TEXT
MUC1 is a structural membranous bound mucin, expressed on the apical borders of secretory epithelial cells which previously had many names such as DF3, episialin, CA5-3, PAS-O, polymorphic epithelial mucin (PEM), or epithelial membrane antigen[1].
MUC1 gene is located on chromosome 1q21 and has 1201 nucleotides. The N-terminal ectodomain of MUC1 (MUC1-N) consists of variable numbers of 20-amino-acid tandem repeats (VNTRs), with the number of repeats varying from 20 to 120 in different individuals[2]. These sites are subject to O-glycosylation that contributes to form a structure that extends beyond the glycocalyx of the cell. The protein has a protective function by binding to pathogens and also functions in a cell signaling capacity[3].
MUC1-N is tethered to the cell membrane as a heterodimer with the MUC1 C-terminal subunit (MUC1-C), which includes a 58-amino acid extracellular domain, a 28-amino acid transmembrane domain, and a 72-amino acid cytoplasmic tail that contains sites for tyrosine and serine phosphorylation[4]. Over expression, aberrant intracellular localization, and changes in glycosylation of this protein as found in most human carcinomas, confers anchorage-independent growth and tumorigenicity[5]. Other studies have demonstrated that over expression of MUC1 confers resistance to apoptosis induced by oxidative stress and anti-cancer agents[6]. Multiple alternatively spliced transcript variants that encode different isoforms of MUC1 have been reported, but the full-length nature of only some of them has been determined[7].
Several lines of evidence point towards a biological role of mucin and particularly MUC1 in colorectal cancer (CRC). A positive correlation was described between mucin secretion, proliferation, invasiveness, metastasis and bad prognosis[8–10]. When MUC1 was expressed at the deepest tumor invasive portion, lymphatic and venous invasion was more pronounced as well as lymph nodes and liver metastasis[11]. Correlation with bad prognosis was found in mismatch repair (MMR) - proficient colorectal tumors, but not in MLH1 negative tumors or in Lynch syndrome (HNPCC)[12].
But, the role of MUC1 in cancer progression is still controversial and somewhat confusing. While Mukherjee and colleagues, in a very sophisticated way, developed MUC1-specific immune therapy in a CRC model, Lillehoj and co-investigators showed recently that MUC1 inhibits cell proliferation by β-catenin-dependent mechanism[1314]. A similar observation was described by Yuan and co-workers[15].
Interaction of the cytoplasmic tail of MUC1 with β-catenin has a significant effect on cell cycle and proliferation. This process hardly happens in normal polarized epithelium, because MUC1 resides on the apical surface while β-catenin resides on the lateral surface. Loss of polarity during transformation creates a permissive environment for MUC1 and β-catenin interaction[7].
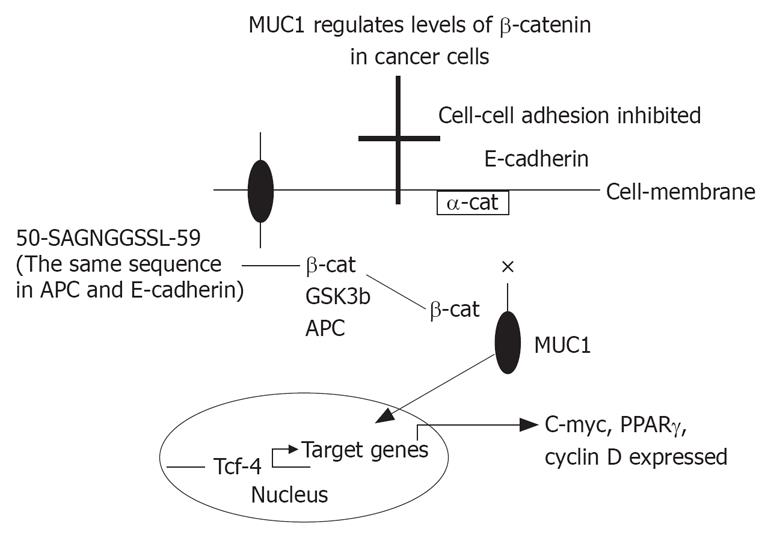
β-catenin can bind directly to the amino acid sequence 50-SAGNGGSSL-59 of the MUC1 cytoplasmic domain (a similar binding site is found on E-cadherin and APC proteins). The binding is promoted by phosphorylation of T41 by Ser/Thr kinase PKCζ and of Y46 by Src or EGFR[16]. Inhibition of β-catenin binding to MUC 1 is the result of phosphorylation of S44 by GSK3β that can also directly degrades β-catenin. Disruption of the β-catenin binding site in MUC1 suppresses its ability to induce anchorage-dependent and independent growth, indicating β-catenin binding to MUC1 is a critical component of its tumorigenc activity. MUC1 also protects β-catenin from degradation by GSK3β, and when co-localized with β-catenin in the nucleus co activates transcription of Wnt target genes[17–19]. MUC1 binding to β-catenin suppresses its ability to interact with E-cadherin at adherent junctions, leading to a breakdown in cell-cell interactions. GSK3β-mediated disruption of the complex restores the E-cadherin/β-catenin interaction[20].
In carcinoma cells the polarization of MUC1 is lost, and the protein is over expressed at high levels over the entire cell surface. A competitive interaction between MUC1 and E-cadherin, through β-catenin binding, disrupts E-cadherin-mediated cell-cell interactions at sites of MUC1 expression. In addition, the complex of MUC1-β-catenin enters the nucleus and activates T-cell factor/leukocyte enhancing factor 1 (Tcf/LEF-1) transcription factors and activates gene expression[18]. This enhances proliferation, and decreases cell-cell adhesion which may both increase carcinogenesis and metastasis. GSK3β interacts with the STDRSPYE motif in MUC1, phosphorylates the serine in this domain, and prevents binding of β-catenin[16].
It is proposed that APC binding prevents the formation of β-catenin-Tcf complex, and that MUC1 binding prevents β-catenin-α-catenin-E-cadherin complex. GSK3β interacts with β-catenin to restore β-catenin-E-cadherin complex, and with APC to bind β-catenin and prevent β-catenin-Tcf complex (Figure 1). The exact mechanism of MUC1 associated cancer cell proliferation and carcinogenesis is not well understood. MUC1 can bind β-catenin, prevents its entering the nucleus or activating Tcf/LEF-1, and inhibits proliferation. On the other hand, MUC1-C complex with β-catenin may enter the nucleus and the opposite action will take place. In both cases, β-catenin binding to MUC1 will prevent its binding to E-cadherin or to APC.
Figure 1 It is proposed that APC binding prevents the formation of β-catenin-Tcf complex, and that MUC1 binding prevents β-catenin-α-catenin-E-cadherin complex.
GSK3β interacts with β-catenin to restore β-catenin-E-cadherin complex, and with APC to bind β-catenin and prevent β-catenin-Tcf complex. × = inhibition; arrow = enhancing.
The cytoplasmic tail of MUC1 contains 4 tyrosine residues that are phosphorylated before binding β-catenin. It is speculated that MUC1 is a membranous receptor which maintains cell cycle progression or enhances apoptosis. Activating MUC1 will result in phosphorylation of the tyrosines on the cytoplasmic tail and binding β-catenin. This will prevent β-catenin from binding E-cadherin or activating Tcf/LEF-1 pathway. This mechanism may be similar to that just described for DCC and UNC5H, which induced apoptosis when not engaged with their ligand netrin, but mediate signals for proliferation, differentiation or migration when ligand bound[21].