Published online Apr 7, 2008. doi: 10.3748/wjg.14.2065
Revised: December 14, 2007
Published online: April 7, 2008
AIM: To evaluate treatment safety and hemodynamic changes during a single 6-h treatment with the Prometheus™ liver assist system in a randomized, controlled study.
METHODS: Twenty-four patients were randomized to either the study group or to one of two control groups: Fractionated Plasma Separation Adsorption and Dialysis, Prometheus™ system (Study group; n = 8); Molecular Adsorbent Recirculation System (MARS)™ (Control group 1, n = 8); or hemodialysis (Control group 2; n = 8). All patients included in the study had decompensated cirrhosis at the time of the inclusion into the study. Circulatory changes were monitored with a Swan-Ganz catheter and bilirubin and creatinine were monitored as measures of protein-bound and water-soluble toxins.
RESULTS: Systemic hemodynamics did not differ between treatment and control groups apart from an increase in arterial pressure in the MARS group (P = 0.008). No adverse effects were observed in any of the groups. Creatinine levels significantly decreased in the MARS group (P = 0.03) and hemodialysis group (P = 0.04). Platelet count deceased in the Prometheus group (P = 0.04).
CONCLUSION: Extra-corporal liver support with Prometheus is proven to be safe in patients with end-stage liver disease but does not exert the beneficial effects on arterial pressure as seen in the MARS group.
- Citation: Dethloff T, Tofteng F, Frederiksen HJ, Hojskov M, Hansen BA, Larsen FS. Effect of Prometheus liver assist system on systemic hemodynamics in patients with cirrhosis: A randomized controlled study. World J Gastroenterol 2008; 14(13): 2065-2071
- URL: https://www.wjgnet.com/1007-9327/full/v14/i13/2065.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.2065
The fact that not all patients with end-stage liver disease are suitable for liver transplantation, and the shortage of grafts enhance the need for supportive liver therapy either to secure time to stabilize hepatic functions or to enable bridging to liver transplantation. Indeed, extra-corporal liver support has turned out to be a valuable supplement to standard medical therapy (SMT)[1]. Especially, albumin dialysis improves not only the general condition, but also both cardiovascular and renal function[2–7] as well as the degree of hepatic encephalopathy[189]. The Fractionated Plasma Separation, Adsorption and Dialysis (Prometheus™) system and the Molecular Adsorbent Recirculation System (MARS™) both represent such treatment modalities.
End-stage liver disease is often accompanied by a hyper-dynamic systemic circulation[10–13]. This circulatory change is caused by a variety of vasoactive factors, such as cytokines, prostacyclins, and nitric oxide[14–18]. Infection or bleeding, which are frequent complications in cirrhotic patients, increase the nitric oxide production and result in aggravation of the hyperdynamic circulation[19]. Studies comparing the hemodynamic alterations in stable patients with cirrhosis during extra-corporal intervention remain scarce though such knowledge might evidently improve patient safety and perhaps lessen reluctance towards the use of extra-corporal liver support at an early time in the treatment.
This randomized, controlled study was designed to clarify the hemodynamic effects of intervention with Prometheus™, using MARS™ and hemodialysis as control groups, in patients with end-stage liver disease.
The clinical and paraclinical characteristics of the patients included in the study are listed in Table 1. The study was approved by the Ethics Committee of Copenhagen (jr. nr. KF 01-186/04) and the study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki. Twenty-six patients (10 females and 16 males) were screened and 24 (9 females and 15 males) were enrolled in the study after obtaining written and oral consent from the patient or next-of-kin. All patients included in the study were under evaluation for liver transplantation and had a right-sided heart catheterization by a Swan-Ganz catheter. Former studies from our department have evaluated the effects of a 6-h albumin dialysis on patients with acute-on-chronic liver failure as well as patients with acute liver failure[2021]. Consequently, the present study was designed accordingly with a 6-h treatment to match and complete the former studies. Patients were divided into three groups each receiving a 6-h extra-corporal treatment: Prometheus treatment with MARS and hemodialysis as control groups. The randomization was performed using sealed, opaque envelopes containing a computerized sequence code. Envelopes were drawn by a third person not involved in the study.
| No | Randomization | Sex | Age(yr) | Cause | MELDscore | Child-Pughscore | Comagrade | INR | Bilirubin(&mgr;mol/L) | Albumin(g/L) | Creatinine(&mgr;mol/L) | Hg(g/L) | Platelet count(× 104/L) | 6-mooutcome |
| 1 | Hemodialysis | M | 51 | Alcoholic cirrhosis | ||||||||||
| 2 | Prometheus | M | 66 | Hemochromatosis | 24 | C/13 | II | 2.5 | 7.1 | 23.6 | 0.7 | 6.2 | 105 | Died |
| 3 | Prometheus | M | 60 | Alcoholic cirrhosis | 22 | B/9 | I | 1.5 | 2.8 | 39.8 | 2.1 | 5.2 | 126 | OLT/survived |
| 4 | MARS | M | 59 | Alcoholic cirrhosis | 25 | C/14 | I | 2.7 | 8.0 | 25.6 | 0.6 | 5.4 | 22 | Died |
| 5 | MARS | F | 44 | Alcoholic cirrhosis | 24 | C/13 | I | 2.2 | 11.9 | 17.8 | 0.8 | 6.0 | 148 | Survived |
| 6 | Hemodialysis | F | 54 | Alcoholic cirrhosis | 32 | C/14 | I | 2.7 | 41.0 | 19.3 | 0.7 | 6.5 | 66 | Died |
| 7 | Hemodialysis | F | 57 | PBC | ||||||||||
| 8 | Prometheus | M | 39 | Porphyria | 22 | C/10 | 0 | 1.4 | 22.8 | 31.8 | 0.4 | 8.0 | 70 | OLT/survived |
| 9 | Prometheus | F | 25 | Alcoholic cirrhosis | 31 | C/12 | 0 | 2.3 | 26.9 | 20.0 | 1.3 | 6.2 | 109 | OLT/survived |
| 10 | Prometheus | F | 59 | Autoimmune hepatitis | 35 | C/12 | I | 1.8 | 15.6 | 34.0 | 3.3 | 6.2 | 71 | Died |
| 11 | Prometheus | F | 49 | Alcoholic cirrhosis | 25 | C/12 | I | 2.4 | 9.6 | 29.2 | 0.7 | 7.1 | 63 | Survived |
| 12 | MARS | F | 43 | Alcoholic cirrhosis | 29 | C/12 | I | 1.8 | 35.8 | 25.0 | 1.3 | 6.3 | 123 | Survived |
| 13 | MARS | F | 45 | Cholest. stor. dis. | 22 | C/11 | I | 2.2 | 5.0 | 29.3 | 1.1 | 5.2 | 57 | OLT/survived |
| 14 | MARS | M | 59 | Autoimmune hepatitis | 26 | C/10 | 0 | 1.7 | 35.1 | 31.3 | 0.9 | 8.8 | 31 | Survived |
| 15 | MARS | M | 63 | Alcoholic cirrhosis | 11 | B/8 | 0 | 1.2 | 1.9 | 31.7 | 0.8 | 7.0 | 65 | Survived |
| 16 | Hemodialysis | M | 54 | Hepatitis C | 20 | C/10 | I | 1.9 | 1.1 | 35.2 | 1.8 | 7.5 | 87 | Survived |
| 17 | Hemodialysis | M | 67 | Alcoholic cirrhosis | 12 | B/9 | I | 1.4 | 1.6 | 30.4 | 0.7 | 6.6 | 91 | OLT/survived |
| 18 | Hemodialysis | M | 61 | Alcoholic cirrhosis | 12 | B/8 | I | 1.2 | 1.2 | 35.1 | 1.4 | 8.3 | 140 | Died |
| 19 | Prometheus | F | 63 | Unknown | 10 | B/8 | I | 1.3 | 1.1 | 40.8 | 0.8 | 8.0 | 146 | Survived |
| 20 | Prometheus | M | 57 | Alcoholic cirrhosis | 48 | C/13 | II | 6.0 | 36.6 | 28.5 | 2.3 | 6.0 | 74 | Died |
| 21 | MARS | M | 65 | Alcoholic cirrhosis | 18 | B/7 | 0 | 1.3 | 1.6 | 39.0 | 2.0 | 7.2 | 99 | Survived |
| 22 | MARS | M | 57 | Hepatitis C | 38 | C/12 | I | 2.2 | 36.8 | 32.1 | 2.5 | 5.8 | 37 | Died |
| 23 | Hemodialysis | M | 59 | Alcoholic cirrhosis | 23 | C/14 | I | 2.4 | 5.5 | 24.6 | 0.4 | 5.6 | 46 | Died |
| 24 | Hemodialysis | M | 52 | Alcoholic cirrhosis | 23 | C/10 | I | 1.5 | 4.0 | 41.8 | 0.5 | 6.8 | 221 | Survived |
Inclusion criteria were pre-existing liver disease with decompensated cirrhosis (verified by histological examination and/or CT/MRI scanning), ascites and a history of hepatic encephalopathy or repeated variceal bleeding.
Exclusion criteria were uncontrolled systemic or intracranial bleeding, uncontrolled systemic infection, extra-hepatic cholestasis, necrotic pancreatitis, cardiovascular failure necessitating > 0.05 &mgr;g/kg per minute of norepine-phrine, and a history of albumin dialysis within the last 7 d before entering the study.
All patients were treated in our liver ward but they were admitted to our liver intensive care unit for the duration of the extra-corporal treatment. Red blood cells, platelets and fresh frozen plasma were transfused according to the attending physician’s orders. Pre-treatment albumin levels were not corrected (Table 1). Measurements of pulmonary artery core temperature, cardiac output (CO), pulmonary artery mean pressure (PAPM), pulmonary artery wedged pressure (PAWP) and central venous pressure (CVP) were obtained through thermo dilution technique using a four-lumen balloon-tipped catheter (Swan-Ganz; Baxter, Copenhagen, Denmark). Using ultrasound supervision, all patients were equipped with a double-lumen catheter in the femoral vein (24 cm, med. COMP, Harlysville PA, USA) as well as a Swan-Ganz catheter in the right internal jugular vein.
Extra-corporal treatment: It was performed using the 4008 hemodialysis machine (Fresenius Medical Care Denmark A/S, Albertslund, Denmark) capable of performing both conventional hemodialysis as well as Prometheus treatments. The 4008 machine was also combined with a MARS monitor to perform MARS treatments. The thermostat of the machine was set to 36.5°C to avoid cooling of the patients. Patients in all three groups were treated with identical blood and dialysate flow rates (225 mL/min and 500 mL/min, respectively).
Anticoagulation: Citrate/calcium-anticoagulation was used for all patients using the 4008 hemodialysis machine’s built-in citrate/calcium algorithm. Calcium is automatically infused with a rate of 3.33 mmol citrate per litre of perfused blood. The citrate dose is calculated from the ionized calcium content in the patient’s venous blood. Venous blood samples were taken every 30 min and immediately analyzed in heparinized syringes (Radiometer, Copenhagen, Denmark). Blood tests before and after the treatment included creatinine, platelet count and bilirubin.
The Prometheus system removes albumin bound toxins in the patient’s blood by combining fractionated plasma separation and adsorption (FPSA) with conventional dialysis. The patient’s blood first passes trough a plasma separator with a pore size of 250 kDa. The filtered plasma fraction then passes over two adsorption columns, a neutral resin and an anion exchange resin adsorber, before it is filtered back to the systemic circulation. The blood then passes through a high-flux dialyzer (F60S, Fresenius, Denmark). The flow rate in the plasma circuit was set to 300 mL/min according to the manufacturer’s recommendations.
MARS is an extra-corporal high-flux hemofiltration that removes albumin-bound toxins from the blood over a specialized hybrid (albumin-impermeable) membrane into an albumin-enriched dialysate (500 mL of 200 g/L albumin). MARS combines a standard dialysis machine with a closed-loop albumin circuit, which is re-circulated by the MARS monitor (Gambro, Lyon, France). The albumin dialysate is passed through a conventional dialyzer and afterwards a charcoal and an anion-exchanger column. The flow rate in the closed albumin circuit was set to the maximum rate of 250 mL/min.
Hemodialysis was performed using a high-flux dialyzer (F60S, Fresenius, Denmark).
All measurements determined heart rate (HR), systolic/diastolic and mean arterial blood pressure (MAP), stroke volume (SV), CO, CVP, PAMP, and PAWP. Baseline measurements were performed 30 min before starting the extra-corporal treatment. Throughout the 6-h treatment, hemodynamic measurements were performed and registered approximately every hour. Blood samples before and after the treatment included platelet count, international normalized ratio (INR), bilirubin, creatinine and alanine transaminase (ALT). During the treatment, we monitored the venous levels of ionized calcium as well as potassium and sodium, hemoglobin, glucose and magnesium. If necessary, substitution by continuous infusion was performed.
Calculations were performed as follows: Cardiac index (CI) (L/min•m2) = CO divided by the body surface area. SV (mL/beat) = CO/HR; SVRI (DS/m2•cm5) = 80 × (MAP - CVP)/CI; PVRI (DS/m2•cm5) = 80 × (PAPM - PAWP)/CI. Unless stated differently, data were expressed as mean ± SEM.
Comparison within a group was performed using the paired t-test or Wilcoxon’s rank sum test. For comparison between groups, the one-way-ANOVA or Kruskal-Wallis rank sum test were applied. P values < 0.05 were considered statistically significant.
Of the 24 patients included, 22 patients completed the 6-h treatment without complications. Patient number 1 and 7, both randomized to hemodialysis treatment, dropped out of the study due to repeated clotting of the dialysis filters. During the treatment, we recorded no serious adverse events, i.e. no drop in MAP and no hemolysis or bleeding requiring therapeutic intervention. Patient number 15 from the MARS group experienced bleeding after removal of the dialysis catheter 10 h after termination of the treatment. None of the conscious patients complained of any discomfort that could be related to the extra-corporal treatment. Nine patients were discharged 2 to 19 d after the treatment; 5 patients underwent orthotopic liver transplantation (OLT) and were alive 6 mo after the transplantation, whereas 11 patients died within 4 to 143 d after participating in the study (Table 1). There was no statistical correlation between the study group and the 6-mo outcome (P = 0.397).
All hemodynamic measurements are listed in Table 2. A one-way analysis of variance between the three groups comparing baseline values of age, MELD score, INR, bilirubin, albumin, creatinine, hemoglobin, and platelets showed no significant differences. Pre-treatment MAP values were within the normal range (mean 76 mmHg; range 57-98 mmHg), while the other hemodynamic parameters were in accordance with the normal findings in end-stage liver disease: CO was elevated (mean 8.9 L/min; range 4.4-13.9 L/min), CI high (mean 4.6 L/min• m2; range 2.3-7.1 L/min•m2), and SVRI low (mean 1231 Ds*s/cm5•m2; range 139-3616).
| Hemodialysis | MARS | Prometheus | |||||
| Variables | Pre-treatment | Post-treatment | Pre-treatment | Post-treatment | Pre-treatment | Post-treatment | |
| CO | L/min | 8.26 ± 1.07 | 8.37 ± 1.01 | 8.66 ± 0.87 | 8.74 ± 0.88 | 9.52 ± 1.26 | 9.36 ± 1.4 |
| HR | beats/min | 74.5 ± 5.89 | 78.5 ± 8.45 | 79.5 ± 6.06 | 81.13 ± 6.53 | 82.25 ± 6.06 | 81.63 ± 6.88 |
| MAP | mmHg | 75.17 ± 6.99 | 76.5 ± 5.13 | 68.88 ± 3.82 | 78.13 ± 4.87 | 74 ± 3.52 | 74.38 ± 5 |
| PAPM | mmHg | 14.17 ± 2.98 | 12.83 ± 2.23 | 18.5 ± 2.25 | 19.88 ± 2.09 | 24.88 ± 5.71 | 23.25 ± 5.32 |
| PAWP | mmHg | 7.17 ± 1.56 | 5.83 ± 1.74 | 11.38 ± 1.61 | 13 ± 2.1 | 13.63 ± 2.24 | 14.63 ± 1.86 |
| CVPM | mmHg | 6.83 ± 2.8 | 3.83 ± 1.38 | 9.5 ± 2.15 | 8.63 ± 1.69 | 13.25 ± 1.81 | 12 ± 2.05 |
| SV | mL | 107.78 ± 11.86 | 112.18 ± 10.95 | 106.95 ± 8.78 | 107.56 ± 7.28 | 116.11 ± 13.54 | 110.91 ± 13.49 |
| CI | L/min•m2 | 4.12 ± 0.5 | 4.26 ± 0.49 | 4.71 ± 0.56 | 4.71 ± 0.51 | 4.9 ± 0.56 | 4.9 ± 0.67 |
| SVRI | DS*m2•cm5 | 1454.17 ± 478.92 | 1541.17 ± 353.82 | 1223.38 ± 344.76 | 1372.25 ± 271.5 | 1073.5 ± 126.69 | 1177.38 ± 194.2 |
| PVRI | DS*m2•cm5 | 138.5 ± 32.22 | 119.67 ± 29.05 | 121.5 ± 30.65 | 100.05 ± 28.13 | 236 ± 141.71 | 210.63 ± 133.63 |
Only small hemodynamic changes from pre- to post-treatment in all three groups were noted. However, the MARS group showed, during the treatment, a significant increase in the systolic and diastolic blood pressure of 10.5% and 15.2%, respectively. Heart rate, CI and SV remained constant.
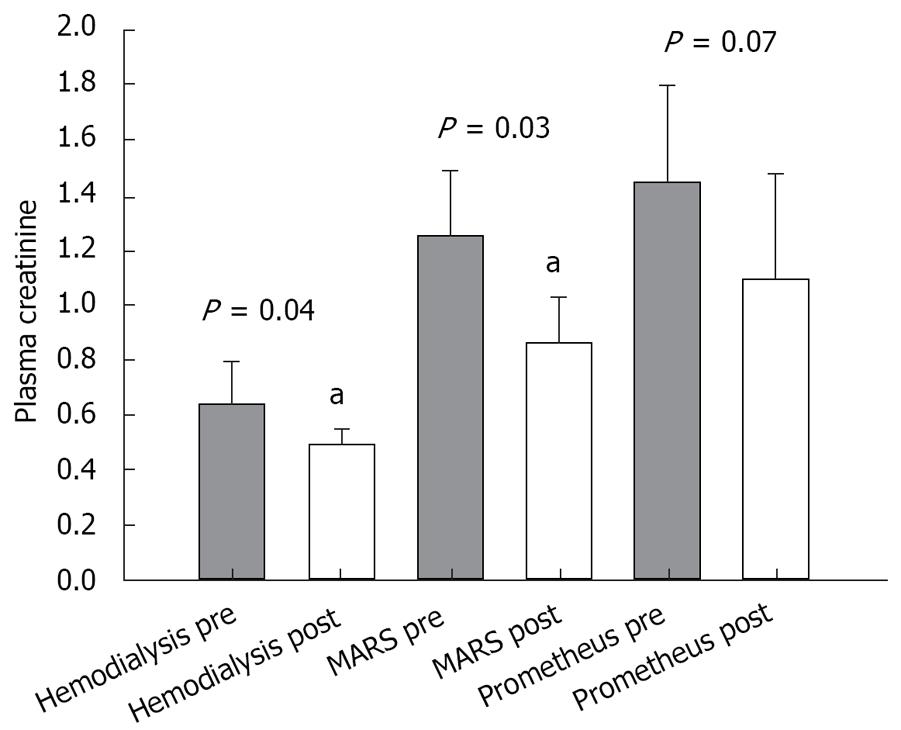
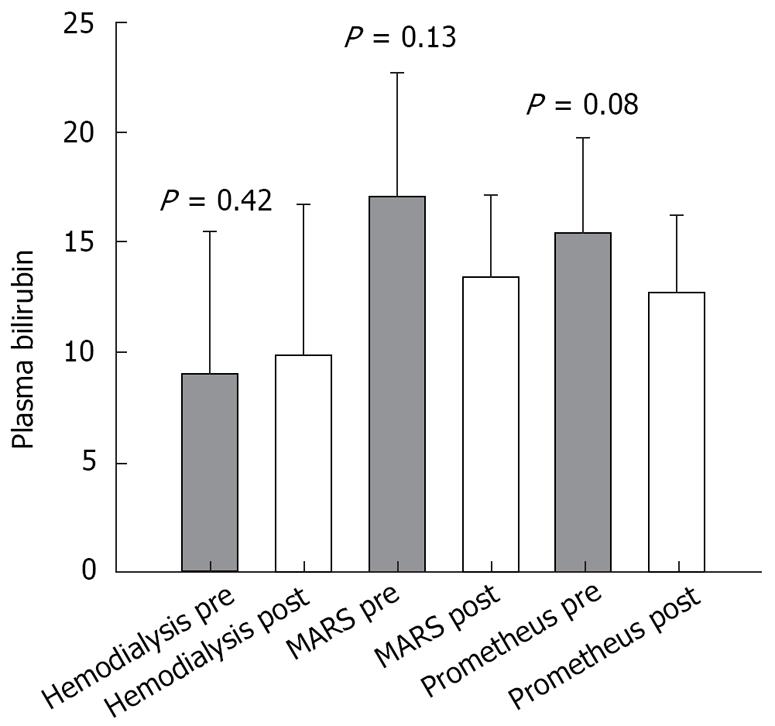
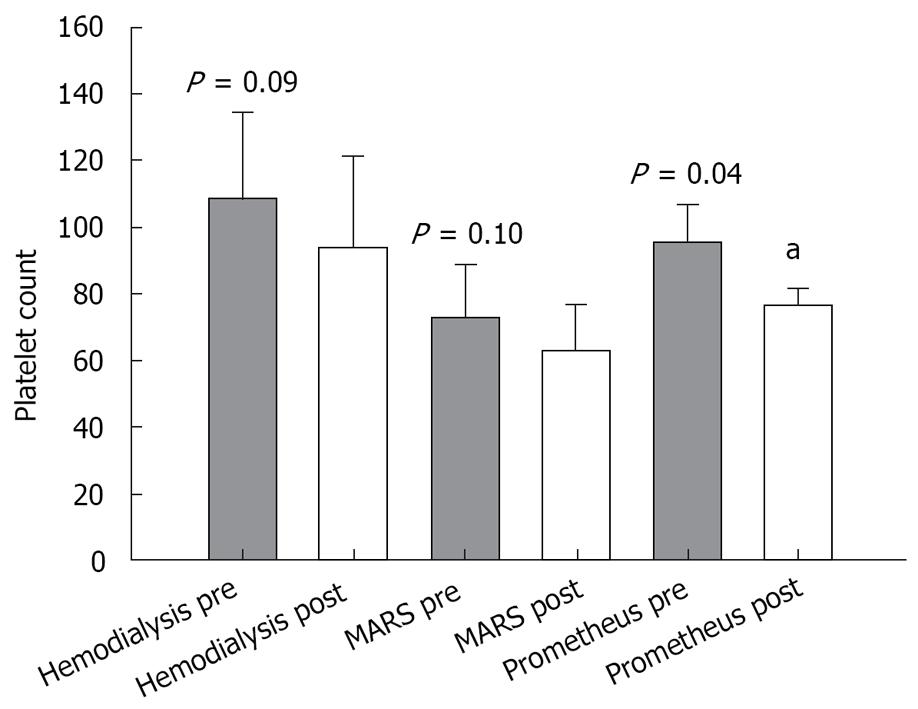
Paraclinical values of bilirubin, creatinine, and platelet count measured before and after the treatment were as follows: (1) Prometheus group: pre-treatment values: bilirubin (mean 15.3 × 10-2 g/L; SEM 4.4 × 10-2 g/L), creatinine (mean 1.44 × 10-2 g/L; SEM 0.35 × 10-2 g/L) and platelet count (mean 96 × 109/L; SEM 11 × 109/L); post-treatment values: bilirubin (mean 12.6 × 10-2 g/L; SEM 3.6 × 10-2 g/L), creatinine (mean 1.09 × 10-2 g/L; SEM 0.39 × 10-2 g/L) and platelet count (mean 76 × 109/L; SEM 11 × 109/L); (2) MARS group: pre-treatment values: bilirubin (mean 17.0 × 10-2 g/L; SEM 5.6 × 10-2 g/L), creatinine (mean 1.2 × 10-2 g/L; SEM 0.24 × 10-2 g/L) and platelet count (mean 73 × 109/L; SEM 16 × 109/L); post-treatment values: bilirubin (mean 13.4 × 10-2 g/L; SEM 3.8 × 10-2 g/L), creatinine (mean 0.86 × 10-2 g/L; SEM 0.17 × 10-2 g/L) (P = 0.03), and platelet count (mean 63 × 109/L; SEM 14 × 109/L). (3) Hemodialysis group: pre-treatment values: bilirubin (mean 9.1 × 10-2 g/L; SEM 6.4 × 10-2 g/L), creatinine (mean 0.64 × 10-2 g/L; SEM 0.16 × 10-2 g/L) and platelet count (mean 109 × 109/L; SEM 25 × 109/L); post-treatment values: bilirubin (mean 9.9 × 10-2 g/L; SEM 6.8 × 10-2 g/L), creatinine (mean 0.49 × 10-2 g/L; SEM 0.06 × 10-2 g/L) and platelet count (mean 94 × 109/L; SEM 28 × 109/L).
The results of the paired t-test comparing pre- and post-treatment values in each group are shown in Figures 1, 2, 3.
Anticoagulation was performed using citrate/calcium infusion. In all patients, we followed the 4008 machine’s built-in algorithm, which calculates the citrate infusion rate from the patient’s ionized calcium. Both during and after the treatment, none of the patients required calcium or citrate supplements and no correction of the pH was necessary.
In this study, we compared the systemic circulatory changes during a single treatment of Prometheus with MARS and hemodialysis as control groups in patients with end-stage liver disease. The hemodynamic differences between the three groups showed insignificant differences besides a rise in MAP in the MARS treated group. None of the patients experienced any serious adverse events. Though we have some experience both about the clinical and the hemodynamic effects of the available extra-corporal treatments, i.e. MARS and Prometheus, there is a growing recognition that patients may benefit from the initiation of extra-corporal liver support before treatment for multi-organ failure in the ICU setting is needed[22].
We have been using both MARS and Prometheus for several years in our liver failure unit. Former studies from our unit have determined the hemodynamic changes during a single 6-h MARS treatment both in patients with acute-on-chronic liver failure (AoCLF) as well as hyper acute liver failure[2021]. None of the patients in this study had an acute exacerbation at the time of randomization. Thus our patients represent a group that differs from AoCLF patients but can be characterized as having a chronic liver failure with decompensation. The Child-Pugh score for our patients supports this view with 6 group B and 18 group C patients.
Arterial hypotension is a well-described adverse effect induced by hemodialysis. In the light of this fact, it is interesting that hemodynamic studies have indicated that the MARS system exerts a beneficial influence on CO, SV, SVRI[20–23] and MAP[20–26]. The present study cannot demonstrate such beneficial changes, neither in the Prometheus nor in the hemodialysis group; however, there was a significant increase in MAP in the MARS group. The overall lack of significant hemodynamic changes (especially on CO, SV, and SVRI as would have been expected in the MARS group) could be attributed to the fact that the patients in the present study were treated early before deterioration of their chronic liver disease: the plasma bilirubin in our patient group was 42% of the value seen in the study by Schmidt et al[20] and 48% of the mean value of all three groups in the study by Laleman et al[2].
Both the Prometheus and MARS systems are capable of removing both albumin-bound as well as water-soluble substances. As shown in Figure 2, both albumin dialysis systems, as expected, decreased the plasma bilirubin level but the removal did not reach statistical significance. This was most likely due to heterogeneity regarding pre-treatment bilirubin levels among our group of patients and a low pre-treatment bilirubin level. In addition, the absolute amount of bilirubin eliminated during a treatment depends on blood concentration: higher pre-treatment values will yield higher clearances.
The toxin concentration in the blood is generally thought to cause the hyperdynamic circulation seen in liver failure. If we consider the bilirubin level merely as an indicator for the general level of albumin-bound toxins in the blood, it could also explain why potential positive hemodynamic effects could not be demonstrated. In our study, the pre-treatment bilirubin level was low and the toxin level would accordingly also be low. Therefore, no significant removal of bilirubin/toxins could be achieved and possible beneficial hemodynamic changes would thus become difficult to demonstrate.
No significant removal of creatinine could be demonstrated in the Prometheus group (Figure 1). This is most likely due to a low pre-treatment concentration and a high standard deviation as mentioned above. As expected, the survival of the patients was not statistically correlated to the randomized study group.
Regarding the safety of the treatment, none of the patients experienced arterial hypotension requiring cessation of the treatment. As a standard routine, the initial blood flow on the 4008 machine was set to 120 mL/min for the first 10 min and was then increased to 225 over the next 5-10 min. As mentioned, one episode of post-treatment bleeding occurred. The event was related to the removal of the dialysis catheter and occurred despite vessel compression for 15 min and a 30-min rest after dialysis-catheter removal, all according to standard guidelines.
Both the study group and the two control groups exhibited a drop in platelet count, yet, it was only statistically significant in the Prometheus group (Figure 3). Low platelet count is well known in cirrhotic patients. In addition, thrombelastography (TEG) often discloses dysfunction of the platelets. Consequently, monitoring the platelet count closely before, during, and after an extra-corporal treatment, especially when using Prometheus treatment, seems advisable as well as substitution, if necessary. Two patients in the hemodialysis group dropped out of the study due to repeated clotting. Patient number 7 showed obvious signs of hyper-coagulation: apart from clotting three hemodialysis filters, the dialysis catheter also clotted during a blood pump stop of 3-5 min. The clotting problems in the hemodialysis group opposed our former clinical experience, which had pointed at patients treated with the Prometheus system in combination with citrate anticoagulation as being most prone to clotting problems. The paraclinical data from patient number 1 and 7 did not account for the repeated clotting. Apart from the reported data, the activated prothrombin time values were within normal range for both patients (data not shown).
In conclusion, the decision to use extra-corporal liver support in the treatment of patients with end-stage liver disease hinges on many factors, with safety considerations as a major concern. The choice of treatment will depend on the risk of adverse events, and possible positive or negative hemodynamic influences between the available treatment modalities. Our study adds to the clarification of these considerations in showing that the Prometheus system does not aggravate the systemic hemodynamics; however, in our study, Prometheus does not exert an equally beneficial influence on MAP as seen during MARS treatment. We conclude that an intervention using extra-corporal liver support with albumin-dialysis should not be withheld merely because of safety considerations.
Patients with end-stage liver disease often need supportive liver therapy either to secure time to stabilize hepatic functions or to enable bridging to liver transplantation. Albumin dialysis improves not only the general condition, but also both cardiovascular and renal function as well as the degree of hepatic encephalopathy. Studies comparing the hemodynamic alterations in stable patients with cirrhosis during extra-corporal intervention remain scarce though such knowledge might evidently improve patient safety and perhaps lessen reluctance towards the use of extra-corporal liver support at an early time in the treatment. This randomized, controlled study was designed to clarify the hemodynamic profile of intervention with Prometheus™, using MARS™ and hemodialysis as control groups, in patients with end-stage liver disease.
This paper is related to the scientific efforts to develop clinically valuable artificial liver assist devices to support patients with fulminant liver failure.
http://www.ncbi.nlm.nih.gov/sites/entrez. See under “liver assist devices” or Sen S, Williams R, Jalan R. Emerging indications ofr albumin dialysis. Am J Gastroenterol 2005; 100: 468-475.
The decision to use extra-corporal liver support in the treatment of patients with end-stage liver disease hinges on many factors, with safety considerations as a major concern. The choice of treatment will depend on the risk of adverse events, and possible positive or negative hemodynamic influences between the available treatment modalities. Our study adds to the clarification of these considerations in showing that the Prometheus system does not aggravate the systemic hemodynamics; however, in our study, Prometheus does not exert an equally beneficial influence on arterial pressure as seen during MARS treatment. We conclude that an intervention using extra-corporal liver support with albumin-dialysis should not be withheld merely because of safety considerations.
Acute-on-chronic liver failure is defined as an acute deterioration in liver function in a patient with cirrhosis that results in dysfunction of other organs, such as the brain or the kidneys.
This is an interesting and well performed study. The abstract gives a clear delineation of the research background, aim, materials and methods, results and conclusion. The design of the study is rational and reliable. The work is of the practical importance.
| 1. | Hassanein TI, Tofteng F, Brown RS Jr, McGuire B, Lynch P, Mehta R, Larsen FS, Gornbein J, Stange J, Blei AT. Randomized controlled study of extracorporeal albumin dialysis for hepatic encephalopathy in advanced cirrhosis. Hepatology. 2007;46:1853-1862. |
| 2. | Laleman W, Wilmer A, Evenepoel P, Elst IV, Zeegers M, Zaman Z, Verslype C, Fevery J, Nevens F. Effect of the molecular adsorbent recirculating system and Prometheus devices on systemic haemodynamics and vasoactive agents in patients with acute-on-chronic alcoholic liver failure. Crit Care. 2006;10:R108. |
| 3. | Stefoni S, Coli L, Bolondi L, Donati G, Ruggeri G, Feliciangeli G, Piscaglia F, Silvagni E, Sirri M, Donati G. Molecular adsorbent recirculating system (MARS) application in liver failure: clinical and hemodepurative results in 22 patients. Int J Artif Organs. 2006;29:207-218. |
| 4. | Jalan R, Sen S, Steiner C, Kapoor D, Alisa A, Williams R. Extracorporeal liver support with molecular adsorbents recirculating system in patients with severe acute alcoholic hepatitis. J Hepatol. 2003;38:24-31. |
| 5. | Lai WK, Haydon G, Mutimer D, Murphy N. The effect of molecular adsorbent recirculating system on pathophysiological parameters in patients with acute liver failure. Intensive Care Med. 2005;31:1544-1549. |
| 6. | Sorkine P, Ben Abraham R, Szold O, Biderman P, Kidron A, Merchav H, Brill S, Oren R. Role of the molecular adsorbent recycling system (MARS) in the treatment of patients with acute exacerbation of chronic liver failure. Crit Care Med. 2001;29:1332-1336. |
| 7. | Sen S, Williams R, Jalan R. Emerging indications for albumin dialysis. Am J Gastroenterol. 2005;100:468-475. |
| 8. | Camus C, Lavoue S, Gacouin A, Le Tulzo Y, Lorho R, Boudjema K, Jacquelinet C, Thomas R. Molecular adsorbent recirculating system dialysis in patients with acute liver failure who are assessed for liver transplantation. Intensive Care Med. 2006;32:1817-1825. |
| 9. | Sen S, Davies NA, Mookerjee RP, Cheshire LM, Hodges SJ, Williams R, Jalan R. Pathophysiological effects of albumin dialysis in acute-on-chronic liver failure: a randomized controlled study. Liver Transpl. 2004;10:1109-1119. |
| 10. | Catalina MV, Barrio J, Anaya F, Salcedo M, Rincon D, Clemente G, Banares R. Hepatic and systemic haemodynamic changes after MARS in patients with acute on chronic liver failure. Liver Int. 2003;23 Suppl 3:39-43. |
| 11. | Schmidt LE, Svendsen LB, Sorensen VR, Hansen BA, Larsen FS. Cerebral blood flow velocity increases during a single treatment with the molecular adsorbents recirculating system in patients with acute on chronic liver failure. Liver Transpl. 2001;7:709-712. |
| 12. | Clemmesen JO, Larsen FS, Ejlersen E, Schiodt FV, Ott P, Hansen BA. Haemodynamic changes after high-volume plasmapheresis in patients with chronic and acute liver failure. Eur J Gastroenterol Hepatol. 1997;9:55-60. |
| 13. | Larsen FS, Ejlersen E, Hansen BA, Mogensen T, Tygstrup N, Secher NH. Systemic vascular resistance during high-volume plasmapheresis in patients with fulminant hepatic failure: relationship with oxygen consumption. Eur J Gastroenterol Hepatol. 1995;7:887-892. |
| 14. | Liu H, Gaskari SA, Lee SS. Cardiac and vascular changes in cirrhosis: pathogenic mechanisms. World J Gastroenterol. 2006;12:837-842. |
| 15. | Wang JJ, Gao GW, Gao RZ, Liu CA, Ding X, Yao ZX. Effects of tumor necrosis factor, endothelin and nitric oxide on hyperdynamic circulation of rats with acute and chronic portal hypertension. World J Gastroenterol. 2004;10:689-693. |
| 16. | Guarner C, Soriano G. Prostaglandin and portal hypertension. Prostaglandins Leukot Essent Fatty Acids. 1993;48:203-206. |
| 18. | Wong F, Bernardi M, Balk R, Christman B, Moreau R, Garcia-Tsao G, Patch D, Soriano G, Hoefs J, Navasa M. Sepsis in cirrhosis: report on the 7th meeting of the International Ascites Club. Gut. 2005;54:718-725. |
| 19. | Mohammed NA, Abd El-Aleem S, Appleton I, Maklouf MM, Said M, McMahon RF. Expression of nitric oxide synthase isoforms in human liver cirrhosis. J Pathol. 2003;200:647-655. |
| 20. | Schmidt LE, Sorensen VR, Svendsen LB, Hansen BA, Larsen FS. Hemodynamic changes during a single treatment with the molecular adsorbents recirculating system in patients with acute-on-chronic liver failure. Liver Transpl. 2001;7:1034-1039. |
| 21. | Schmidt LE, Wang LP, Hansen BA, Larsen FS. Systemic hemodynamic effects of treatment with the molecular adsorbents recirculating system in patients with hyperacute liver failure: a prospective controlled trial. Liver Transpl. 2003;9:290-297. |
| 22. | Chiu A, Fan ST. MARS in the treatment of liver failure: controversies and evidence. Int J Artif Organs. 2006;29:660-667. |
| 23. | Lai WK, Haydon G, Mutimer D, Murphy N. The effect of molecular adsorbent recirculating system on pathophysiological parameters in patients with acute liver failure. Intensive Care Med. 2005;31:1544-1549. |
| 24. | Sorkine P, Ben Abraham R, Szold O, Biderman P, Kidron A, Merchav H, Brill S, Oren R. Role of the molecular adsorbent recycling system (MARS) in the treatment of patients with acute exacerbation of chronic liver failure. Crit Care Med. 2001;29:1332-1336. |
| 25. | Jalan R, Sen S, Steiner C, Kapoor D, Alisa A, Williams R. Extracorporeal liver support with molecular adsorbents recirculating system in patients with severe acute alcoholic hepatitis. J Hepatol. 2003;38:24-31. |
| 26. | Stefoni S, Coli L, Bolondi L, Donati G, Ruggeri G, Feliciangeli G, Piscaglia F, Silvagni E, Sirri M, Donati G. Molecular adsorbent recirculating system (MARS) application in liver failure: clinical and hemodepurative results in 22 patients. Int J Artif Organs. 2006;29:207-218. |