Published online Apr 7, 2008. doi: 10.3748/wjg.14.2003
Revised: February 21, 2008
Published online: April 7, 2008
AIM: To determine the effect of tetrahydrocurcumin (THC) on tumor angiogenesis compared with curcumin (CUR) by using both in vitro and in vivo models of human hepatocellular carcinoma cell line (HepG2).
METHODS: The 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyl-tetrazolium bromide (MTT) assay was used for testing the anti-proliferating activities of CUR and THC. In male BALB/c nude mice, 2 × 106 human HepG2 cells were inoculated onto a dorsal skin-fold chamber. One day after HepG2 inoculation, the experimental groups were fed oral daily with CUR or THC (300 mg/kg or 3000 mg/kg). On d 7, 14 and 21, the tumor microvasculature was observed using fluorescence videomicroscopy and capillary vascularity (CV) was measured.
RESULTS: Pathological angiogenic features including microvascular dilatation, tortuosity, and hyper-permeability were observed. CUR and THC could attenuate these pathologic features. In HepG2-groups, the CV were significantly increased on d 7 (52.43%), 14 (69.17%), and 21 (74.08%), as compared to controls (33.04%, P < 0.001). Treatment with CUR and THC resulted in significant decrease in the CV (P < 0.005 and P < 0.001, respectively). In particular, the anti-angiogenic effects of CUR and THC were dose-dependent manner. However, the beneficial effect of THC treatment than CUR was observed, in particular, from the 21 d CV (44.96% and 52.86%, P < 0.05).
CONCLUSION: THC expressed its anti-angiogenesis without any cytotoxic activities to HepG2 cells even at the highest doses. It is suggested that anti-angiogenic properties of CUR and THC represent a common potential mechanism for their anti-cancer actions.
- Citation: Yoysungnoen P, Wirachwong P, Changtam C, Suksamrarn A, Patumraj S. Anti-cancer and anti-angiogenic effects of curcumin and tetrahydrocurcumin on implanted hepatocellular carcinoma in nude mice. World J Gastroenterol 2008; 14(13): 2003-2009
- URL: https://www.wjgnet.com/1007-9327/full/v14/i13/2003.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.2003
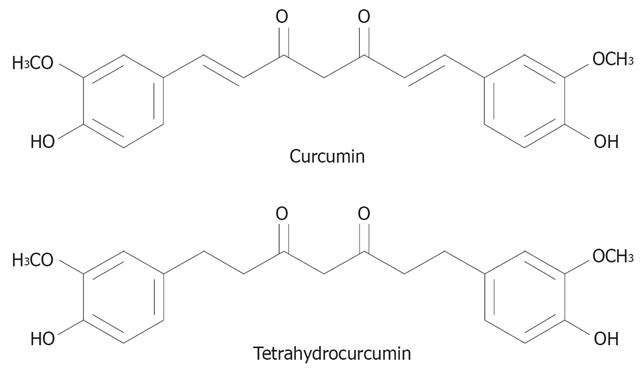
Hepatocellular carcinoma (HCC) is a highly malignant tumor characterized by active neovascularization[1]. Since HCC recruit new blood vessels to support tumor growth, an anti-angiogenic agent is one of the goal drugs to treatment of HCC. Cancer cells have a very high rate of mutation in contrast to endothelial cells, which are the main components of blood vessels and have a lower rate of mutation. Because of this genetic stability, anti-cancer treatmenting tumor-induced angiogenesis is expected to be less vulnerable to such drug tolerance. Moreover, it may work on a broad spectrum of solid tumors because all these tumors need to induce angiogenesis for their processes. It is of great interest to apply the idea of anti-angiogenesis treatment to the prevention of cancer. If food factors that can inhibit angiogenesis were to be found, such factors could be used to stop small cancers from progression. Curcumin (CUR) is considered to be among such candidates. Curcumin (diferuloylmethane) is a phenolic compound from the plant Curcuma longa. A variety of pharmacological effects of curcumin have been reported, including anti-inflammatory[2], anti-oxidant[34], and anti-carcinogenic activities[5–7]. Recently, it has been shown that the anti-cancer property of curcumin is mediated in part by its anti-angiogenic activity[8–11]. As the active metabolite of curcumin obtained in gastrointestinal tract, tetrahydrocurcumin (THC) is a reduced analog of curcumin with phenolic and β-diketo moieties as well as curcumin (Figure 1). Sugiyama et al[12] demonstrated that THC exhibited similar physiological and pharmacological properties, in particular, THC has possessed strong anti-oxidant action than curcuminoids including curcumin, demethoxycurcumin, and bisdemethoxycurcumin[13]. Although the role of THC in anti-cancer activity has been implicated[13], its possible mechanism(s) and efficacy related to curcumin anti-cancer responsibility are still controversial. For instance, THC has been reported to be a less effective chemopreventive agent in mouse skin than curcumin[14]. In contrast, 0.5% THC mixed diet showed a stronger inhibitory effect on 1,2-dimethylhydrazine induced mouse colon carcinogenesis than curcumin[15]. Therefore, the present study was aimed to determine the effect of THC on tumor angiogenesis in comparison with curcumin by using both in vitro and in vivo models of human hepatocellular carcinoma cell line (HepG2).
The curcuminoid mixture obtained from the rhizomes of Curcuma longa was subjected to silica gel column chromato-graphy, using hexane-dichloromethane, dichloromethane and dichloromethane-methanol as eluents to afford curcumin (CUR) as the major constituent. Recrystallization was accomplished by dissolving the evaporated eluate with a small quantity of dichloromethane and ethanol was then added. CUR crystallized out as yellow needles, melting point (m.p.) 181-183°C. THC was synthesized from CUR by catalytic hydrogenation reaction, with palladium on charcoal as a catalyst. The product was purified by silica gel column chromatography followed by recrystallization with dichloromethane-hexane to give 75% yield of THC as colorless needles, m.p. 93-94°C. The spectroscopic (IR, 1H-NMR and mass spectra) data of the synthesized THC were consistent with the reported values[16].
The effects of CUR and THC on the growth and survival of human hepatocellular carcinoma cell lines were measured using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) assay. Briefly, HepG2 cells (7.5 × 104 per well) were plated in 0.2 mL medium containing 10% FBS in triplicate in 96 well plate after 24 h medium was removed and then treated with 0.2 mL medium containing the indicated concentrations of CUR or THC at 37°C for 24 h. At the end of incubation, 0.050 mL of MTT solution (5 mg/mL) was added to each well. After 20 min incubated at 37°C, 0.030 mL of isopropanol was added to dissolve the formazan crystals. The absorbance of the MTT formazan was determined at 570 nm in an enzyme-linked immunosorbent assay (ELISA) reader. Cell growth index was defined as a percentage of the absorbance of treated cells to untreated cells.
The experiments were performed in BALB/c-nude mice (b.w. 20-25 g; n = 90). The animal experiment was conducted according to the guideline of experimental animals by The National Research Council of Thailand (1999). The mice were bred and maintained in a specific pathogen germ-free environment.
The mice were divided into four groups: (1) normal (control) mice with vehicle treatment (Con, n = 15), (2) HepG2-induced tumor mice (HepG2, n = 15), (3) HepG2-induced tumor mice with CUR treatment (HepG2-CUR, n = 30) and (4) HepG2-induced tumor mice with THC treatment (HepG2-THC, n = 30). In order to implant HepG2 a dorsal skin-fold chamber (7 mm diameter)[16] was used. After the anesthetization by sodium pentobarbital (50 mg/100 g BW, i.p.), 30 &mgr;L of 2 × 10-6 HepG2 cells were inoculated in the middle area of dorsal skin-fold chamber and then covered with 7 mm glass slip. All surgical procedures were performed under aseptic conditions. The animals were then housed one animal per cage with free access to sterile water and standard laboratory chow.
In the CUR and THC treated groups (HepG2-CUR and HepG2-THC groups), the mice were daily oral treated by 2 mL of 300 and 3000 mg/kg BW CUR and THC dissolved in 0.1% dimethyl sulfoxide (DMSO; Sigma, USA). These treatments were started twenty-four hours after the inoculation. In the control group (Con and HepG2), the mice received vehicle (0.1% DMSO) instead.
The experiments were performed d 7, 14 and 21 after vehicle, CUR or THC treatments. The mice were anesthetized with an intraperitoneal injection of sodium pentobarbital (50 mg/kg BW). A catheter was inserted into a jugular vein for application of fluorescence tracers. Then, the dorsal skin-fold chamber was removed and skin area around the chamber was fixed with modeling wax on a plate.
The microcirculation within a studied area was observed under an intravital fluorescence microscope using a 10 × objective. During the experiment, a videocamera (Sony, Japan) was used to project the image onto a monitor (Sony, Japan) and to record the interested areas within the tumor-bearing chambers by using a video-recorder (Sony, Japan). The videotape of each experiment was then analyzed off-line using digital image processing software (Global Lab II).
For visualization of the microvascular lumen, a bolus of 0.1 mL of 5% fluorescein isothiocyanate-labeled dextran (FITC-dextran) was injected into the jugular vein 5 min prior to the recording. The recorded videoimages were analyzed and calculated for capillary vascularity, using digital image processing software (Global Lab II) and then expressed in percentage as described previously[9]. The capillary vascularity (CV) level was used as an index of angiogenesis.
Based on the recorded video image, we measured capillary vascularity (CV) defined as follows: CV = (number of pixels within the capillaries) × 100 (%)/total number of pixels within the selected window area.
In each mouse, we observed and recorded at 5 positions on the surface of tumors by moving the microscopic stage. Figure 2 shows an example of 5 windows selected on one low-magnified image. Each window (video frame of 100 ×100 pixels) was selected so as to cover any no large vessel. By determining both minimum and maximum intensities of pixels, we counted the total number of pixels over all capillaries in each window, using digital image processing software (Global Lab II) and expressing the CV as percents of capillary area to total area. Averaging the CV’s (CVi, i = 1-5) measured over 5 video-frames (positions), we calculated the mean CV in one mouse: Mean CV = (1/5) Σ CV(1-5).
Results were shown as mean ± SE. One-way ANOVA was used to evaluate the difference of means. The statistical differences were considered at the probability level (P value) of less than 0.05.
The anti-proliferation activity of CUR and its analog, THC, were examined in HepG2 cell lines by MTT assay. It was found that CUR is a more potent anti-proliferative agent than THC. The IC50 of CUR and THC were 85.98 and 233.12 &mgr;mol/L, respectively (Figure 3).
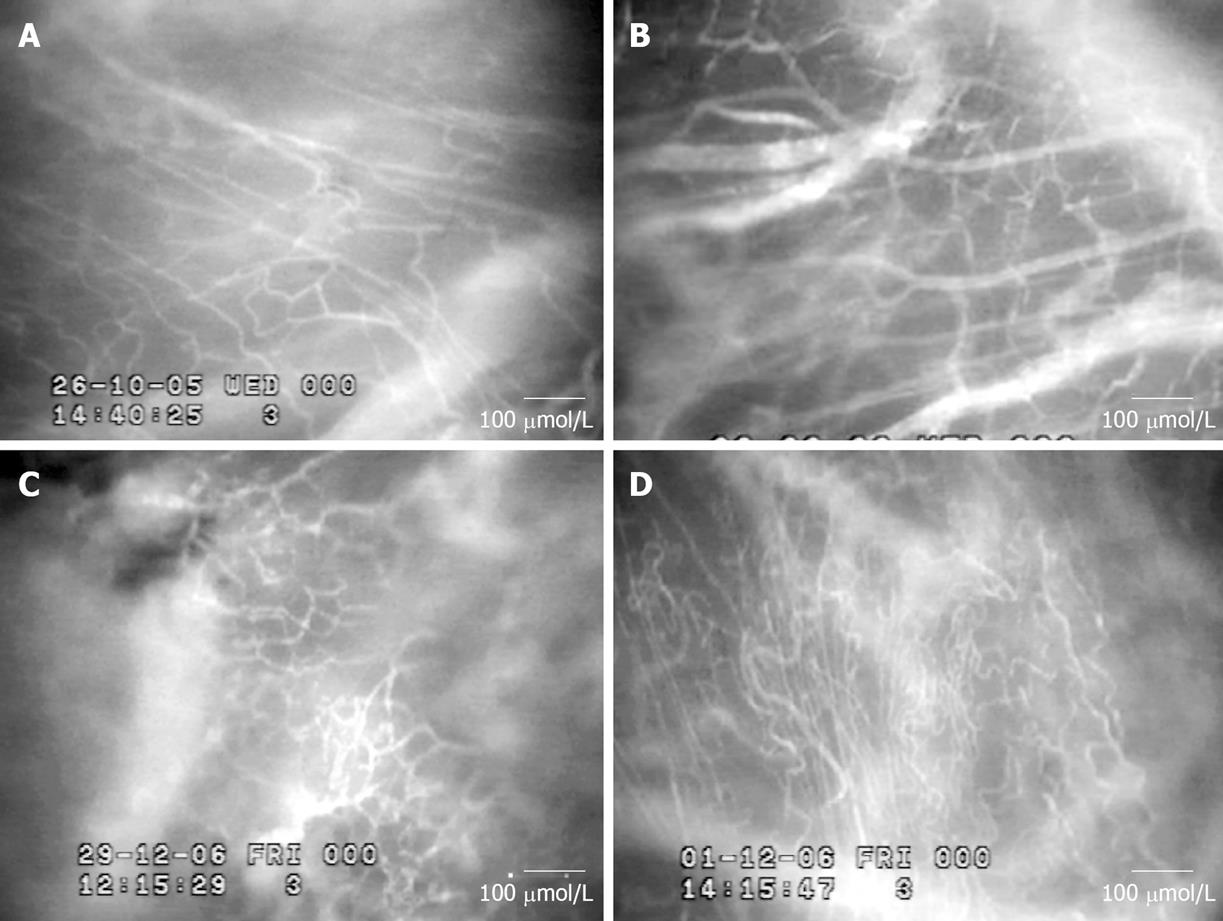
Intravital fluorescence microscopic observation demon-strated a number of neocapillaries in HepG2 groups. Figure 4 shows fluorescence videoimages of the microvascula-ture for control and HepG2 groups on d 7, 14 and 21 after tumor cells implantation. In addition, pathological angiogenic features including abrupt changes in the diameter, tortuosity, and hyper-permeability were also observed in HepG2 groups.
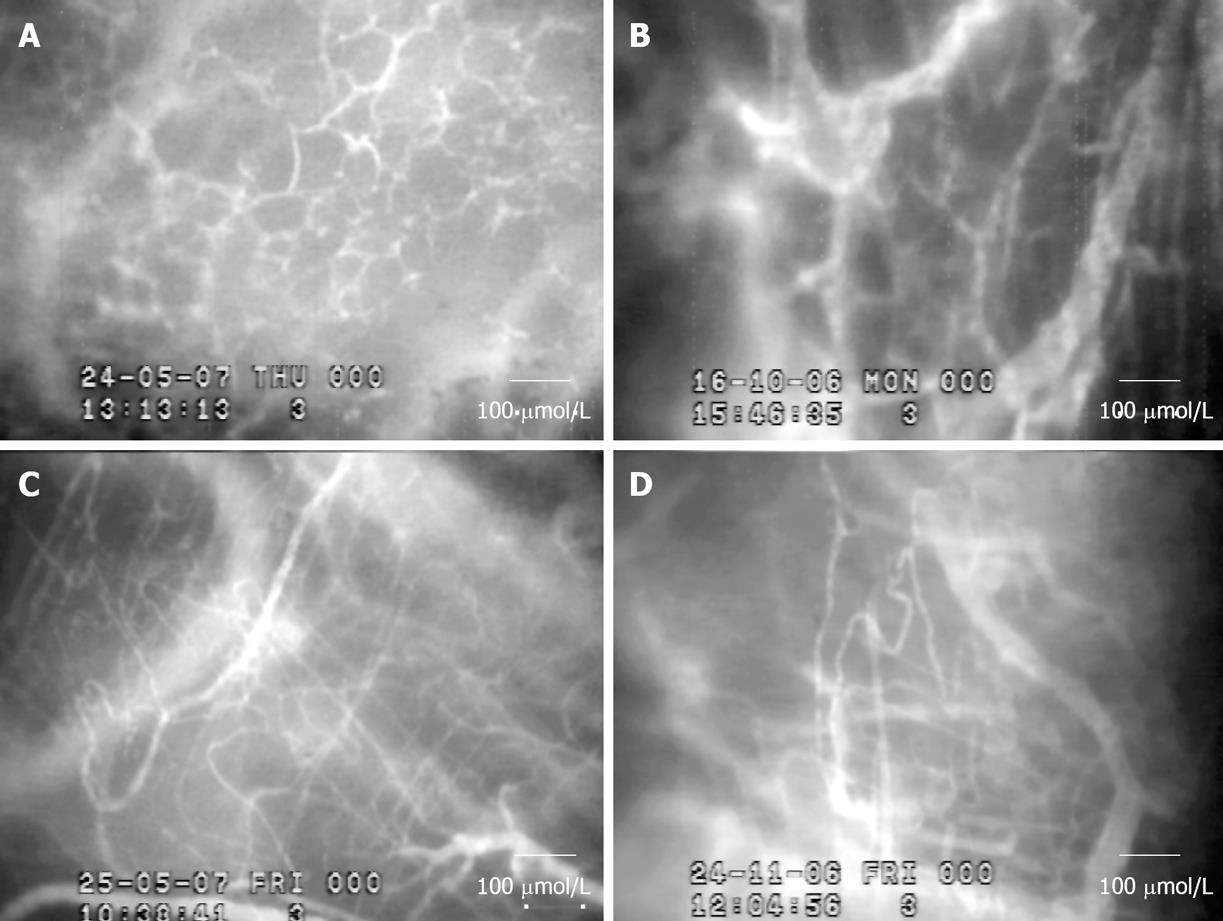
Figure 5 demonstrated the intravital fluorescent microscopic observation of tumor angiogenesis affected by CUR and THC treatment. The result showed that the appearance of neocapillaries induced by HepG2 was markedly reduced on 14 and 21 d after treatment of CUR and THC (3000 mg/kg BW). In addition, the abnormalities of neocapillary network pattern were attenuated after both treatments.
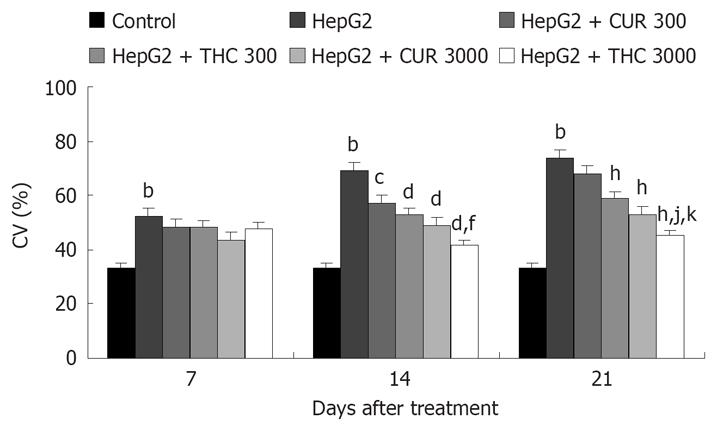
For analysis of microvascular parameters, CV in the surface area of tumor was calculated at different periods after tumor cell inoculation. Figure 6 shows CV of 7, 14 and 21 d after vehicle, CUR or THC treatment in control and HepG2 groups. In HepG2-group, the percentage of CV was significantly increased on d 7 (52.43%), 14 (69.17%), and 21 (74.08%), as compared to age-matched controls (33.04%, P < 0.001). Treatment with CUR and THC showed significant decrease in the percentage of CV (P < 0.005 and P < 0.001, respectively). In particular, the anti-angiogenic effects of CUR and THC were dose-dependent manner. However, the beneficial effect of (3000 mg/kg) THC treatment than CUR was suggested, in particular, from the 21 d percent of neocapillaries density (44.96% and 52.86%, P < 0.05).
By using MTT assay, the anti-proliferation properties of CUR and THC were examined in HepG2 cell lines. It was found that CUR has more potent anti-proliferation properties than THC. The IC50 of CUR and THC were 85.98 and 233.12 &mgr;mol/L, respectively. CUR has been shown to inhibit cell proliferation in a wide variety of human cancer cell lines in vitro[18] and in various xenotransplant and orthotopic models of human cancer in rodent[1819]. CUR suppresses the activation of several transcription factors that are implicated in carcinogenesis[18], including nuclear factor kappa B (NF-κB)[20], activator protein 1 (AP-1)[21], and at least two of the signal transducer and activator of transcription proteins (STAT3, STAT5), and modulates the expression of early growth response protein 1 (Erg-1), peroxisome proliferators-associated receptor gamma (PPAR-γ)[22]. It also suppresses the expression of cyclin D1[23] and induces apoptosis of tumor cells[2425]. According to the inhibitory effects of CUR on these cell signaling pathways, CUR may mediate its anti-proliferation by inhibiting either expression or activation of proteins that required for cell survival or cell proliferation. Furthermore, it might imply that THC could be able to suppress these key factors of signaling pathways at lesser efficacies than CUR.
By using intravital fluorescence videomicroscopy, the results showed that: (1) there was a significant increase in the numbers of CV with the heterogeneous network in HepG2 groups as compared to controls as which consistency with our previous reports[910]. (2) In this study, it was confirmed that more neocapillary density was observed as a time-dependent manner during tumor progression (CV of 21 d > CV of 14 d > CV of 7 d). (3) THC is a stronger anti-angiogenic agent than CUR.
Therefore, in the present study, CUR and THC could exert both direct and indirect actions by inhibiting tumor cell proliferation and by inhibiting tumor angiogenesis, respectively. Although the precise mechanisms that lead to tumor angiogenesis are not fully understood, several studies have shown that tumor angiogenesis which is the common process necessary for every tumor types requires the expressions of cyclooxygenase-2 (COX-2), vascular endothelial growth factor (VEGF), and matrix metalloproteinase-9 (MMP-9). Similar to our findings, CUR has been shown to suppress the proliferation of human vascular endothelial cells in vitro[26] and to abrogate angiogenic response in vivo[10]. It has also been shown that CUR inhibited Akt activation and down-regulated the expression of 5-lipooxygenase[20].
The significant finding initiated from the current study is that THC has efficacy in anti-angiogenic activity than CUR. Although THC is shown to be less anti-proliferative activity than CUR, several studies agreed to demonstrate that THC is a more potent anti-oxidant than CUR[1227], and the mechanism could be implied by its β-diketo moiety[12]. A number of evidence also suggested that tumor-mediated inflammatory response could generate an intensive local accumulation of reactive oxygen species (ROS). ROS may play a role as the mediator for the consequence of tumor induced the expression of tumor biomarkers involved tumor angiogenesis. The activation for VEGF and angiopoietin-1 induced EC migration and/or proliferation through an increase in ROS mainly proposed by a number of researchers[2829]. It was found that ethanol stimulated actin cytoskeletal reorganization, cell motility and tube formation in a ROS-dependent manner in ECs[30]. Furthermore, Leptin, a circulating adipocytokine, upregulated VEGF mRNA and stimulates cell proliferation through an increase in ROS in ECs[31].
Based on the idea of ROS, several other antioxidants such as green tea catechins, vitamin E, and natural polyphenols from red wine have been documented as the inhibitors of tumor angiogenic responses[32]. According to the more potent anti-oxidant activity of THC, the more potent anti-angiogenic activity of THC than CUR could be used to describe the different anti-angiogenic results.
Finally, it was concluded that both CUR and THC have shown to produce both anti-proliferation and anti-angiogenesis at different manner. More potent tumor anti-angiogenesis was observed for THC, and it might be due to its higher anti-oxidant activity than CUR. It is implied that THC might be a promising candidate for tumor anti-angiogenesis in the near future.
Anti-angiogenesis, the postulated mechanism of anti-cancer activities of tetrahydrocurcumin (THC) and curcumin (CUR), was examined using hepatocellular carcinoma cells (HepG2)-implanted nude mice. CUR has been found to be an angiogenic inhibitor. However, THC, a potent anti-oxidative agent is responsible for the reported effect is still to be determined. The present study was aimed to determine the effect of THC on tumor angiogenesis in comparison with CUR by using both in vitro and in vivo models of HepG2.
THC contains both a phenolic moiety and a β-diketone moiety in the same structure. THC exhibits many of the same physiologic and pharmacological activities as CUR and in some systems may exert greater anti-oxidant activity than CUR. Therefore, the role of THC in preventive and therapeutic of cancer is gain interest. Anti-angiogenic therapy is one of the most promising strategies for cancer treatment. In this study, anti-angiogenic activity was investigated by evaluating the density of neovascularization induced by Hepatocellular carcinoma cell (HepG2) in nude mice, using intravital fluorescence videomicroscope. The results showed that THC exerts significant anti-angiogenic activity. Therefore, THC might be a promising candidate for tumor anti-angiogenesis in the near future.
The present study showed the anti-cancer and anti-angiogenic activities of CUR and THC. CUR and THC have shown to produce both anti-proliferation and anti-angiogenesis at different manner. More potent tumor anti-angiogenesis was observed for THC, and it might be due to its higher anti-oxidant activity than CUR.
The findings from this study support the idea that anti-oxidative substances can be a therapeutic target for treating cancer.
In this study, THC, a novel type of anti-oxidant showed anti-angiogenic activities without any cytotoxic effect. Importantly, our results have provided originally an in vivo evidence for anti-angiogenic activity of THC, in particular by using hepatocellular-carcinoma inoculated skin-chamber model.
| 1. | Abou-Shady M, Baer HU, Friess H, Zimmermann A, Buchler MW. Molecular aspects of hepatocellular carcinoma. Swiss Surg. 1999;5:102-106. |
| 2. | Huang MT, Lysz T, Ferraro T, Abidi TF, Laskin JD, Conney AH. Inhibitory effects of curcumin on in vitro lipoxygenase and cyclooxygenase activities in mouse epidermis. Cancer Res. 1991;51:813-819. |
| 3. | Jovanovic SV, Boone CW, Steenken S, Trinoga M, Kaskey RB. How curcumin works preferentially with water soluble antioxidants. J Am Chem Soc. 2001;123:3064-3068. |
| 4. | Sandur SK, Ichikawa H, Pandey MK, Kunnumakkara AB, Sung B, Sethi G, Aggarwal BB. Role of pro-oxidants and antioxidants in the anti-inflammatory and apoptotic effects of curcumin (diferuloylmethane). Free Radic Biol Med. 2007;43:568-580. |
| 5. | Huang MT, Ma W, Yen P, Xie JG, Han J, Frenkel K, Grunberger D, Conney AH. Inhibitory effects of topical application of low doses of curcumin on 12-O-tetradecanoylphorbol-13-acetate-induced tumor promotion and oxidized DNA bases in mouse epidermis. Carcinogenesis. 1997;18:83-88. |
| 6. | Singh SV, Hu X, Srivastava SK, Singh M, Xia H, Orchard JL, Zaren HA. Mechanism of inhibition of benzo[a]pyrene-induced forestomach cancer in mice by dietary curcumin. Carcinogenesis. 1998;19:1357-1360. |
| 7. | Singletary K, MacDonald C, Iovinelli M, Fisher C, Wallig M. Effect of the beta-diketones diferuloylmethane (curcumin) and dibenzoylmethane on rat mammary DNA adducts and tumors induced by 7,12-dimethylbenz[a]anthracene. Carcinogenesis. 1998;19:1039-1043. |
| 8. | Li L, Braiteh FS, Kurzrock R. Liposome-encapsulated curcumin: in vitro and in vivo effects on proliferation, apoptosis, signaling, and angiogenesis. Cancer. 2005;104:1322-1331. |
| 9. | Yoysungnoen P, Wirachwong P, Bhattarakosol P, Niimi H, Patumraj S. Antiangiogenic activity of curcumin in hepatocellular carcinoma cells implanted nude mice. Clin Hemorheol Microcirc. 2005;33:127-135. |
| 10. | Yoysungnoen P, Wirachwong P, Bhattarakosol P, Niimi H, Patumraj S. Effects of curcumin on tumor angiogenesis and biomarkers, COX-2 and VEGF, in hepatocellular carcinoma cell-implanted nude mice. Clin Hemorheol Microcirc. 2006;34:109-115. |
| 11. | Lin YG, Kunnumakkara AB, Nair A, Merritt WM, Han LY, Armaiz-Pena GN, Kamat AA, Spannuth WA, Gershenson DM, Lutgendorf SK. Curcumin inhibits tumor growth and angiogenesis in ovarian carcinoma by targeting the nuclear factor-kappaB pathway. Clin Cancer Res. 2007;13:3423-3430. |
| 12. | Sugiyama Y, Kawakishi S, Osawa T. Involvement of the beta-diketone moiety in the antioxidative mechanism of tetrahydrocurcumin. Biochem Pharmacol. 1996;52:519-525. |
| 13. | Pari L, Murugan P. Protective role of tetrahydrocurcumin against erythromycin estolate-induced hepatotoxicity. Pharmacol Res. 2004;49:481-486. |
| 14. | Huang MT, Ma W, Lu YP, Chang RL, Fisher C, Manchand PS, Newmark HL, Conney AH. Effects of curcumin, demethoxycurcumin, bisdemethoxycurcumin and tetrahydrocurcumin on 12-O-tetradecanoylphorbol-13-acetate-induced tumor promotion. Carcinogenesis. 1995;16:2493-2497. |
| 15. | Kim JM, Araki S, Kim DJ, Park CB, Takasuka N, Baba-Toriyama H, Ota T, Nir Z, Khachik F, Shimidzu N. Chemopreventive effects of carotenoids and curcumins on mouse colon carcinogenesis after 1,2-dimethylhydrazine initiation. Carcinogenesis. 1998;19:81-85. |
| 16. | Lee SL, Huang WJ, Lin WW, Lee SS, Chen CH. Preparation and anti-inflammatory activities of diarylheptanoid and diarylheptylamine analogs. Bioorg Med Chem. 2005;13:6175-6181. |
| 17. | Lehr HA, Leunig M, Menger MD, Nolte D, Messmer K. Dorsal skinfold chamber technique for intravital microscopy in nude mice. Am J Pathol. 1993;143:1055-1062. |
| 18. | Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 2003;23:363-398. |
| 19. | Aggarwal BB, Shishodia S, Takada Y, Banerjee S, Newman RA, Bueso-Ramos CE, Price JE. Curcumin suppresses the paclitaxel-induced nuclear factor-kappaB pathway in breast cancer cells and inhibits lung metastasis of human breast cancer in nude mice. Clin Cancer Res. 2005;11:7490-7488. |
| 20. | Aggarwal S, Ichikawa H, Takada Y, Sandur SK, Shishodia S, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates expression of cell proliferation and antiapoptotic and metastatic gene products through suppression of IkappaBalpha kinase and Akt activation. Mol Pharmacol. 2006;69:195-206. |
| 21. | Tomita M, Kawakami H, Uchihara JN, Okudaira T, Masuda M, Takasu N, Matsuda T, Ohta T, Tanaka Y, Mori N. Curcumin suppresses constitutive activation of AP-1 by downregulation of JunD protein in HTLV-1-infected T-cell lines. Leuk Res. 2006;30:313-321. |
| 22. | Chen A, Xu J. Activation of PPAR{gamma} by curcumin inhibits Moser cell growth and mediates suppression of gene expression of cyclin D1 and EGFR. Am J Physiol Gastrointest Liver Physiol. 2005;288:G447-G456. |
| 23. | Mukhopadhyay A, Banerjee S, Stafford LJ, Xia C, Liu M, Aggarwal BB. Curcumin-induced suppression of cell proliferation correlates with down-regulation of cyclin D1 expression and CDK4-mediated retinoblastoma protein phosphorylation. Oncogene. 2002;21:8852-8861. |
| 24. | Choudhuri T, Pal S, Das T, Sa G. Curcumin selectively induces apoptosis in deregulated cyclin D1-expressed cells at G2 phase of cell cycle in a p53-dependent manner. J Biol Chem. 2005;280:20059-20068. |
| 25. | Sandur SK, Ichikawa H, Pandey MK, Kunnumakkara AB, Sung B, Sethi G, Aggarwal BB. Role of pro-oxidants and antioxidants in the anti-inflammatory and apoptotic effects of curcumin (diferuloylmethane). Free Radic Biol Med. 2007;43:568-580. |
| 26. | Singh AK, Sidhu GS, Deepa T, Maheshwari RK. Curcumin inhibits the proliferation and cell cycle progression of human umbilical vein endothelial cell. Cancer Lett. 1996;107:109-115. |
| 27. | Osawa T, Sugiyama Y, Inayoshi M, Kawakishi S. Antioxidative activity of tetrahydrocurcuminoids. Biosci Biotechnol Biochem. 1995;59:1609-1612. |
| 28. | Harfouche R, Malak NA, Brandes RP, Karsan A, Irani K, Hussain SN. Roles of reactive oxygen species in angiopoietin-1/tie-2 receptor signaling. FASEB J. 2005;19:1728-1730. |
| 29. | Ikeda S, Ushio-Fukai M, Zuo L, Tojo T, Dikalov S, Patrushev NA, Alexander RW. Novel role of ARF6 in vascular endothelial growth factor-induced signaling and angiogenesis. Circ Res. 2005;96:467-475. |
| 30. | Qian Y, Luo J, Leonard SS, Harris GK, Millecchia L, Flynn DC, Shi X. Hydrogen peroxide formation and actin filament reorganization by Cdc42 are essential for ethanol-induced in vitro angiogenesis. J Biol Chem. 2003;278:16189-16197. |
| 31. | Yamagishi S, Amano S, Inagaki Y, Okamoto T, Takeuchi M, Inoue H. Pigment epithelium-derived factor inhibits leptin-induced angiogenesis by suppressing vascular endothelial growth factor gene expression through anti-oxidative properties. Microvasc Res. 2003;65:186-190. |
| 32. | Tang FY, Meydani M. Green tea catechins and vitamin E inhibit angiogenesis of human microvascular endothelial cells through suppression of IL-8 production. Nutr Cancer. 2001;41:119-125. |