Published online Apr 7, 2008. doi: 10.3748/wjg.14.2110
Revised: January 30, 2008
Published online: April 7, 2008
AIM: To examine the expression of connective tissue growth factor (CTGF), also known as CCN2, in gastric carcinoma (GC), and the correlation between the expression of CTGF, clinicopathologic features and clinical outcomes of patients with GC.
METHODS: One hundred and twenty-two GC patients were included in the present study. All patients were followed up for at least 5 years. Proteins of CTGF were detected using the Powervision two-step immunostaining method.
RESULTS: Of the specimens from 122 GC patients analyzed for CTGF expression, 58 (58/122, 47.5%) had a high CTGF expression in cytoplasm of gastric carcinoma cells and 64 (64/122, 52.5%) had a low CTGF expression. Patients with a high CTGF expression showed a higher incidence of lymph node metastasis than those with a low CTGF expression (P = 0.032). Patients with a high CTGF expression had significantly lower 5-year survival rate than those with a low CTGF expression (27.6% vs 46.9%, P = 0.0178), especially those staging I + II + III (35.7% vs 65.2%, P = 0.0027).
CONCLUSION: GC patients with an elevated CTGF expression have more lymph node metastases and a shorter survival time. CTGF seems to be an independent prognostic factor for the successful differentiation of high-risk GC patients staging I + II + III. Over-expression of CTGF in human GC cells results in an increased aggressive ability.
- Citation: Liu LY, Han YC, Wu SH, Lv ZH. Expression of connective tissue growth factor in tumor tissues is an independent predictor of poor prognosis in patients with gastric cancer. World J Gastroenterol 2008; 14(13): 2110-2114
- URL: https://www.wjgnet.com/1007-9327/full/v14/i13/2110.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.2110
Gastric cancer (GC), one of the most common malignant diseases, is the second leading cause for cancer-related death both in China and in the world (700 000 deaths annually)[12].
TNM staging system is used worldwide to predict the prognosis and direct therapeutic decisions of patients with GC[3]. The 5-year survival rate of GC patients at stages I and IV is close to 90% and less than 30%, respectively[4]. GC exhibits markedly heterogenous in histologic feature and biologic behavior, especially at advanced stages. It was reported that the biological behavior and prognosis of GC can be significantly different among GC patients at the same stage[5]. Some studies showed that some biomarkers could provide additional information for predicting the biological behavior and prognosis of GC. More specific and effective markers and therapies should be identified and developed for improving the survival of GC patients.
Connective tissue growth factor (CTGF), also known as CCN2, is a member of the CCN family, including cysteine-rich protein 61 (Cyr61), also known as CCN1, and nephroblastoma-overexpressed gene (Nov), also known as CCN3, as well as Wisp-1/elm1 (CCN4), Wisp-2/rCop1 (CCN5) and Wisp-3 (CCN6)[67]. The primary translational products of CCN family members are 343-381 residues, which generate proteins of Mr 35 000-40 000 with homologies ranging from 60% to 90%. All members of the CCN gene family possess a secretory signal peptide at the NH2 terminus, indicating that they are secreted proteins. CTGF can bind to integrins on cell surface[6], and is a potent stimulator of endothelial cell adhesion, proliferation, migration and angiogenesis in vivo[9–11]. CTGF is believed to be a multifunctional signaling modulator involved in a wide variety of biologic or pathologic processes, such as angiogenesis, osteogenesis, fibrosis in kidneys and skin, and tumor development[6–812–15]. It was reported that CTGF plays an important role in the progression of several types of cancer[16]. Elevated CTGF levels have been detected in a number of cancers including pancreatic cancer[1617], breast cancer[1819], prostate cancer[20], esophageal adenocarcinoma[21], glioma[22] and melanoma[23]. However, little information on the association between expression of CTGF and GC prognosis is available.
In this study, we examined the expression of CTGF in gastric carcinoma in order to analyze its correlation with histologic type, clinicopathologic feature, and clinical outcome of gastric carcinoma patients.
A consecutive series of 122 patients with gastric carcinoma were studied. All patients were treated at the Department of Surgery, Affiliated Hospital of Binzhou Medical Collage, between July 1994 and December 2000. All patients gave their written informed consent to participate in this study. There were 88 males and 34 females with a mean age of 56.6 years (range 25-80 years). All patients underwent radical gastrectomy and none of the patients received chemotherapy or radiation therapy prior to operation. Age and sex of the patients, maximum tumor size, histologic grade, status of lymph node metastasis and distant metastasis were obtained from histopathology reports. Stage of GC was defined according to the 1997 tumor-node-metastasis (TNM) classification of malignant tumors by the International Union against Carcinoma[24]. All patients were followed-up until May 2007.
The tissue, fixed in 10% neutral formalin and embedded in paraffin, was cut into 4-&mgr;m thick sections. CTGF expression was examined by immunostaining using the Powervision two-step immunostaining method. Briefly, the sections were treated with a 3% hydrogen peroxide solution for 10 min to block the endogenous peroxidase activity after deparaffinized in xylene and rehydrated in a graded ethanol series. Antigen retrieval was performed in 1 mmol/L EDTA (pH 8.0) in an autoclave for 3 min. The monoclonal antibodies used were clone 88430 (1:100, R&D Systems Inc, Minneapolis, MN, USA) which recognizes CTGF. The sections were incubated overnight at 4°C with primary antibody. The primary antibody was detected using the Powervision two-step histostaining reagent-peroxidase-labeled goat anti-mouse immunoglobulin (PV-6002, DAKO, Glostrop, Denmark) for 1 h at room temperature. After peroxidase activity was developed with 3, 3’-diaminobenzidine tetrachloride (DAB), slides were counterstained with haematoxylin and observed under a light microscope. Positive and negative immunohistochemistry controls were routinely used.
Three experienced pathologists, unaware of the information on the clinicopathologic data and clinical outcomes of the patients, independently examined the CTGF staining. A scoring system was devised to assign a staining intensity score for CTGF expression from 0 (no expression) to 3 (highest intensity staining). Immunostaining was classified into two groups according to both intensity and extent. Low expression was defined as no staining present (staining intensity score: 0) or positive staining detected in ≤ 10% of the cells (staining intensity score: 1) and high expression was defined as positive immunostaining present in 10%-50% of the cells (staining intensity score: 2) or > 50% of the cells (staining intensity score: 3)[25].
All data were analyzed using SPSS 10.0 software. The association of CTGF expression with various clinicopathologic features was analyzed using the Pearson χ2 test. Cumulative survival was estimated with the Kaplan-Meier method and the difference in survival curves was analyzed by the log-rank test. The influence of each variable on survival was analyzed with the multivariate analysis of Cox proportional hazard model (backward, stepwise). All statistical tests were two-sided. P < 0.05 was considered statistically significant.
The clinicopathologic features of the patients are summarized in Table 1. The follow-up time ranged from 2 mo to 121 mo (median, 27 mo). The 5-year survival rate of patients at stages I, II, III and IV was 88.9%, 66.7%, 28.3% and 2.9%, respectively. The overall 5-year survival rate was 37.7%.
| Factors | Cases | CTGF expression | P value1 | |
| Low expression | High expression | |||
| Age (yr) | 0.628 | |||
| < 60 | 68 | 37 | 31 | |
| ≥ 60 | 54 | 27 | 27 | |
| Sex | 0.251 | |||
| Male | 88 | 49 | 39 | |
| Female | 34 | 15 | 19 | |
| Tumor size (cm) | 0.555 | |||
| < 5 | 56 | 31 | 25 | |
| ≥ 5 | 66 | 33 | 33 | |
| Differentiation | 0.014 | |||
| Well | 19 | 6 | 13 | |
| Moderate | 32 | 13 | 19 | |
| Poor | 71 | 45 | 26 | |
| Lauren type | 0.045 | |||
| Intestinal type | 40 | 15 | 25 | |
| Diffuse type | 64 | 40 | 24 | |
| Mixed type | 18 | 9 | 9 | |
| TNM stage | 0.391 | |||
| I | 18 | 11 | 7 | |
| II | 24 | 15 | 9 | |
| III | 46 | 20 | 26 | |
| IV | 34 | 18 | 16 | |
| Lymph nodes metastasis | 0.032 | |||
| Absent | 32 | 22 | 10 | |
| Present | 90 | 42 | 48 | |
| Metastasis | 0.821 | |||
| Absent | 104 | 55 | 49 | |
| Present | 18 | 9 | 9 | |
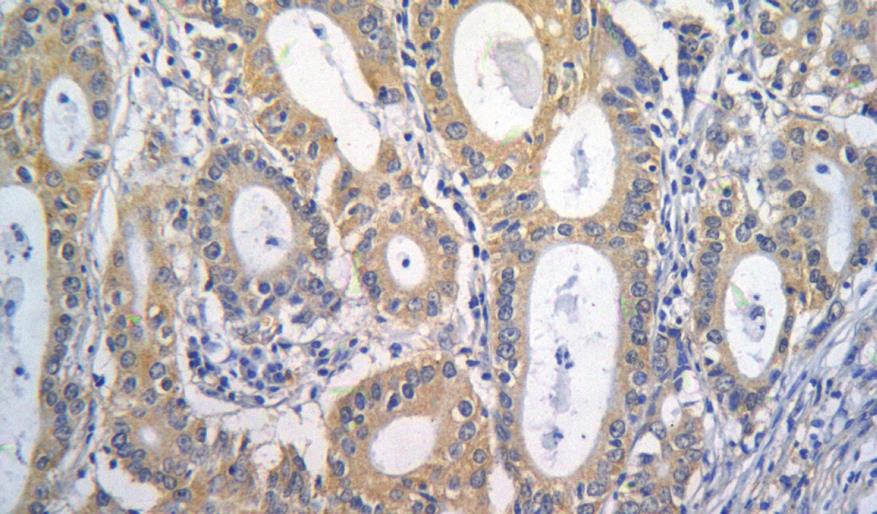
The CTGF protein was predominantly localized in cytoplasm or membrane of normal or tumor cells. No CTGF expression was detected in normal gastric epithelial cells, but deep glands and fibroblasts were positively stained. Glands in some cases were positively stained in intestinal metaplasia and dysplasia gastric mucosa.
Of the 122 specimens from GC patients analyzed for CTGF expression, 58 (58/122, 47.5%) had a high CTGF expression in cytoplasm of gastric carcinoma cells, 43 (43/122, 35.2%) had a score of 2, and 15 (15/122, 12.3%) a score of 3, while 64 (64/122, 52.5%) had a low CTGF expression, 37 (37/122, 30.3%) had a score of 0 and 27 (27/122, 22.1%) a score of 1 (Figure 1).
CTGF was highly expressed more frequently in well-differentiated GC than in moderately- or poorly- differentiated GC (P = 0.014) and in intestinal-type carcinoma than in diffuse-type or mixed-type carcinoma (P = 0.045). Patients with a high CTGF expression had a higher incidence of lymph node metastasis than those with a low CTGF expression (P = 0.032). No significant relationship was found between the level of CTGF expression and the age and sex, tumor size, TNM stage and distance metastasis of GC patients (Table 1).
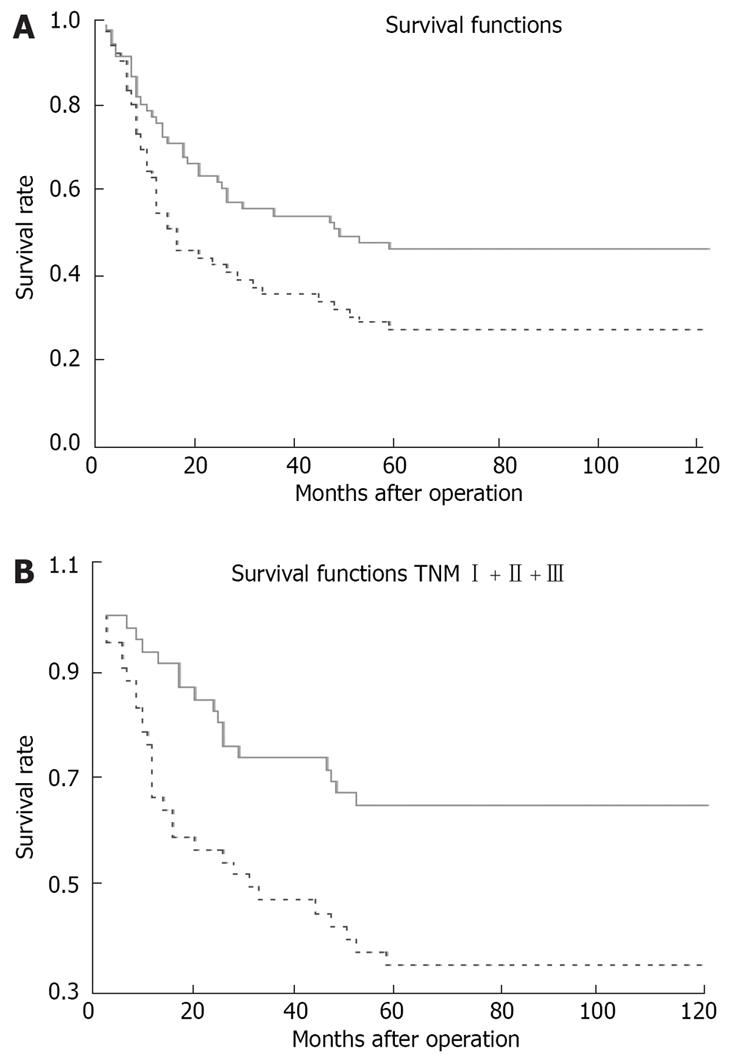
Patients with a high CTGF expression had a significantly lower cumulative 5-year survival rate (27.6%) than those with a low CTGF expression (46.9%, two-sided log-rank test, P = 0.0178; Figure 2A). The prognostic significance of CTGF expression in patients at TNM stage I + II + III was analyzed. Patients at stage I + II + III had a high CTGF expression and a significantly lower 5-year survival rate (35.7%) than those with a low CTGF expression (65.2%, two-sided log-rank test, P = 0.0027; Figure 2B).
Multivariate analysis revealed that CTGF expression, TNM stage, differentiation were independent prognostic indicators for the overall survival of the patients after adjustment for sex, age, tumor size, grade of differentiation, Lauren types, TNM stages, lymph node metastasis and distant metastasis (P < 0.05, Table 2).
| Variables | B | SE | RR (95% CI) | P |
| TNM stage | < 0.001 | |||
| II vs I | 1.162 | 0.792 | 3.197 (0.677-15.099) | 0.142 |
| III vsI | 2.202 | 0.734 | 9.039 (2.143-38.136) | 0.003 |
| IV vs I | 3.561 | 0.746 | 35.208 (8.165-151.830) | < 0.001 |
| Differentiation | 0.067 | |||
| Moderate vs Well | 0.771 | 0.381 | 2.162 (1.024-4.567) | 0.043 |
| Poor vs Well | 0.929 | 0.414 | 2.533 (1.126-5.699) | 0.025 |
| CTGF expression | ||||
| High vs Low | 0.565 | 0.265 | 1.760 (1.047-2.958) | 0.033 |
In the present study, we detected CTGF expression in GC patients. High CTGF expression was closely related with lymph node metastasis, grade of differentiation, and Lauren type. Univariate and multivariate analyses revealed that high CTGF expression was a powerful independent predictor for the poor survival of GC patients, especially for those at stage I + II + III. The overall 5-year survival rate of GC patients with a higher CTGF expression and a lower CTGF expression was 27.6% and 46.9%, respectively (P = 0.0178). The 5-year survival rate of GC patients with a higher CTGF expression and a lower CTGF expression at stage I + II + III was 35.7% and 65.2%, respectively (P = 0.0027), indicating that over-expression of CTGF could promote the aggressive behavior of GC.
CTGF is a novel, potent angiogenic factor[910], which was first identified as a mitogen, detected in conditioned medium from human umbilical vein endothelial cells[26]. Integrin is an important receptor for CCN proteins, and receptor activation may produce a variety of effects. CTGF protein can bind directly to integrins αvβ3 and αIIbβ3[1011]. Shimo et al[9] and Babic et al[10] reported that CTGF mediates endothelial cell adhesion and migration through binding to integrin αvβ3, prolong endothelial cell survival, and induce angiogenesis in vivo. Yang et al[20] reported that CTGF is a downstream mediator of TGF-β1 action in cancer-associated reactive stroma, and one of the key promoters of angiogenesis in tumor-reactive stromal microenvironment, and plays an important role in prostate carcinogenesis. Breast cancer stage is positively associated with tumor size, lymph node metastasis status and over-expression of CTGF[19]. In our study, high CTGF expression was related with lymph node metastasis, depending on the ability of CTGF to induce angiogenesis.
CTGF is believed to be a multifunctional signaling modulator involved in a wide variety of biologic or pathologic processes. CTGF proteins exhibit diverse cellular functions, such as regulation of cell division, proliferation, mitogenesis, differentiation, survival, adhesion and migration, apoptosis, motility, and ion transport. CTGF plays a role in the development and progression of cancer. Recently, Dornhöfer et al[16] showed that CTGF promotes anchorage-independent pancreatic cancer cell growth. Furthermore, anti-CTGF treatment inhibits anchorage-independent growth in vitro, primary tumor growth in vivo and macroscopic lymph node metastases[16]. In contrast to the above results, CTGF is a new autocrine survival and differentiation factor for human rhabdomyosarcoma cells[27]. It was reported that over-expression of CTGF suppresses the growth of oral squamous carcinoma cells transplanted into mice[28]. Furthermore, apoptosis of MCF-7 cells induced by TGF-β appears to be mediated by CTGF, suggesting that CTGF may play an important role in human breast cancer cell growth[29]. Elevated level of CTGF is significantly correlated with a good prognosis of colorectal cancer[30] and lung adenocarcinoma[25], suggesting that the role of CTGF in different types of cancer may vary considerably, depending on the tissue involved. The question of how cell or tissue context determines the action of CTGF protein is interesting and deserves further investigation.
The present study showed that high CTGF expression was a powerful independent predictor for the poor overall survival of GC patients, especially for those at stage I + II + III. Multi-mechanisms are involved in aggressive behaviors of tumors at stage IV. The 5-year survival rate was only about 10% of GC patients at stage IV. Additional biomarkers might be helpful in predicting the prognosis of GC patients and more specific and effective therapies should be developed to improve the survival of GC patients at stage I + II + III. However, the value of additional biomarkers for predicting the prognosis of GC patients at stage IV is poor.
In conclusion, GC patients with an elevated CTGF expression have more lymph node metastases and a shorter survival time. CTGF seems to be an independent prognostic factor that allows successful differentiation of high-risk GC patients at stage I + II + III. Over-expression of CTGF in human GC cells results in an increased aggressive ability of cancer.
Connective tissue growth factor (CTGF), also known as CCN2, is a member of the CCN family, which is believed to be a multifunctional signaling modulator involved in a wide variety of biologic or pathologic processes. CTGF plays an important role in the progression of several types of cancer. However, little information on the association between CTGF expression and GC prognosis is available.
In this study, we examined the expression of CTGF in gastric carcinoma in order to analyze its correlation with histologic type, clinicopathologic feature, and clinical outcomes of gastric cancer (GC) patients.
GC, one of the most common malignant diseases, is the second leading cause for cancer-related death both in China and in the world. It has been shown that its biologic behavior and prognosis can be significantly different in GC patients at the same stage. CTGF seems to be an independent prognostic factor that allows differentiation of high-risk patients at stageI + II + III. Over-expression of CTGF in human GC cells results in an increased aggressive ability of GC.
CTGF may represent a potential novel target for treatment of GC. Inhibition of CTGF may control primary tumor growth and lymph node metastasis.
In this study, the authors showed that CTGF was a prognostic factor for GC patients. This paper is well-written.
| 1. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. |
| 2. | Gallo A, Cha C. Updates on esophageal and gastric cancers. World J Gastroenterol. 2006;12:3237-3242. |
| 3. | Hyung WJ, Noh SH, Yoo CH, Huh JH, Shin DW, Lah KH, Lee JH, Choi SH, Min JS. Prognostic significance of metastatic lymph node ratio in T3 gastric cancer. World J Surg. 2002;26:323-329. |
| 5. | Zhang XF, Huang CM, Lu HS, Wu XY, Wang C, Guang GX, Zhang JZ, Zheng CH. Surgical treatment and prognosis of gastric cancer in 2,613 patients. World J Gastroenterol. 2004;10:3405-3408. |
| 6. | Lau LF, Lam SC. The CCN family of angiogenic regulators: the integrin connection. Exp Cell Res. 1999;248:44-57. |
| 7. | Bork P. The modular architecture of a new family of growth regulators related to connective tissue growth factor. FEBS Lett. 1993;327:125-130. |
| 8. | Zeng ZJ, Yang LY, Ding X, Wang W. Expressions of cysteine-rich61, connective tissue growth factor and Nov genes in hepatocellular carcinoma and their clinical significance. World J Gastroenterol. 2004;10:3414-3418. |
| 9. | Shimo T, Nakanishi T, Nishida T, Asano M, Kanyama M, Kuboki T, Tamatani T, Tezuka K, Takemura M, Matsumura T. Connective tissue growth factor induces the proliferation, migration, and tube formation of vascular endothelial cells in vitro, and angiogenesis in vivo. J Biochem. 1999;126:137-145. |
| 10. | Babic AM, Chen CC, Lau LF. Fisp12/mouse connective tissue growth factor mediates endothelial cell adhesion and migration through integrin alphavbeta3, promotes endothelial cell survival, and induces angiogenesis in vivo. Mol Cell Biol. 1999;19:2958-2966. |
| 11. | Brigstock DR. Regulation of angiogenesis and endothelial cell function by connective tissue growth factor (CTGF) and cysteine-rich 61 (CYR61). Angiogenesis. 2002;5:153-165. |
| 12. | Brigstock DR. The connective tissue growth factor/cysteine-rich 61/nephroblastoma overexpressed (CCN) family. Endocr Rev. 1999;20:189-206. |
| 13. | Perbal B. The CCN family of genes: a brief history. Mol Pathol. 2001;54:103-104. |
| 14. | Perbal B. NOV (nephroblastoma overexpressed) and the CCN family of genes: structural and functional issues. Mol Pathol. 2001;54:57-79. |
| 15. | Planque N, Perbal B. A structural approach to the role of CCN (CYR61/CTGF/NOV) proteins in tumourigenesis. Cancer Cell Int. 2003;3:15. |
| 16. | Dornhofer N, Spong S, Bennewith K, Salim A, Klaus S, Kambham N, Wong C, Kaper F, Sutphin P, Nacamuli R. Connective tissue growth factor-specific monoclonal antibody therapy inhibits pancreatic tumor growth and metastasis. Cancer Res. 2006;66:5816-5827. |
| 17. | Wenger C, Ellenrieder V, Alber B, Lacher U, Menke A, Hameister H, Wilda M, Iwamura T, Beger HG, Adler G. Expression and differential regulation of connective tissue growth factor in pancreatic cancer cells. Oncogene. 1999;18:1073-1080. |
| 18. | Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordon-Cardo C, Guise TA, Massague J. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3:537-549. |
| 19. | Xie D, Nakachi K, Wang H, Elashoff R, Koeffler HP. Elevated levels of connective tissue growth factor, WISP-1, and CYR61 in primary breast cancers associated with more advanced features. Cancer Res. 2001;61:8917-8923. |
| 20. | Yang F, Tuxhorn JA, Ressler SJ, McAlhany SJ, Dang TD, Rowley DR. Stromal expression of connective tissue growth factor promotes angiogenesis and prostate cancer tumorigenesis. Cancer Res. 2005;65:8887-8895. |
| 21. | Koliopanos A, Friess H, di Mola FF, Tang WH, Kubulus D, Brigstock D, Zimmermann A, Büchler MW. Connective tissue growth factor gene expression alters tumor progression in esophageal cancer. World J Surg. 2002;26:420-427. |
| 22. | Xie D, Yin D, Wang HJ, Liu GT, Elashoff R, Black K, Koeffler HP. Levels of expression of CYR61 and CTGF are prognostic for tumor progression and survival of individuals with gliomas. Clin Cancer Res. 2004;10:2072-2081. |
| 23. | Kubo M, Kikuchi K, Nashiro K, Kakinuma T, Hayashi N, Nanko H, Tamaki K. Expression of fibrogenic cytokines in desmoplastic malignant melanoma. Br J Dermatol. 1998;139:192-197. |
| 24. | de Manzoni G, Verlato G, Guglielmi A, Laterza E, Tomezzoli A, Pelosi G, Di Leo A, Cordiano C. Classification of lymph node metastases from carcinoma of the stomach: comparison of the old (1987) and new (1997) TNM systems. World J Surg. 1999;23:664-669. |
| 25. | Chang CC, Shih JY, Jeng YM, Su JL, Lin BZ, Chen ST, Chau YP, Yang PC, Kuo ML. Connective tissue growth factor and its role in lung adenocarcinoma invasion and metastasis. J Natl Cancer Inst. 2004;96:364-375. |
| 26. | Bradham DM, Igarashi A, Potter RL, Grotendorst GR. Connective tissue growth factor: a cysteine-rich mitogen secreted by human vascular endothelial cells is related to the SRC-induced immediate early gene product CEF-10. J Cell Biol. 1991;114:1285-1294. |
| 27. | Croci S, Landuzzi L, Astolfi A, Nicoletti G, Rosolen A, Sartori F, Follo MY, Oliver N, De Giovanni C, Nanni P. Inhibition of connective tissue growth factor (CTGF/CCN2) expression decreases the survival and myogenic differentiation of human rhabdomyosarcoma cells. Cancer Res. 2004;64:1730-1736. |
| 28. | Moritani NH, Kubota S, Nishida T, Kawaki H, Kondo S, Sugahara T, Takigawa M. Suppressive effect of overexpressed connective tissue growth factor on tumor cell growth in a human oral squamous cell carcinoma-derived cell line. Cancer Lett. 2003;192:205-214. |
| 29. | Hishikawa K, Oemar BS, Tanner FC, Nakaki T, Luscher TF, Fujii T. Connective tissue growth factor induces apoptosis in human breast cancer cell line MCF-7. J Biol Chem. 1999;274:37461-37466. |
| 30. | Lin BR, Chang CC, Che TF, Chen ST, Chen RJ, Yang CY, Jeng YM, Liang JT, Lee PH, Chang KJ. Connective tissue growth factor inhibits metastasis and acts as an independent prognostic marker in colorectal cancer. Gastroenterology. 2005;128:9-23. |