1 by post-translational regulation that is important for cervical cancer cell invasiveness. J Cell Physiol. 2008;214:810-819. |
| 25. | Stock C, Mueller M, Kraehling H, Mally S, Noel J, Eder C, Schwab A. pH nanoenvironment at the surface of single melanoma cells. Cell Physiol Biochem. 2007;20:679-686. |
| 26. | Friday E, Oliver R 3rd, Welbourne T, Turturro F. Role of epidermal growth factor receptor (EGFR)-signaling versus cellular acidosis via Na+/H+ exchanger1(NHE1)-inhibition in troglitazone-induced growth arrest of breast cancer-derived cells MCF-7. Cell Physiol Biochem. 2007;20:751-762. |
| 27. | Waibel M, Kramer S, Lauber K, Lupescu A, Manns J, Schulze-Osthoff K, Lang F, Wesselborg S. Mitochondria are not required for death receptor-mediated cytosolic acidification during apoptosis. Apoptosis. 2007;12:623-630. |
| 28. | Turturro F, Driscoll M, Friday E, Welbourne T. ALK-mediated Na+/H+ exchanger-dependent intracellular alkalinization: does it matter for oncogenesis? Haematologica. 2007;92:706-707. |
| 29. | Heming TA, Bidani A. Intracellular pH regulation in U937 human monocytes: roles of V-ATPase and Na+/H+ exchange. Immunobiology. 2003;207:141-148. |
| 30. | Rebillard A, Tekpli X, Meurette O, Sergent O, LeMoigne-Muller G, Vernhet L, Gorria M, Chevanne M, Christmann M, Kaina B. Cisplatin-induced apoptosis involves membrane fluidification via inhibition of NHE1 in human colon cancer cells. Cancer Res. 2007;67:7865-7874. |