Published online Apr 7, 2008. doi: 10.3748/wjg.14.2029
Revised: February 21, 2008
Published online: April 7, 2008
AIM: To evaluate the effects of three potentially anti-inflammatory probiotic bacteria from three different genera on immune variables in healthy adults in a clinical setting based on previous in vitro characterization of cytokine responses.
METHODS: A total of 62 volunteers participated in this randomized, double-blind and placebo-controlled parallel group intervention study. The volunteers were randomized to receive a milk-based drink containing either Lactobacillus rhamnosus GG (LGG), Bifidobacterium animalis ssp. lactis Bb12 (Bb12), or Propionibacterium freudenreichii ssp. shermanii JS (PJS) or a placebo drink for 3 wk. Venous blood and saliva samples were taken at baseline and on d 1, 7 and 21. Fecal samples were collected at baseline and at the end of intervention.
RESULTS: The serum hsCRP expressed as the median AUC0-21 (minus baseline) was 0.018 mg/L in the placebo group, -0.240 mg/L in the LGG group, 0.090 mg/L in the Bb12 group and -0.085 mg/L in the PJS group (P = 0.014). In vitro production of TNF-α from in vitro cultured peripheral blood mononuclear cells (PBMC) was significantly lower in subjects receiving LGG vs placebo. IL-2 production from PBMC in the Bb12 group was significantly lower compared with the other groups.
CONCLUSION: In conclusion, probiotic bacteria have strain-specific anti-inflammatory effects in healthy adults.
- Citation: Kekkonen RA, Lummela N, Karjalainen H, Latvala S, Tynkkynen S, Järvenpää S, Kautiainen H, Julkunen I, Vapaatalo H, Korpela R. Probiotic intervention has strain-specific anti-inflammatory effects in healthy adults. World J Gastroenterol 2008; 14(13): 2029-2036
- URL: https://www.wjgnet.com/1007-9327/full/v14/i13/2029.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.2029
Probiotics are defined as living microorganisms that have beneficial effects on human health[1]. The immunomodula-tory effects of probiotics have mostly been studied in certain disease conditions, such as allergies[2] and inflammatory diseases[34], though the general, healthy population mostly consumes probiotics. The immunomodulatory effects of probiotics in healthy populations have not been fully established and only a few randomized, double blind, placebo-controlled studies have addressed this question[5–9]. Also, there are few studies where the effects of different probiotic bacteria have been compared within the same clinical setting. Isolauri et al[10] and Viljanen et al[11] have compared the effects of two different probiotics or a probiotic mixture with placebo in allergic infants. Schiffrin et al[12] and Gill et al[13] evaluated the effects of two different probiotics in healthy adults, but these studies did not have a placebo group. Efforts trying to compare the in vitro results of one probiotic to its results in an in vivo setting are even more scarce and are at the moment limited to comparisons between in vitro and experimental animal studies[14–16].
In our previous studies, we have characterized the capacity of potentially probiotic bacteria to induce cytokine production in human leukocyte cell culture and found that probiotic bacteria direct immune responses to either the Th1 type or the anti-inflammatory direction in a manner specific to the bacterial genera[17]. Based on these findings we selected probiotic bacteria from three different genera for the present study and compared their effects on immune variables in healthy adults in a 3-wk intervention trial.
The subjects were healthy adults recruited by an advertisement in the Helsinki area. The inclusion criteria were to be healthy (no chronic illnesses), to exercise regularly (at least three times per week), and to not be participating in any other clinical trials. The exclusion criteria was comprised of milk allergies (due to the nature of the study products), use of antibiotics during the two months before the study, acute gastrointestinal disorders during the two months before the study, gastrointestinal diseases and related medications, pregnancy, and lactation. Before entering the study, the subjects gave their written informed consent. The study protocol was approved by the Ethics Committee of the Hospital District of Helsinki and Uusimaa.
A total of 68 subjects were recruited for the study. Six subjects withdrew from the study during the run-in period and were not included in the analysis. The mean age for the subjects was 44 years (range 23-58) and their mean BMI was 24 kg/m2 (range 18-30). Of these 62 subjects (45 females, 17 males), one subject withdrew from the study due to a back injury after two study visits and one subject due to an antibiotic treatment after four study visits. These two subjects were included in the statistical analysis.
The study was a randomized, double-blind and placebo-controlled parallel group intervention study. Prior to the intervention period, there was a 3-wk run-in period during which no probiotic-containing products were allowed. Thereafter the subjects received either Lactobacillus rhamnosus GG (n = 13), Bifidobacterium animalis ssp. lactis Bb12 (n = 16), Propionibacterium freudenreichii ssp. shermanii JS (n = 17) or placebo (n = 16) drink for 3 wk. After the intervention period, subjects were followed up for 3 wk without any study drink. A list of probiotic-containing products was given to the subjects, and they were asked not to consume any other probiotic-containing products at any time during the study.
The subjects were advised to consume a 250 mL milk-based fruit drink daily for 3 wk containing either: L. rhamnosus GG (ATCC 53103) (LGG) bacteria, on average 6.2 × 107 cfu/mL (daily dose of 1.6 × 1010 cfu); B. animalis ssp. lactis Bb12 (Bb12) bacteria, 1.4 × 108 cfu/mL (daily dose of 3.5 × 1010 cfu); P. freudenreichii ssp. shermanii JS (DSM 7067) (PJS) bacteria, 1.3 × 108 cfu/mL (daily dose of 3.3 × 1010 cfu); or a placebo drink without any probiotic bacteria. The subjects consumed the study drinks throughout the 3-wk intervention period after the baseline blood sampling. The amount of probiotic bacteria in the study drinks was measured right after packaging and after 3 wk. The appearance and taste of the study drinks were the same.
Venous blood samples from the antecubital vein were taken at baseline, on 1, 7 and 21 d, and after the 3-wk follow-up period after an overnight fast. The samples were taken into standard serum tubes and EDTA tubes, centrifuged, and the plasma/serum was collected and stored at -20°C for further analyses. Three EDTA tubes were used in the purification of PBMC.
Blood cells and immunoglobulins: Blood cells (leukocytes, monocytes, and lymphocytes) from all time points were determined using an electronic counter (Coulter MAXM Hematology Analyzer, Beckman Coulter, Fullerton, CA, USA). Immunoglobulins (IgA, IgG and IgM) from all time points were measured by immunoturbidimetric method with Tina-quant Roche/Hitachi System reagent using a Roche Hitachi 912 analyzer (Roche Diagnostics GmbH, Mannheim, Germany).
Highly sensitive C-reactive protein: Serum levels of C-reactive protein (CRP) were measured at all time points by a highly sensitive particle-enhanced immunoturbidimetric CRP (hsCRP) assay using a Tina-quant C-reactive protein (latex) high sensitive reagent and a Roche Hitachi 912 analyzer (Roche Diagnostics GmbH) with a detection limit of 0.04 mg/L.
Cytokine levels from serum: Baseline and 21 d cytokine levels (TNF-α, IL-6, IFN-γ and IL-10) in serum were determined using Quantikine HS, Human TNF-α/TNFSF1A (Catalog Number HSTA00D), IL-6 (HS600B), IFN-γ (DIF50) and IL-10 (HS100B) immunoassays purchased from R&D Systems (Minneapolis MN, USA). These assays were carried out according to the manufacturer’s instructions. The detection limit was 0.5 pg/mL for TNF-α, 0.16 pg/mL for IL-6, 15.6 pg/mL for IFN-γ and 0.78 pg/mL for IL-10. For TNF-α, 94% of the samples were over the detection limit, and for IL-6, 89%. For statistical analyses, a detection limit divided by two was given as a value for those samples under the detection limit. None of the IFN-γ samples and only 39% of the IL-10 samples was over the detection limit and were therefore not further analyzed.
Purification: Human PBMC were purified by density gradient centrifugation over a Ficoll-Paque gradient (Amersham-Pharmacia Biotech, Uppsala, Sweden), as described previously[18], from freshly collected EDTA blood on the study days (baseline, d 1, 7 and 21 wk and 3 wk after intervention). After washing, the cells were resuspended in RPMI 1640 medium (Sigma, USA) containing 10% heat-inactivated fetal calf serum (FCS) (Integro, Zaandam, Holland) and supplemented with 2 mmol/L L-glutamine (Sigma), 100 U/mL penicillin and 100 &mgr;g/mL streptomycin (Gibco BRL, Paisley, Scotland). In stimulation experiments, purified leukocytes (2 × 106 cells/mL) were incubated with stimulants in a final volume of one ml in 24-well plates (Nunc, Roskilde, Denmark) for 24 h in 5% CO2 at 37°C.
Stimulations: During the stimulation experiments, the PBMC were maintained in RPMI-1640 medium containing 10% FCS. PBMC were left unstimulated or were stimulated with one of three different stimulants, simulating Gram-positive bacteria, a Gram-negative bacteria or a virus. Live Group A streptococci S. pyogenes serotype T1M1 obtained from the National Public Health Institute, Helsinki, Finland, grown as previously described[19], was used as a Gram-positive bacteria at 1:1 host-cell:bacteria ratio; lipopolysaccharide (LPS) from E. coli serotype 0111:B4 (L-3024, Sigma) was used as a model for Gram-negative bacteria at a concentration of 100 ng/mL; and Influenza A H3N2 virus (A/Beijing/353/89) was used to infect cells at a multiplicity of infection of 5. Cell culture supernatants were collected individually at the 24 h time point and stored at -20°C before analysis.
Cytokine levels from cell culture supernatants of stimulated PBMC: Cytokine levels (TNF-α, IFN-γ, IL-1β, Il-2, IL-4, IL-5, IL-6, IL-8, IL-10 and IL-12p70) in cell culture supernatants from each time point (baseline, 1 d, 7 d, 21 d and 3 wk after intervention) were determined using the FlowCytomix human Th1/Th2 10 plex kit II(BMS716FFCE) from Bender MedSystems (Vienna, Austria) according to manufacturer’s instructions. The detection limit was 4.5 pg/mL for IL-1β, 8.9 pg/mL for IL-2, 6.4 pg/mL for IL-4, 5.3 pg/mL for IL-5, 4.7 pg/mL for IL-6, 6.4 pg/mL for IL-8, 6.9 pg/mL for IL-10, 7.9 pg/mL for TNF-α, 9.7 pg/mL for IL-12p70 and 7.0 pg/mL for IFN-γ. Only those cytokines from which over 80% of the samples were above the detection limit were statistically analyzed. Therefore, all unstimulated samples, IL-4 and IL-5 in all stimulated samples, and IFN-γ in LPS stimulated samples were not included in further analyses. For statistical analyses, samples under the detection limit were replaced by the values obtained by dividing the detection limit by two.
An unstimulated saliva sample was taken at every visit (at baseline, d 1, 7 and 21 and 3 wk after the intervention) after the blood sampling. The saliva samples were placed in Eppendorf tubes, chilled, and stored at -20°C until secretory IgA was analyzed. SIgA from saliva was determined with an ELISA assay (catalog number K8870) purchased from Gentaur (Brussels, Belgium) according to the manufacturers’ instructions.
The fecal samples were collected at home at baseline and at the end of the 3-wk intervention period. Immediately after the collection the subjects were asked to deep-freeze (-20°C) the samples at home. They were subsequently transported to the study center on the morning of the study day and the samples were immediately put on dry ice and stored at -70°C until analysis. The amounts of the probiotic strains L. rhamnosus GG, B. animalis ssp. lactis Bb12 and P. freudenreichii ssp. shermanii JS in the fecal samples were analyzed with a previously described real-time quantitative PCR method[20].
Subjects were asked to fill in a structured study diary throughout the study. The study diary included questions about the use of the study product, the presence of any symptoms of respiratory infection, gastrointestinal symptoms or any other symptoms, the amount of exercise, and the use of any medication. No respiratory tract infections or major symptoms were recorded by the subjects during the study. The amount of weekly exercise carried out by the study subjects remained the same throughout the study.
The intention-to-treat population (all randomized patients who took at least one dose of the study product) was included in the analysis. The last-observation-carried-forward (LOCF) approach was used for missing data and for subjects who withdrew early.
The main outcome measures were the serum hsCRP levels and the cytokines produced by PBMCs. The responses for these outcomes were calculated as the area under the curve from the 0, 1 d, 7 d and 21 d, subtracted by the baseline value (AUC0-21 minus baseline).
Data is presented as mean with standard deviation (SD) or as median with interquartile range (IQR). The differences between the groups were tested using the Kruskal-Wallis test or median regression analysis with Holm’s adjustment for pair wise comparisons. A P-value below 0.05 was regarded as statistically significant, but no adjustment was made for multiple testing.
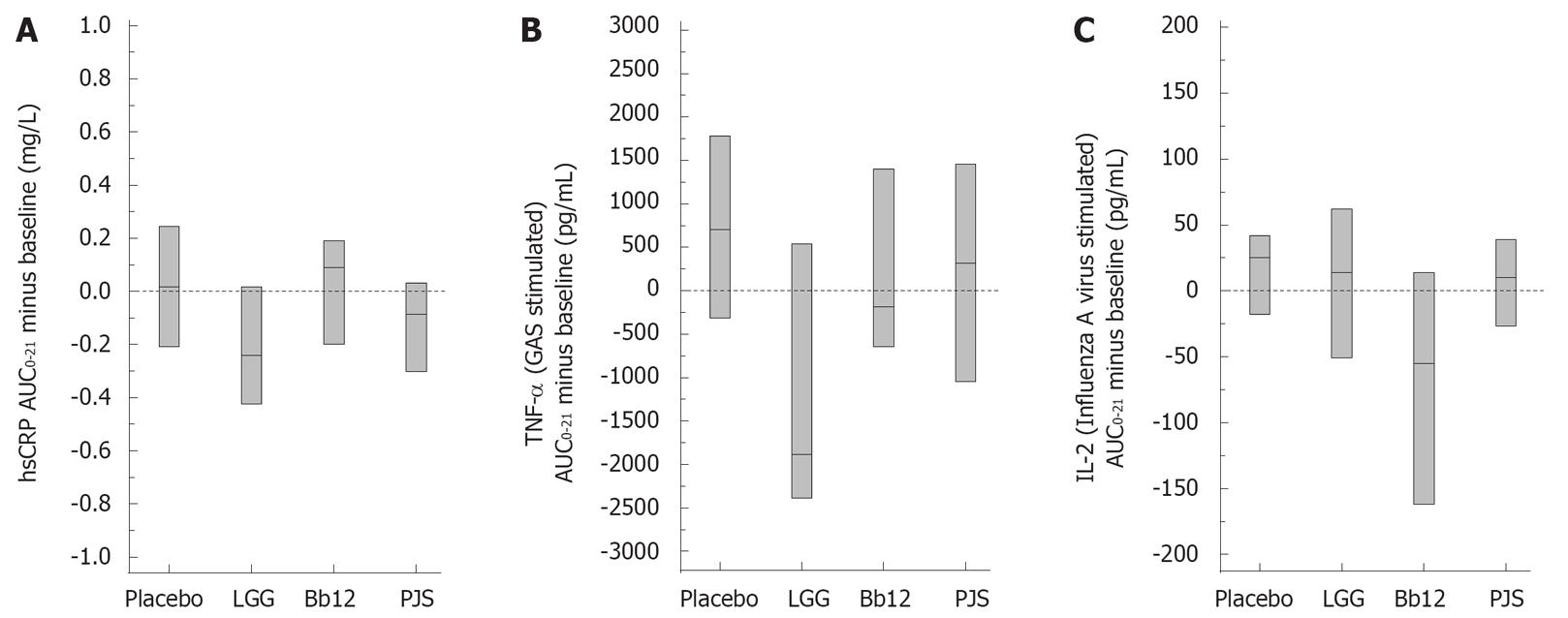
In order to study the effect of probiotic bacteria on inflammatory markers, we determined serum CRP levels at different time points during the intervention. The median AUC0-21 minus baseline (IQR) for hsCRP was 0.018 (-0.209-0.244) mg/L in the placebo group, -0.240 (-0.424-0.017) mg/L in the LGG group, 0.090 (-0.199-0.191) mg/L in the Bb12 group and -0.085 (-0.303-0.032) mg/L in the PJS group (P = 0.014); a statistically significant difference was observed between LGG and Bb12 group by pair wise comparisons. In the LGG and PJS groups, hsCRP appeared to be at a lower level during the 3-wk intervention period compared with the Bb12 and placebo groups (Figure 1A).
The baseline values for pro-inflammatory cytokine TNF-α in serum were 1.2 pg/mL in the placebo group, 1.0 pg/mL in the LGG, 1.0 pg/mL in the Bb12 and 0.8 pg/mL in the PJS. The change (median with IQR) from baseline to the end of 3-wk intervention for TNF-α in these study groups was 0.1 (-0.1-0.3) pg/mL, 0.1 (-0.02-0.2) pg/mL, 0.3 (-0.04-0.4) pg/mL and 0.0 (-0.1-0.3) pg/mL, respectively (P = 0.44).
The baseline values for pro-inflammatory cytokine IL-6 were 0.3 pg/mL in the placebo group, 0.6 pg/mL in the LGG, 0.3 pg/mL in the Bb12 and 0.4 pg/mL in the PJS. The change (median with IQR) from baseline to the end of 3-wk intervention for IL-6 in these study groups was -0.5 (-0.6-0.0) pg/mL, -0.2 (-0.3-0.2) pg/mL, 0.1 (-0.3-0.3) pg/mL and -0.04 (-0.3-0.1) pg/mL, respectively (P = 0.26). There were no statistically significant differences between the study groups with respect to serum cytokine levels.
Baseline values for leukocytes, monocytes, neutrophils, basophils, lymphocytes and immunoglobulins are presented in Table 1. There were no differences in these variables between the groups during the intervention.
| Placebo (n = 16) | LGG (n = 13) | Bb12 (n = 16) | PJS (n = 17) | P value1 | |
| Leukocytes | 4.90 (3.90-7.05) | 5.20 (4.90-6.40) | 5.25 (4.60-6.00) | 4.90 (4.35 -5.70) | 0.55 |
| Monocytes | 5.00 (4.25-6.75) | 5.00 (5.00-6.00) | 6.00 (5.00-6.00) | 6.00 (4.50-6.50) | 0.84 |
| Neutrophils | 2.05 (1.67-3.72) | 3.10 (2.50-3.30) | 2.95 (2.12-3.47) | 3.10 (2.15-3.45) | 0.39 |
| Basophils | 0.05 (0.00-0.10) | 0.10 (0.00-0.10) | 0.05 (0.00-0.10) | 0.00 (0.00-0.10) | 0.73 |
| Eosinophils | 4.00 (3.00-5.75) | 3.00 (2.00-6.00) | 3.00 (2.25-4.75) | 2.00 (1.50-3.00) | 0.077 |
| Lymphocytes | 38.0 (33.5-48.7) | 35.0 (31.5-37.5) | 34.0 (30.0-40.5) | 31.0 (26.0-40.5) | 0.24 |
| IgM | 1.28 (0.97-1.65) | 0.87 (0.69-1.32) | 1.10 (0.73-1.66) | 1.44 (0.80-1.77) | 0.31 |
| IgG | 10.7 (9.5-12.2) | 10.3 (9.3-11.7) | 10.6 (9.1-12.1) | 10.4 (8.4-11.7) | 0.72 |
| IgA | 2.65 (2.45-3.21) | 2.42 (2.04-3.44) | 2.20 (1.73-2.94) | 2.44 (1.53-2.85) | 0.22 |
| sIgA | 0.23 (0.15- 0.34) | 0.27 (0.14-0.42) | 0.40 (0.27-0.88) | 0.28 (0.17-0.49) | 0.065 |
We also determined whether the use of probiotic bacteria has an effect on the overall responsiveness of PBMC to various microbial stimuli in in vitro cultured cells. The microbe-induced cytokine production by PBMC is presented in Table 2. S. pyogenes-stimulated production of pro-inflammatory cytokine TNF-α was significantly different between the groups (P = 0.025); a statistically significant difference was observed between LGG and placebo groups by pair wise comparisons (Figure 1B). Influenza A virus-stimulated production of Th1 cytokine IL-2 was significantly different between the groups (P < 0.001); the statistically significant difference was observed between Bb12 and other groups (Figure 1C). There were no significant differences between the study groups with respect to the other cytokines produced by PBMC.
| Placebo (n = 16) | LGG (n = 13) | Bb12 (n = 16) | PJS (n = 17) | P value1 | Localization | |
| TNF-α | ||||||
| Streptococcus | 703 (-315-1784) | -1883 (-2389-540) | -645 (-1843-1403) | 315 (-1045-1460) | 0.025 | LGG vs placebo |
| Influenza | 31 (-3-66) | 6 (-83-84) | -27 (-101-37) | 29 (-39-83) | 0.32 | |
| LPS | 11 (-16-38) | -15 (-31-10) | 14 (-36-48) | -6 (-78-36) | 0.53 | |
| IFN-γ | ||||||
| Streptococcus | -19 (-221-97) | -10 (-227-284) | 71 (-161-360) | -23 (-223-155) | 0.6 | |
| Influenza | 117 (29-284) | -72 (-221-194) | -7 (-436-189) | 102 (-59-218) | 0.25 | |
| LPS | NA | NA | NA | NA | ||
| IL-1β | ||||||
| Streptococcus | 2308 (45-5222) | -1324 (-5609-352) | 444 (-6152-5000) | 649 (-3412-3747) | 0.49 | |
| Influenza | 166 (13-478) | 83 (-183-273) | -15 (-817-170 ) | 156 (-75-791) | 0.69 | |
| LPS | 67 (-29-138) | 5 (-147-114) | 51 (-40-122) | 17 (-43-199) | 0.66 | |
| IL-2 | ||||||
| Streptococcus | 5 (-23-79) | -46 (-176-0) | 1 (-135-97) | 39 (-105-213) | 0.33 | |
| Influenza | 25 (-18-42) | 14 (-51-62) | -55 (-162-14) | 10 (-27-39) | < 0.001 | Bb12 vs others |
| LPS | 2 (-18-35) | 27 (-25-57) | 1 (-42-51) | 6 (-35-25) | 0.54 | |
| IL-6 | ||||||
| Streptococcus | 793 (-691-4829) | -379 (-3955-397) | 205 (-2757-1553) | 175 (-4344-909) | 0.82 | |
| Influenza | 1530 (166-4632) | 1288 (-4329-4073) | 550 (-7178-1905) | 1644 (-327-3344) | 0.74 | |
| LPS | 947 (-418-2527) | -2189 (-3675-4053) | 329 (-1949-3950) | 639 (-1217-1607) | 0.13 | |
| IL-8 | ||||||
| Streptococcus | 240 (-1585-3914) | 34 (-3066-3143) | 400 (-3638-2891) | 132 (-2667-2976) | 0.96 | |
| Influenza | -455 (-1966-1670) | -193 (-2953-1520) | -1675 (-4245 to -601) | -1148 (-2550-1707) | 0.31 | |
| LPS | -742 (-3384-180) | -149 (-1608-1517) | -334 (-2692-739) | -1111 (-2457-836) | 0.78 | |
| IL-10 | ||||||
| Streptococcus | 907 (263-2149) | -4 (-881-2420) | 452 (-2982-1735) | 226 (-86-950) | 0.29 | |
| Influenza | 95 (48-284) | -57 (-159-233) | 4 (-301-130) | 95 (13-350) | 0.25 | |
| LPS | 381 (19-602) | 78 (-298-656) | 347 (-403-796) | 187 (28-1440) | 0.51 | |
| IL-12 | ||||||
| Streptococcus | 26 (-18-75) | -32 (-99-36) | 22 (-36-67) | 35 (-73-172) | 0.46 | |
| Influenza | 0 (-4-16) | 7 (-9-30) | 8 (-19-51) | 5 (-25-37) | 0.88 | |
| LPS | 15 (1-36) | 3 (-7-28) | 0 (-6-29) | 0 (-62-20) | 0.23 |
In order to determine whether the ingested bacteria could also be found in the fecal samples, the bacterial DNA levels were determined at the baseline and after the 3-wk intervention. The baseline levels for all three studied probiotics were low in fecal samples (Table 3). Despite the 3-wk run-in with probiotic restriction, a detectable level of the probiotic strains, especially LGG, was harbored in some of the subjects at baseline before the probiotic ingestion (Table 3). The amount of studied probiotic in feces in a given probiotic intervention group increased significantly from the baseline values during the intervention (P < 0.001). In the placebo group, the levels of different probiotics in feces remained low during the whole intervention period.
| Strain | Fecal samples | |||||||
| Placebo (n = 16) | LGG (n = 13) | Bb12 (n = 16) | PJS (n = 17) | |||||
| Baseline | After intervention | Baseline | After intervention | Baseline | After intervention | Baseline | After intervention | |
| L. rhamnosus GG | ||||||||
| Number of subjects1 | 7 | 10 | 7 | 13 | 10 | 5 | 6 | 9 |
| Mean (SD)2 | 4.7 (1.2) | 5.1 (1.1) | 5.1 (1.4) | 8.6 (0.6) | 5.2 (1.3) | 4.6 (1.5) | 4.5 (1.2) | 5.0 (1.4) |
| B. animalis ssp. lactis Bb12 | ||||||||
| Number of subjects1 | 6 | 5 | 5 | 2 | 7 | 16 | 2 | 4 |
| Mean (SD)2 | 5.4 (1.6) | 5.3 (1.7) | 5.3 (1.4) | 4.9 (1.3) | 5.4 (1.6) | 8.6 (0.5) | 4.7 (1.1) | 5.1 (1.6) |
| P. freudenreichii ssp. shermanii JS | ||||||||
| Number of subjects1 | 4 | 2 | 2 | 2 | 4 | 4 | 1 | 16 |
| Mean (SD)2 | 4.5 (1.4) | 4.0 (0.7) | 4.1 (0.8) | 4.0 (0.6) | 4.4 (1.6) | 4.2 (0.9) | 3.8 (0.3) | 8.3 (1.0) |
Three weeks after the intervention period, follow-up samples were taken. The levels for blood cells, immunoglobulins, hsCRP and cytokines produced by PBMC were at the baseline levels.
In the present study, we studied the in vivo effects of three probiotic bacteria from three different genera on immune variables in healthy adults in a randomized, double-blind, placebo-controlled setting. The selection of these probiotics was based on our previous findings showing that, in human leukocyte cell cultures, probiotic bacteria readily induce cytokine production in PBMCs, but different bacteria are able to direct immune responses to either the Th1 type or the anti-inflammatory side in a genera-specific manner[17]. Based on the cell culture results, two potentially anti-inflammatory strains (a Bifidobacterium and a Propionibacterium strain) and a well-studied L. rhamnosus GG strain[21] as a reference probiotic were selected. Our data indicates that in vivo probiotics differ in their ability to induce anti-inflammatory and cytokine responses and may have a weak, genera-specific anti-inflammatory effect reflected as a decrease in serum hsCRP levels in healthy adults. In addition, we observed that, during the intervention, S. pyogenes-induced TNF-α responses and influenza A virus-induced IL-2 responses in in vitro cultured PBMC were reduced, indicating a clear anti-inflammatory potential of some probiotic bacteria.
To our knowledge, this is the first study to show that probiotics may reduce serum hsCRP levels in healthy adults in a randomized, double-blind, placebo-controlled setting. It appeared that in the L. rhamnosus GG and P. freudenreichii ssp. shermanii JS treated groups, the hsCRP level tended to be lower during the intervention, whereas in B. animalis ssp. lactis Bb12 and the placebo groups, serum hsCRP levels remained unchanged. CRP is a sensitive marker of inflammation[22] and provides an easy way to measure the anti-inflammatory potential of probiotics and other biological or pharmacological substances. This result was somewhat contradictory to our previous findings in leukocyte cell culture[17], where B. animalis ssp. lactis Bb12 and P. freudenreichii ssp. shermanii JS were both good inducers of anti-inflammatory cytokines, whereas L. rhamnosus GG was a rather poor inducer of any cytokine. Previously, the effect of probiotics on CRP has only been studied in immunocompromised patients[23–27], allergic children[28] and patients suffering from rheumatoid arthritis[29]. In immunocompromised patients, a combination of L. casei, B. breve and prebiotic galactooligosaccharides[26] and B. longum[30] have reduced serum CRP levels and also resulted in improvement in the overall clinical appearance of chronic inflammation[30]. In contrast to the studies above and to our results in the present study, Lactobacillus rhamnosus GG increased serum hsCRP levels compared to placebo in infants with IgE-associated atopic eczema dermatitis syndrome[28]. However, L. rhamnosus GG had no effect on serum CRP levels in patients with rheumatoid arthritis[29]. It is of interest that a combination of four probiotic bacteria (L. rhamnosus GG, L. rhamnosus Lc705, B. breve 99, P. freudenreichii ssp. shermanii JS) did not have an effect on sensitive CRP[28] in the same clinical setting with allergic children. In immunocompromised patients undergoing surgical procedures, L. plantarum 299V[2325] or a combination of L. acidophilus La5, B. animalis ssp. lactis Bb12, S. thermophilus and L. bulgaricus[2427] did not change serum CRP concentrations, either. It appears that the effect of probiotics on CRP is controversial, and it is very difficult to compare the effects due to the differences in the measurement technique (highly sensitive vs normal CRP measurement), the different patient materials (healthy vs various diseases) and the different probiotic strains that have been used. It seems that age, the immunological status of the individual and the probiotic strain used in the study has a great impact on the immunomodulatory effects. Probiotics may have a strain-specific ability to lower serum CRP levels, thus having anti-inflammatory effects in apparently healthy adults and in patients suffering from different inflammatory conditions. In allergic patients, however, probiotics seem to induce a low-grade inflammatory response, as evidenced by increased serum CRP levels, and thus the treatment may have a beneficial effect on the host Th1/Th2 balance.
We found that L. rhamnosus GG was also able to reduce pro-inflammatory TNF-α production in the Gram-positive bacteria-stimulated PBMC. TNF-α is secreted by the monocytes, and it acts as an inflammatory mediator activating many types of cells. In our previous work with leukocyte cell culture, L. rhamnosus GG was found to be a relatively poor inducer of TNF-α, IL-12, IFN-γ and IL-10[17]. Our present findings are supported by another clinical study carried out in healthy adults showing that L. rhamnosus GG treatment leads to decreased TNF-α production in PBMC[31]. In addition, when the cytokine expression pattern in the small bowel mucosa was studied, it was found that L. rhamnosus GG induced the expression of genes involved in immune response and inflammation (TGF-beta and TNF family members, cytokines, nitric oxide synthase 1, defensin alpha 1)[32]. Schultz and coworkers[31] observed a decreased IL-6 and IFN-γ and an increased IL-10 and IL-4 production in PBMC obtained from L. rhamnosus GG treated individuals. We, however, did not find any significant changes in bacteria-induced production of cytokines apart from the TNF-α in the PBMC cultures of our study subjects after L. rhamnosus GG treatment. In another study with healthy adults and with patients with Crohn’s disease, L. rhamnosus GG decreased the production of IL-2, IL-10 and IL-4 from PBMCs sorted as naïve and memory T cells[33]. It seems that L. rhamnosus GG has a role in modulating the cytokine responses and may possess an anti-inflammatory potential in healthy individuals.
In the present study, we also found that B. animalis ssp. lactis Bb-12 decreased the T lymphocyte growth factor IL-2 in the influenza-virus-stimulated PBMC, indicating an anti-inflammatory effect, which is consistent with our previous findings in human leukocyte cell culture[17]. Our finding is a new one since, in healthy adults, a combination of B. animalis ssp. lactis Bb-12 and L. paracasei ssp. paracasei CRL-431 had no effect in in vitro-stimulated blood cytokine production[7]. IL-2 is a very important cytokine in viral infections and inflammatory responses since it activates NK cells and induces activation and proliferation of T lymphocytes. Therefore, IL-2 production might be an important factor for a probiotic fighting against respiratory tract infections. Based on our present results, the Bifidobacterium strain might not be the most optimal strain against respiratory infections. Indeed, it is mainly probiotic strains from Lactobacillus genera-L. rhamnosus GG[34], L. casei DN-114001[35], a combination of L. gasseri PA 16/8, B. longum SP 07/3 and B. bifidum MF 20/5[56], and L. reuteri[36]-that have reduced the incidence or symptoms of common cold or respiratory tract infections. However, the immunomodulatory effects underlying the results observed in these studies have not been fully elucidated.
In conclusion, it appears that probiotics have an anti-inflammatory potential seen as a decrease in serum CRP levels and as a reduction in bacteria-induced production of pro-inflammatory cytokines in PBMC in healthy adults. However, all of the markers were in the normal range, and therefore the real impact of probiotics as anti-inflammatory substances warrants further evaluation in studies during inflammatory processes and with individuals suffering from various types of inflammatory or autoimmune diseases.
Probiotics have been mostly studied in the prevention and treatment of different gastrointestinal diseases and allergy. Probiotic products, however, are usually consumed by the general, healthy population but not much is known what kind of effects they have on immune system in healthy adults.
It is not fully clarified how probiotics exert their health effects, but one of the most probable action mechanisms is the modulation of immune responses via gut mucosal immune system.
In the present study the immunomodulatory effects of probiotics were studied in healthy adults. Probiotic bacteria had strain-specific anti-inflammatory effects reflected in reduced sensitive C-reactive protein, which is a new finding, and decreased proinflammatory cytokine production in peripheral blood mononuclear cells (PBMC).
Understanding of the specific immunomodulatory effects of probiotics may help in designing future probiotics for targeted purposes. As the effects in the present study were investigated in healthy adults, the real impact of probiotics on inflammatory variables warrants further evaluation during inflammatory processes and with individuals suffering from various types of inflammatory or autoimmune diseases.
The paper by Kekkonen and co-workers investigated the effects of three probiotic bacteria on immune variables in healthy adults. They observed strain-specific anti-inflammatory effects for distinct bacteria. Overall this paper is interesting and it has clearly stated aims, the sample size and the overall designs of the study are fair, the results adequate to provide experimental evidence and to support valid conclusions. As placebo per se could cause effects on immune response, a further control group, formed by healthy subjects, would be advisable in order to analyze the basic fluctuation of all the parameters studied.
| 1. | FAO/WHO. Guidelines for the evaluation of probiotics in food. Report of a joint FAO/WHO working group on drafting guidelines for the evaluation of probiotics in food. World Health Organization, London Ontario, Canada. 2002;Page 8. ftp://ftp.fao.org/es/esn/food/wgreport2.pdf. |
| 2. | Vaarala O. Immunological effects of probiotics with special reference to lactobacilli. Clin Exp Allergy. 2003;33:1634-1640. |
| 3. | Limdi JK, O’Neill C, McLaughlin J. Do probiotics have a therapeutic role in gastroenterology? World J Gastroenterol. 2006;12:5447-5457. |
| 4. | Ewaschuk JB, Dieleman LA. Probiotics and prebiotics in chronic inflammatory bowel diseases. World J Gastroenterol. 2006;12:5941-5950. |
| 5. | de Vrese M, Winkler P, Rautenberg P, Harder T, Noah C, Laue C, Ott S, Hampe J, Schreiber S, Heller K. Effect of Lactobacillus gasseri PA 16/8, Bifidobacterium longum SP 07/3, B. bifidum MF 20/5 on common cold episodes: a double blind, randomized, controlled trial. Clin Nutr. 2005;24:481-491. |
| 6. | Winkler P, de Vrese M, Laue Ch, Schrezenmeir J. Effect of a dietary supplement containing probiotic bacteria plus vitamins and minerals on common cold infections and cellular immune parameters. Int J Clin Pharmacol Ther. 2005;43:318-326. |
| 7. | Christensen HR, Larsen CN, Kaestel P, Rosholm LB, Sternberg C, Michaelsen KF, Frokiaer H. Immunomodulating potential of supplementation with probiotics: a dose-response study in healthy young adults. FEMS Immunol Med Microbiol. 2006;47:380-390. |
| 8. | Olivares M, Diaz-Ropero MA, Gomez N, Lara-Villoslada F, Sierra S, Maldonado JA, Martin R, Lopez-Huertas E, Rodriguez JM, Xaus J. Oral administration of two probiotic strains, Lactobacillus gasseri CECT5714 and Lactobacillus coryniformis CECT5711, enhances the intestinal function of healthy adults. Int J Food Microbiol. 2006;107:104-111. |
| 9. | Klein A, Friedrich U, Vogelsang H, Jahreis G. Lactobacillus acidophilus 74-2 and Bifidobacterium animalis subsp lactis DGCC 420 modulate unspecific cellular immune response in healthy adults. Eur J Clin Nutr. 2008;62:584-593. |
| 10. | Isolauri E, Arvola T, Satas Y, Moilanen E, Salminen S. Probiotics in the management of atopic eczema. Clin Exp Allergy. 2000;30:1604-1610. |
| 11. | Viljanen M, Savilahti E, Haahtela T, Juntunen-Backman K, Korpela R, Poussa T, Tuure T, Kuitunen M. Probiotics in the treatment of atopic eczema/dermatitis syndrome in infants: a double-blind placebo-controlled trial. Allergy. 2005;60:494-500. |
| 12. | Schiffrin EJ, Rochat F, Link-Amster H, Aeschlimann JM, Donnet-Hughes A. Immunomodulation of human blood cells following the ingestion of lactic acid bacteria. J Dairy Sci. 1995;78:491-497. |
| 13. | Gill HS, Rutherfurd KJ, Cross ML. Dietary probiotic supplementation enhances natural killer cell activity in the elderly: an investigation of age-related immunological changes. J Clin Immunol. 2001;21:264-271. |
| 14. | Drouault-Holowacz S, Foligne B, Dennin V, Goudercourt D, Terpend K, Burckel A, Pot B. Anti-inflammatory potential of the probiotic dietary supplement Lactibiane Tolerance: in vitro and in vivo considerations. Clin Nutr. 2006;25:994-1003. |
| 15. | Foligne B, Nutten S, Grangette C, Dennin V, Goudercourt D, Poiret S, Dewulf J, Brassart D, Mercenier A, Pot B. Correlation between in vitro and in vivo immunomodulatory properties of lactic acid bacteria. World J Gastroenterol. 2007;13:236-243. |
| 16. | Hisbergues M, Magi M, Rigaux P, Steuve J, Garcia L, Goudercourt D, Pot B, Pestel J, Jacquet A. In vivo and in vitro immunomodulation of Der p 1 allergen-specific response by Lactobacillus plantarum bacteria. Clin Exp Allergy. 2007;37:1286-1295. |
| 17. | Kekkonen RA, Kajasto E, Miettinen M, Veckman V, Korpela R, Julkunen I. Probiotic Leuconostoc mesenteroides ssp. cremoris and Streptococcus thermophilus induce IL-12 and IFN-gamma production. World J Gastroenterol. 2008;14:1192-1203. |
| 18. | Pirhonen J, Sareneva T, Kurimoto M, Julkunen I, Matikainen S. Virus infection activates IL-1 beta and IL-18 production in human macrophages by a caspase-1-dependent pathway. J Immunol. 1999;162:7322-7329. |
| 19. | Miettinen M, Matikainen S, Vuopio-Varkila J, Pirhonen J, Varkila K, Kurimoto M, Julkunen I. Lactobacilli and streptococci induce interleukin-12 (IL-12), IL-18, and gamma interferon production in human peripheral blood mononuclear cells. Infect Immun. 1998;66:6058-6062. |
| 20. | Myllyluoma E, Kajander K, Mikkola H, Kyronpalo S, Rasmussen M, Kankuri E, Sipponen P, Vapaatalo H, Korpela R. Probiotic intervention decreases serum gastrin-17 in Helicobacter pylori infection. Dig Liver Dis. 2007;39:516-523. |
| 21. | Saxelin M, Tynkkynen S, Mattila-Sandholm T, de Vos WM. Probiotic and other functional microbes: from markets to mechanisms. Curr Opin Biotechnol. 2005;16:204-211. |
| 22. | Volanakis JE. Human C-reactive protein: expression, structure, and function. Mol Immunol. 2001;38:189-197. |
| 23. | McNaught CE, Woodcock NP, MacFie J, Mitchell CJ. A prospective randomised study of the probiotic Lactobacillus plantarum 299V on indices of gut barrier function in elective surgical patients. Gut. 2002;51:827-831. |
| 24. | Anderson AD, McNaught CE, Jain PK, MacFie J. Randomised clinical trial of synbiotic therapy in elective surgical patients. Gut. 2004;53:241-245. |
| 25. | McNaught CE, Woodcock NP, Anderson AD, MacFie J. A prospective randomised trial of probiotics in critically ill patients. Clin Nutr. 2005;24:211-219. |
| 26. | Sugawara G, Nagino M, Nishio H, Ebata T, Takagi K, Asahara T, Nomoto K, Nimura Y. Perioperative synbiotic treatment to prevent postoperative infectious complications in biliary cancer surgery: a randomized controlled trial. Ann Surg. 2006;244:706-714. |
| 27. | Reddy BS, Macfie J, Gatt M, Larsen CN, Jensen SS, Leser TD. Randomized clinical trial of effect of synbiotics, neomycin and mechanical bowel preparation on intestinal barrier function in patients undergoing colectomy. Br J Surg. 2007;94:546-554. |
| 28. | Viljanen M, Pohjavuori E, Haahtela T, Korpela R, Kuitunen M, Sarnesto A, Vaarala O, Savilahti E. Induction of inflammation as a possible mechanism of probiotic effect in atopic eczema-dermatitis syndrome. J Allergy Clin Immunol. 2005;115:1254-1259. |
| 29. | Hatakka K, Martio J, Korpela M, Herranen M, Poussa T, Laasanen T, Saxelin M, Vapaatalo H, Moilanen E, Korpela R. Effects of probiotic therapy on the activity and activation of mild rheumatoid arthritis--a pilot study. Scand J Rheumatol. 2003;32:211-215. |
| 30. | Furrie E, Macfarlane S, Kennedy A, Cummings JH, Walsh SV, O’neil DA, Macfarlane GT. Synbiotic therapy (Bifidobacterium longum/Synergy 1) initiates resolution of inflammation in patients with active ulcerative colitis: a randomised controlled pilot trial. Gut. 2005;54:242-249. |
| 31. | Schultz M, Linde HJ, Lehn N, Zimmermann K, Grossmann J, Falk W, Scholmerich J. Immunomodulatory consequences of oral administration of Lactobacillus rhamnosus strain GG in healthy volunteers. J Dairy Res. 2003;70:165-173. |
| 32. | Di Caro S, Tao H, Grillo A, Elia C, Gasbarrini G, Sepulveda AR, Gasbarrini A. Effects of Lactobacillus GG on genes expression pattern in small bowel mucosa. Dig Liver Dis. 2005;37:320-329. |
| 33. | Braat H, van den Brande J, van Tol E, Hommes D, Peppelenbosch M, van Deventer S. Lactobacillus rhamnosus induces peripheral hyporesponsiveness in stimulated CD4+ T cells via modulation of dendritic cell function. Am J Clin Nutr. 2004;80:1618-1625. |
| 34. | Hatakka K, Savilahti E, Ponka A, Meurman JH, Poussa T, Nase L, Saxelin M, Korpela R. Effect of long term consumption of probiotic milk on infections in children attending day care centres: double blind, randomised trial. BMJ. 2001;322:1327. |
| 35. | Turchet P, Laurenzano M, Auboiron S, Antoine JM. Effect of fermented milk containing the probiotic Lactobacillus casei DN-114001 on winter infections in free-living elderly subjects: a randomised, controlled pilot study. J Nutr Health Aging. 2003;7:75-77. |
| 36. | Tubelius P, Stan V, Zachrisson A. Increasing work-place healthiness with the probiotic Lactobacillus reuteri: a randomised, double-blind placebo-controlled study. Environ Health. 2005;4:25. |