Published online Apr 7, 2008. doi: 10.3748/wjg.14.2106
Revised: February 19, 2008
Published online: April 7, 2008
AIM: To evaluate the long-term effects of gonadotropin-releasing hormone (GnRH)-based vaccine on levels of GnRH antibody and testosterone, and vaccine-induced immunocastration on sexual behavior of male rats.
METHODS: The rats were treated with GnRH-PE40 intraperitoneally every other day for 12 wk. GnRH antibody and testosterone level in rat blood were determined by ELISA and radioimmunoassay, respectively. Morphological changes in testes and sexual behavior of rats were evaluated.
RESULTS: GnRH-PE40 induced a high production in GnRH antibody, decreased the serum testosterone level, testis atrophy and sexual function in rats.
CONCLUSION: Intraperitoneal administration of GnRH-PE40 produces structural and functional castration of male rat reproductive system by inducing anti-GnRH antibody.
- Citation: Yu L, Zhang ZF, Jing CX, Wu FL. Intraperitoneal administration of gonadotropin-releasing hormone-PE40 induces castration in male rats. World J Gastroenterol 2008; 14(13): 2106-2109
- URL: https://www.wjgnet.com/1007-9327/full/v14/i13/2106.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.2106
Gonadotropin releasing hormone (GnRH) is secreted by the hypothalamus that stimulates the anterior pituitary gland to release gonadotropins in mammals. GnRH has been widely used as a single medical agent[1] or a conjugated compound with other macromolecules[2]. Studies indicate that GnRH- based chimerical proteins, consisting of GnRH and toxins, such as tetanus toxoid, diphtheria toxoid and pseudomonas aeruginosa exotoxin A, target specifically at positive tumor cells of GnRH receptor and kill them efficiently[3–7]. It was reported that some of the chimeric proteins can effectively control reproductive (prostate, breast, ovary and endometrium) and digestive neoplasms[8–11]. However, since its application in this field, high GnRH antibody titer always develops along with the treatment, which often impedes the use of these compounds[1213]. Some authors even reported that the high antibody titer induced by chimeric proteins leads to testis atrophy by depleting immunological hormone[14].
Male livestocks are routinely castrated in most countries to prevent their unpleasant odour (known as boar taint), aggressive behavior and unplanned breeding. As we know, intact male animals have superior feed conversion and leaner carcasses than surgically castrated pigs[15]. Therefore, the problem is how to concurrently maintain both the intact of animals and the high quality of meat. If the similar strategy of anti-tumor agents mentioned above is applied to contraceptive vaccine, the problem can be possibly solved. Currently, scientists are trying to develop a substitute for the traditional surgical castration. Many preparations based on this theory have been applied to laboratory animals or pets for their immunological castration[1617]. It has been demonstrated that immunocastration can improve the meat quality and increase growth performance[18–20].
GnRH-PE40, one of the recombinant single-chain fusion proteins consisting of GnRH fused to a binding-defective form of pseudomonas aeruginosa exotoxin A (PE40), has been developed as a preparation with potential functions of immune castration in male reproductive system. We report here the long term usage of GnRH- based chimeric protein which substantially induces castration in male rat reproductive system.
GnRH-PE40 is a genetic engineering product consisting of PE and GnRH from our laboratory.
Rats (specific pathogen-free) of Wistar strain, weighing 180-200 g, bought from Animal Center of Military Academy of Medical Sciences (Beijing, PRC), were housed in plexiglass cages (5 per cage) at temperature of 22°C-26°C and humidity of 60% in a 12 h light/dark cycle with free access to food and water. The experimental protocol was approved by the Animal Research Committee of Jinan University.
Twenty male rats were randomly divided into treatment group and control group and received intraperitoneal injection of 150 &mgr;g/kg of GnRH-PE40 and saline natrium, respectively, every other day for 12 wk. The sexual behaviors of rats were evaluated 12 h after the last injection. The rats were sacrificed under pentobarbital anesthesia 24 h after the last injection. Blood was collected from the heart of comatose rats for hormone or antibody determination. Testes were taken out, weighed, and fixed for histopathological evaluation.
A 96-well microtiter plate was coated with 50 &mgr;L of 10 &mgr;g/mL of GnRH in carbonate bicarbonate buffer (CBB, pH 9.6) overnight at 4°C. After blocked with 3% bovine serum albumin (BSA) in PBS for 1 h at 37°C, the plate was incubated with diluted sera (1:100 to 1:12800) from the rats in different groups in 0.05% Tween 20/PBS with 0.3% (w/v) BSA for 1 h at 37°C. After washing, antibody was detected using horseradish peroxidase (HRP) conjugated goat anti-rat-IgG (BD Pharmingen, San Jose, CA, USA) for 1 h at 37°C. Signals were developed using DAB + substrate (Zhongshan Company, Beijing PRC) and optical density was determined at 490 nm using a BIO-RAD model 550 plate reader. Each measurement of a sample was conducted in duplicate. An absorbance equal to or greater than the mean + 3SE of the control group was considered positive.
Testosterone level in rat blood was measured by radioimmunoassay using a coat-A-count total testosterone kit (Diagnostic Products Corporation, Los Angeles, USA) according to its manufacturer’s instructions. Each measurement of a sample was conducted in duplicate.
Testes were fixed in Bouin’s solution overnight at 4°C, followed by embedding, sectioning, staining with haematoxylin and eosin, and finally examined histopatholo-gically under light microscope.
Ovariectomy was performed for female rats under ethyl ether anesthesia and 15 &mgr;g of estradiol benzoate was subcutaneously injected followed by 500 &mgr;g of progesterone 48 h later. Only those exhibiting a good sexual receptivity of male rats, that is, lordosis in response to mounting and with no reject behavior, were used.
The mating behavior of male rats was evaluated during the dark cycle in a sound-proof room, under a dim red light, according to the standard procedure[21]. After a 10-min adaptation period in a rectangular glass observation cage (60 cm × 50 cm × 40 cm), a stimulus female rat was introduced to a male rat by dropping it gently into the cage. Then following behavioral parameters were recorded or calculated: latency of mount, intromission or ejaculation, and number of mounts, intromissions or ejaculations in a 30-min observation period.
Data were expressed as mean ± SE. Where analysis of variance indicated significant differences between groups with ANOVA, for the preplanned comparison of interest, Student’s t test was applied utilizing the SPSS11.0 version. P < 0.05 was considered statistically significant.
ELISA analysis showed that GnRH-PE40 induced a high production of IgG antibody to GnRH in rats. All the animals treated with GnRH-PE40 showed a positive anti-GnRH response. The antibody titers ranged from 1:800 to 1:3200 with a median of 1:1600 (Table 1).
Intraperitoneal administration of GnRH-PE40 provoked a significant decrease in blood testosterone of rats. The average testosterone level in rats treated with GnRH-PE40 was 5.4 &mgr;g/mL compared with 20.3 &mgr;g/mL in control group as shown in Table 1 (P < 0.01).
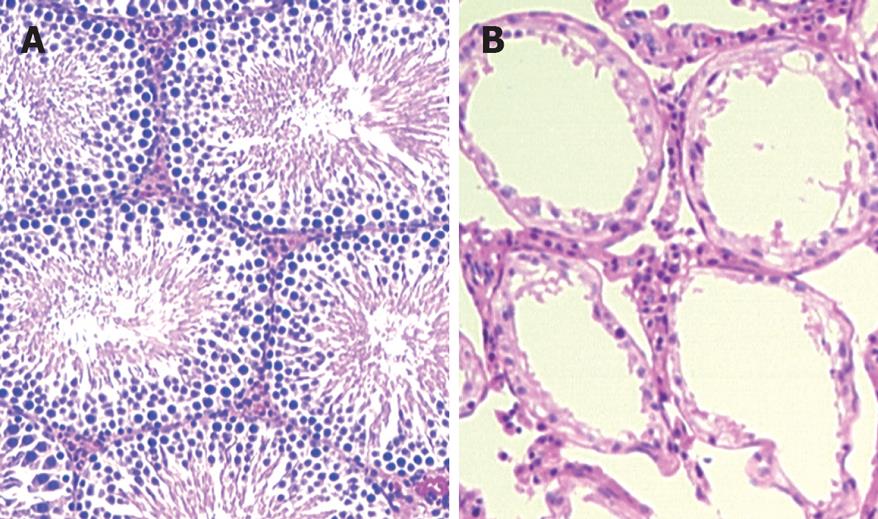
Histopathological examination showed that GnRH resulted in remarkable atrophy of testis in rats. The testis weight of rats treated with 150 &mgr;g/kg of GnRH-PE40 was decreased by 1.45-fold (P < 0.01) (Table 1). The seminiferous epithelium became thinner and the number of spermatogenic cells was decreased compared with that of control group (Figure 1).
Mating behavior test showed that GnRH-PE40 could improve mating performance of male rats. Compared with the controls, rats that received 150 &mgr;g/kg of GnRH-PE40 exhibited an increased mount and an intromission latency, but a reduced mount and an intromission frequency (P < 0.01). No successful ejaculation was observed in rats treated with GnRH-PE40, while the mean ejaculation number in controls was 2.4 in a 30-min observation period (Table 2).
GnRH, a short peptide in its natural form, has a poor immunogenicity. When conjugated to a large carrier, it is likely to become an antigen, inducing antibody response. BcePred is a web-based tool for predicting B cell epitope regions in the sequence of an antigen with physicochemical properties. Identified properties of B cell epitope include hydrophilicity, flexibility/mobility, accessibility, polarity, exposed surface and turns. Using this tool, we predicted GnRH motif of GnRH-PE40 with the above properties individually using the default thresholds suggested by software, and found a potential epitope with a higher polarity than the default threshold of 1.8 at GnRH motif[22]. We hypothesized that this potential epitope was capable of eliciting antibody reaction, which was verified by antibody assay, suggesting that GnRH-PE40 induces a high titer of anti-GnRH antibodies in sera of rats. Hormone determination and histopathological examination showed that intraperitoneal administration of GnRH-PE40 could decrease testosterone level, testis weight and sexual function, which are all indications of testis atrophy[23–25]. Production of GnRH antibody is possibly the cause for testis atrophy[26]. It is well-known that hypothalamus-pituitary-gonadal axis is involved in the control of normal reproductive cycle and maintaining of the structure and function of the reproductive system. Anti-GnRH antibodies reduce the concentration of serum GnRH, which triggers hormone cascades including a lower pituitary LH/FSH and testosterone release, thereby affecting the structure and function of sexual organs[27]. A high production of anti-GnRH antibodies in serum blocks primarily the function of GnRH, leading to the impairment of sexual organs[2829]. Studies showed that anti-GnRH antibodies induce testis atrophy in various laboratory animals and pets immunized with GnRH conjugates[1617].
In another study, we showed that 12-wk intravenous administration of GnRH-PE40 to monkeys produced only a low titer of anti-GnRH antibodies, but no marked testis atrophy was found (results not shown here), providing further evidence that antibodies against GnRH are the reason for testis atrophy.
In conclusion, GnRH-PE40 administration produces immunological castration by inducing anti-GnRH antibodies produced in response to the stimulation of B cell epitope of GnRH motif. GnRH-PE40 can be used in animal sciences as a non-surgical castration substitution for surgical castration[3031].
Surgical castration has been widely used as a routine way to prevent unpleasant odour and aggressive behavior of animals. Compared with surgical castration, immunocastration has more advantages such as easy operation in large scale, meat quality improvement and good animal welfare. Therefore, it is necessary to develop new drugs for animal immunocastration.
Scientists in institutions or pharmaceutical companies are engaged in developing new drugs for animal immunocastraion.
A new compound with potential use as an immunocastration agent was found and its physiological properties and actions were studied.
If the compound reported in this article can be used as an animal immunocastration agent, it will contribute greatly to farmers and production of high quality meat.
Castration means surgical removal or artificial destruction of gonads. Immunocastration refers to castration methods based on immunological processes and techniques, such as use of castration vaccines.
In the present study, the authors described the treatment of rats with GnRH-PE40, which resulted in the production of antibodies against GnRH, lower testosterone levels and sexual behavior. The study was well designed and its results strongly support that GnRH can be used as a potential castration agent.
| 1. | Fister S, Gunthert AR, Emons G, Grundker C. Gonadotropin-releasing hormone type II antagonists induce apoptotic cell death in human endometrial and ovarian cancer cells in vitro and in vivo. Cancer Res. 2007;67:1750-1756. |
| 2. | Hansel W, Enright F, Leuschner C. Destruction of breast cancers and their metastases by lytic peptide conjugates in vitro and in vivo. Mol Cell Endocrinol. 2007;260-262:183-189. |
| 3. | Nagy A, Schally AV. Targeting cytotoxic conjugates of somatostatin, luteinizing hormone-releasing hormone and bombesin to cancers expressing their receptors: a "smarter" chemotherapy. Curr Pharm Des. 2005;11:1167-1180. |
| 4. | Li J, Sun Y, Zhang J. A recombinant protein LHRH-PE40 for tumour therapy: preclinical safety studies. Basic Clin Pharmacol Toxicol. 2006;99:398-404. |
| 5. | Haggerty HG, Warner WA, Comereski CR, Peden WM, Mezza LE, Damle BD, Siegall CB, Davidson TJ. BR96 sFv-PE40 immunotoxin: nonclinical safety assessment. Toxicol Pathol. 1999;27:87-94. |
| 6. | FitzGerald D, Idziorek T, Batra JK, Willingham M, Pastan I. Antitumor activity of a thioether-linked immunotoxin: OVB3-PE. Bioconjug Chem. 1990;1:264-268. |
| 7. | Pai LH, Batra JK, FitzGerald DJ, Willingham MC, Pastan I. Antitumor effects of B3-PE and B3-LysPE40 in a nude mouse model of human breast cancer and the evaluation of B3-PE toxicity in monkeys. Cancer Res. 1992;52:3189-3193. |
| 8. | Pall MK, Mayer I, Borg B. Androgen and behavior in the male three-spined stickleback, Gasterosteus aculeatus. II. Castration and 11-ketoandrostenedione effects on courtship and parental care during the nesting cycle. Horm Behav. 2002;42:337-344. |
| 9. | Limonta P, Moretti RM, Marelli MM, Motta M. The biology of gonadotropin hormone-releasing hormone: role in the control of tumor growth and progression in humans. Front Neuroendocrinol. 2003;24:279-295. |
| 10. | Nechushtan A, Yarkoni S, Marianovsky I, Lorberboum-Galski H. Adenocarcinoma cells are targeted by the new GnRH-PE66 chimeric toxin through specific gonadotropin-releasing hormone binding sites. J Biol Chem. 1997;272:11597-11603. |
| 11. | Ben-Yehudah A, Yarkoni S, Nechushtan A, Belostotsky R, Lorberboum-Galski H. Linker-based GnRH-PE chimeric proteins inhibit cancer growth in nude mice. Med Oncol. 1999;16:38-45. |
| 12. | Ben-Yehudah A, Prus D, Lorberboum-Galski H. I.V. Administration of L-GNRH-PE66 efficiently inhibits growth of colon adenocarcinoma xenografts in nude mice. Int J Cancer. 2001;92:263-268. |
| 13. | Huang PS, Oliff A. Drug-targeting strategies in cancer therapy. Curr Opin Genet Dev. 2001;11:104-110. |
| 14. | Li J, Zhang J. Immunological hormone atrophy by gonadotropin-based drug. Int J Exp Pathol. 2006;87:495-499. |
| 15. | Zamaratskaia G, Rydhmer L, Andersson HK, Chen G, Lowagie S, Andersson K, Lundstrom K. Long-term effect of vaccination against gonadotropin-releasing hormone, using Improvactrade mark, on hormonal profile and behaviour of male pigs. Anim Reprod Sci. 2007;. |
| 16. | Carelli C, Audibert F, Gaillard J, Chedid L. Immunological castration of male mice by a totally synthetic vaccine administered in saline. Proc Natl Acad Sci USA. 1982;79:5392-5395. |
| 17. | Bonneau M, Dufour R, Chouvet C, Roulet C, Meadus W, Squires EJ. The effects of immunization against luteinizing hormone-releasing hormone on performance, sexual development, and levels of boar taint-related compounds in intact male pigs. J Anim Sci. 1994;72:14-20. |
| 18. | Dunshea FR, Colantoni C, Howard K, McCauley I, Jackson P, Long KA, Lopaticki S, Nugent EA, Simons JA, Walker J. Vaccination of boars with a GnRH vaccine (Improvac) eliminates boar taint and increases growth performance. J Anim Sci. 2001;79:2524-2535. |
| 19. | Gong SL, Zhao G, Zhao HG, Lu WT, Liu GW, Zhu P. Ability of luteinizing hormone releasing hormone-Pseudomonas aeruginosa exotoxin 40 binding to LHRH receptor on human liver cancer cells. World J Gastroenterol. 2004;10:2870-2873. |
| 20. | Wu GM, Zhu P, Li JZ. Cytotoxic Activity of GnRH-PE40 Injection to Human Tumor Cell Lines Cultured In Vitro. Chin J Biologicals. 2001;14:221-222. |
| 21. | Benelli A, Frigeri C, Bertolini A, Genedani S. Influence of mirtazapine on the sexual behavior of male rats. Psychopharmacology (Berl). 2004;171:250-258. |
| 22. | Saha S, Raghava GPS; BcePred: Prediction of Continuous B-Cell Epitopes in Antigenic Sequences Using Physicochemical Properties. In Nicosia G, Cutello V, Bentley PJ and Timis J, editors. ICARIS 2004, LNCS 3239, 197, Springer, 2004. Available from: URL: http://www.imtech.res.in/raghava/ bcepred/. |
| 23. | Hannesdottir SG, Han X, Lund T, Singh M, Van Der Zee R, Roitt IM, Delves PJ. Changes in the reproductive system of male mice immunized with a GnRH-analogue conjugated to mycobacterial hsp70. Reproduction. 2004;128:365-371. |
| 24. | Sprenkle PC, Fisch H. Pathologic effects of testosterone deprivation. Curr Opin Urol. 2007;17:424-430. |
| 25. | Millar RP, Lu ZL, Pawson AJ, Flanagan CA, Morgan K, Maudsley SR. Gonadotropin-releasing hormone receptors. Endocr Rev. 2004;25:235-275. |
| 26. | Ulker H, Kanter M, Gokdal O, Aygun T, Karakus F, Sakarya ME, deAvila DM, Reeves JJ. Testicular development, ultrasonographic and histological appearance of the testis in ram lambs immunized against recombinant LHRH fusion proteins. Anim Reprod Sci. 2005;86:205-219. |
| 27. | Huxsoll CC, Price EO, Adams TE. Testis function, carcass traits, and aggressive behavior of beef bulls actively immunized against gonadotropin-releasing hormone. J Anim Sci. 1998;76:1760-1766. |
| 28. | Parthasarathy V, Price EO, Orihuela A, Dally MR, Adams TE. Passive immunization of rams (Ovis aries) against GnRH: effects on antibody titer, serum concentrations of testosterone, and sexual behavior. Anim Reprod Sci. 2002;71:203-215. |
| 29. | Zeng XY, Turkstra JA, Meloen RH, Liu XY, Chen FQ, Schaaper WM, Oonk HB, Guo DZ, van de Wiel DF. Active immunization against gonadotrophin-releasing hormone in Chinese male pigs: effects of dose on antibody titer, hormone levels and sexual development. Anim Reprod Sci. 2002;70:223-233. |
| 30. | Zhang Y, Rozell TG, deAvila DM, Bertrand KP, Reeves JJ. Development of recombinant ovalbumin-luteinizing hormone releasing hormone as a potential sterilization vaccine. Vaccine. 1999;17:2185-2191. |
| 31. | Naz RK, Gupta SK, Gupta JC, Vyas HK, Talwar AG. Recent advances in contraceptive vaccine development: a mini-review. Hum Reprod. 2005;20:3271-3283. |