Published online Mar 28, 2008. doi: 10.3748/wjg.14.1885
Revised: January 19, 2008
Published online: March 28, 2008
AIM: To assess prospectively small bowel stenoses in Crohn’s disease (CD) patients treated with infliximab using Small Intestine Contrast Ultrasonography (SICUS).
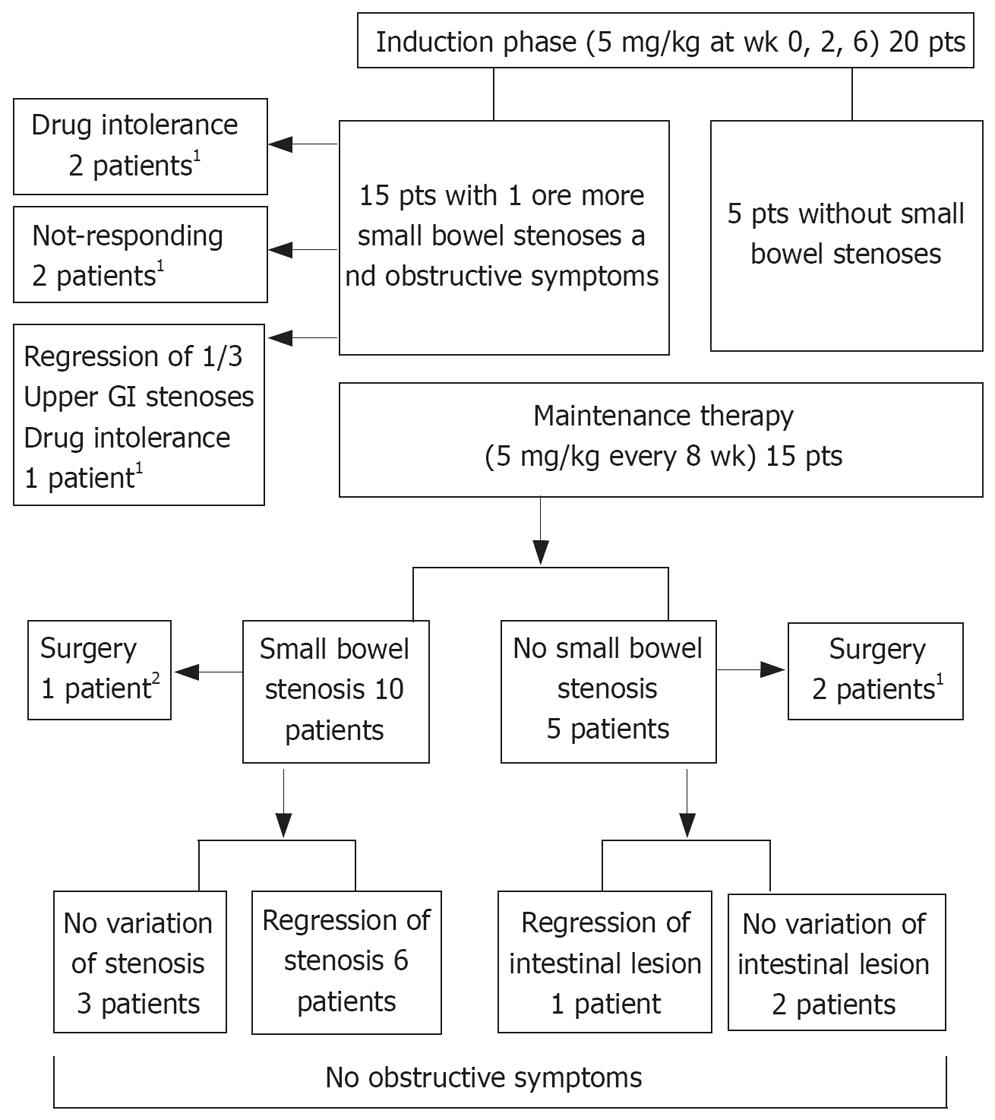
METHODS: Twenty patients (M 12, age, 42.7 ± 11.8 years), 15 of whom showed obstructive symptoms indicating the presence of small bowel stenosis, and 5 without stenosis, were treated with infliximab (5 mg/kg at wk 0, 2, 6 and 5 mg/kg every 8 wk thereafter) for steroid refractoriness, fistulizing disease, or to avoid high-risk surgery. SICUS was performed at the induction phase and at regular time intervals during the follow-up period of 34.7 ± 16.1 mo (range 7-58). Small bowel stenoses were detected by SICUS, endoscopy and MRI.
RESULTS: In no case was progression of stenoses or the appearance of new ones seen. Of the 15 patients with stenosis, 5 stopped treatment after the induction phase (2 for no response, 3 for drug intolerance, one of whom showed complete regression of one stenosis). Among the remaining 10 patients, a complete regression of 8 stenoses (1 stenosis in 5 patients and 3 stenoses in one patient) was observed after 6-22 infliximab infusions.
CONCLUSION: In patients with CD treated with infliximab we observed: (a) No progression of small bowel stenosis and no appearance of new ones, (b) Complete regression of 1/22 stenosis after the induction phase and of 8/15 (53.3%) stenosis after 6-22 infusions during maintenance therapy.
- Citation: Pallotta N, Barberani F, Hassan NA, Guagnozzi D, Vincoli G, Corazziari E. Effect of infliximab on small bowel stenoses in patients with Crohn’s disease. World J Gastroenterol 2008; 14(12): 1885-1890
- URL: https://www.wjgnet.com/1007-9327/full/v14/i12/1885.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.1885
The effect of infliximab on symptomatic Crohn’s intestinal stenosis is controversial and, even though there is no direct evidence indicating anti-TNFα antibodies therapy enhances stricture formation, it is usual practice to withhold infliximab therapy in patients with intestinal stenosis. Indeed, two retrospective studies have reported intestinal obstructions as a possible adverse event[12]. Such obstructive events have been interpreted as the outcome of an accelerated healing process that may trigger fibrosis within the inner layers of the gut wall. Conversely a review of large clinical studies concluded infliximab treatment did not increase the risk of developing strictures in patients with Crohn’s disease (CD)[3].
Because of the lack of non-invasive, radiation-free, techniques to assess transmural small bowel lesions, previous studies were retrospective and relied on assessments of the obstructive symptoms rather than of the stenotic lesion itself[4–7]. Small intestine contrast ultrasonography (SICUS), performed after the ingestion of oral contrast, enables measurement of the wall thickness and luminal diameter of the small bowel[89]. This technique can accurately assess the presence, size, and number of small bowel lesions in CD patients[1011].
In the present study, SICUS was used to assess the time course of small bowel stenosis in CD patients treated with infliximab, in a prospective follow-up investigation.
As a part of a long-term prospective follow-up study, twenty patients (12 males and 8 females, age 42.7± 11.8 years) with CD of the small bowel received infliximab therapy because of the presence of fistulae (11 patients) and/or steroid dependence (7 patients) and/or azathioprine intolerance (5 patients) and/or extra-intestinal manifestation (1 patient with ankylosing spondylitis). Fifteen had small bowel stenoses and obstructive symptoms (6 had previously received extensive small bowel resections).
Diagnosis of CD was based on the criteria adopted in the EC-study[12]. Medical history, including abdominal and extra-abdominal complaints, associated disease, CD behavior and CDAI at the first and last assessment of the follow-up, smoking status at diagnosis and at follow-up, family history, location of CD, duration of the disease, past surgery and endoscopic dilatation, and current and previous medical treatment were enquired and reported. Informed consent was obtained from each subject.
Patients were scheduled to receive an infusion of infliximab (5 mg/kg) at wk 0, 2 and 6 (induction phase) and 5 mg/kg every 8 wk thereafter (maintenance therapy). Each patient was prospectively evaluated with SICUS. SICUS was performed during the infliximab induction phase and at six-month intervals thereafter. Each patient was initially subjected to a standardized clinical interview and a physical examination, performed by one of two certified and experienced gastroenterologists (FB, EC). After an overnight fast, patients were consecutively submitted to SICUS and, on different days and in random order, to an endoscopic examination, with multiple mucosal biopsies, of the entire large bowel and terminal or neoterminal ileum. When deemed necessary additional investigations, including biochemistry, upper GI endoscopy, abdominal CT or MRI, were performed. The sonologist was aware of the diagnosis and clinical data, including bowel surgery, but was blinded to the results of endoscopy and of other investigations, and did not review the results of the previous SICUS examinations at each follow-up assessment.
At the end of the US investigation small bowel abnormalities were reported on a standardized form, with particular reference to presence, anatomical site and extension in centimeters of intestinal wall, and lumen alterations. Fistulas and abscesses were looked for and reported.
Real-time US was performed using Toshiba Tosbee (Tokyo, Japan) equipment with 3.5 MHz convex and 5 MHz linear array transducers. The sonologist (NP) had experience exceeding 7000 sonographic examinations of the whole abdomen and 4000 examinations of SICUS.
SICUS was performed according to a previously published[8] method. Briefly, after the ingestion of 375 mL of macrogol contrast oral solution (Promefarm, Milan, Italy) and after the contrast was seen to flow through the terminal ileum into the colon, a retrograde follow-through assessment of the entire small bowel was performed visualizing, in a caudo-cranial sequence, the contrast-filled ileal and jejunal loops. The body positions of patients were changed and abdominal compression with the US transducer was used whenever required to improve visualization of any single loop and detection of intestinal abnormalities after the ingestion of the oral contrast. All examinations were recorded on VHS to be re-examined at will.
Wall thickness and lumen diameter were measured at several sites (proximal, middle and distal) of the small bowel at the level of the maximally distended, and not contracting, intestinal loops.
The criteria for presence of CD ileal lesions were as follows[10]: (1) Increased wall thickness (more than 3 mm) and lack and/or distortion of the intestinal folds; (2) presence of bowel stenosis defined as a lumen diameter of less than 1 cm, measured at the level of maximally distended loop, independent of the presence of pre-stenotic dilatation; and (3) bowel dilatation defined as lumen diameter more than 2.5 cm[89]. The extension of the stenoses was expressed as the length of the segment with a lumen diameter of less than 1 cm[89]. To distinguish a stenosis from a dynamic reversible reduction of luminal diameter due to intestinal contraction, multiple and prolonged, more than 15 min, observations of the narrowed tract were performed. Furthermore the presence of stenoses located in the terminal and neoterminal ileum were confirmed at endoscopy based on their inability to pass an 11 mm caliber endoscope; those in the more proximal small bowel segments were confirmed on at least two consecutive follow-up observations at SICUS and MRI.
For each detected lesion, site, number and length were reported on the record chart. Regression of intestinal stenosis was defined as an intestinal lumen with a diameter more than 1 cm as assessed by at least 2 follow-up SICUS evaluations, and confirmed at endoscopy for the lesions located in the terminal ileum, and at MRI for those located in the more proximal small bowel segment.
Fifteen patients had one or more stenosis of the small bowel; five patients did not have stenoses. In two female patients with penetrating CD behavior, infliximab therapy was discontinued for intolerance after the first i.v. administration. In an additional 2 female patients, one with entero-enteric fistulae and one with recto-vaginal fistula, treatment was discontinued after the induction period owing to a lack of response to the treatment. After the first 3 induction infusions there was a complete regression of one of the three upper GI stenoses in one male patient, who discontinued treatment owing to intolerance.
Fifteen patients then received maintenance therapy with infliximab. The main characteristics of patients receiving maintenance therapy are reported in Table 1 and Figure 1. During the follow-up period of 34.7 ± 16.1 mo (range 7-58 mo, median 38 mo), SICUS and, when required, endoscopic and imaging investigations, were performed at 10.7 ± 3.7 mo intervals in all but 3 patients who were assessed at induction and regularly subjected to follow-up starting at 21, 24 and 28 mo after the induction phase. Three patients were referred for surgery as they did not respond to maintenance therapy; one for entero-cutaneous fistulas after 3 infusions, one for severe recurrence after 12 infusions and one who previously received surgical operations and endoscopic dilatations for recurrent obstructive episodes after 6 infusions. Thirteen patients remained on maintenance therapy.
| Gender | Smoker | IB | DD | Age | Previous | Associated | CDAI | FU | Infusions2 | Stenosis | |||||||
| (yr) | (yr)1 | surgery | therapy | Inclusion | Last | months | number | Site | Number | Length (cm) | Ø (mm) | WT (mm) | PSD | Regression5 | |||
| M | + | B3 | 11 | 24 | 3 | - | 74 | 13 | 26 | 11 | PAI | 1 | 10 | 5 | 5 | - | 8 |
| M | - | B3 | 2.5 | 38 | - | 5-ASA | 139.2 | 40.4 | 24 | 12 | PI | 1 | 20 | 7 | 10 | + | 8 |
| M | + | B3 | 5.6 | 52 | - | - | 96 | 39.2 | 43 | 22 | DI/TI | 3 | 6 | 8 | 9 | - | 9 |
| 3.5 | 9 | 6 | - | 22 | |||||||||||||
| 4.5 | 8 | 5 | - | 22 | |||||||||||||
| M | - | B2 | 21.5 | 22 | 1 | - | 28 | 28 | 54 | 27 | PAI | 1 | 7 | 9 | 8.5 | - | 6 |
| M | + | B2 | 21 | 30 | - | AZT/5-ASA | 202 | 119 | 25 | 15 | DI | 1 | 5 | 4 | 6 | + | 15 |
| F | + | B3 | 9 | 29 | - | AZT/5-ASA | 167 | 32 | 58 | 12 | TI | 1 | 5 | 5 | 7 | + | 12 |
| M4 | + | B2 | 31 | 21 | 3 | - | 54 | 28 | 47 | 223 | PAI | 1 | 7 | 7 | 6 | + | - |
| M | - | B2 | 15.5 | 22 | 1 | 5-ASA | 330.2 | 267.2 | 50 | 19 | UGIT | 4 | 3 | 4 | 9 | + | - |
| 2 | 4 | 9 | + | - | |||||||||||||
| 6 | 4 | 9 | + | - | |||||||||||||
| 3 | 4 | 9 | + | - | |||||||||||||
| F | - | B3 | 30 | 18 | 3 | 5-ASA | 241 | 12 | 51 | 4 | PAI | 1 | 15 | 9 | 7 | + | - |
| M | + | B2 | 11 | 30 | - | - | 134.6 | 78.6 | 42 | 23 | TI | 1 | 25 | 7 | 9 | + | - |
| M | - | B2 | 4.5 | 52 | 1 | 5-ASA | 126 | 71.8 | 14 | 9 | No stenosis | ||||||
| F | + | B3 | 3.2 | 26 | - | 5-ASA | 154.6 | 0 | 38 | 9 | No stenosis | ||||||
| M4 | - | B3 | 5.6 | 19 | - | - | 35.6 | 384 | 24 | 14 | No stenosis | ||||||
| M4 | - | B3 | 15 | 38 | - | 5-ASA | 306 | 144 | 7 | 6 | No stenosis | ||||||
| F | + | B3 | 14.4 | 18 | - | AZT | 134 | 103 | 17 | 9 | No stenosis | ||||||
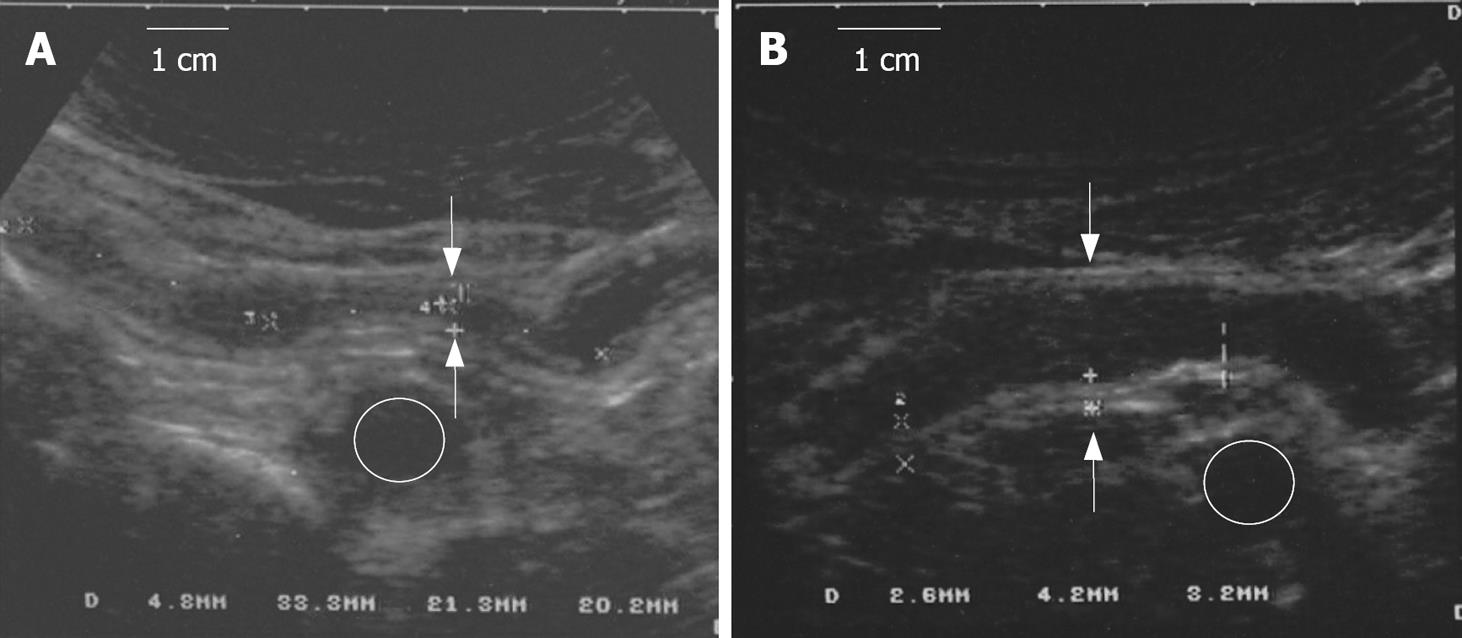
Nine patients had 1 or more small bowel stenoses (1 patient with 4 jejunal stenoses; 1 patient with 1 stenosis at the level of proximal ileum; 1 patient with 3 stenoses at the level the distal and terminal ileum; 1 patient with 1 stenosis at the level of distal ileum; 2 patients with 1 stenosis at the level of terminal ileum; 3 patients with 1 stenosis at the level of neo-terminal ileum). Complete regression of 8 stenoses, 3 of which showed prestenotic dilatation, was observed in 6 patients; in 2 of these, the stenosis was at the level of the neo-terminal ileum. In one patient with three stenoses, the most proximal one regressed after 9 cycles of therapy, whereas the remaining two regressed after 22 cycles (Figure 2). Regression of all stenoses was confirmed in subsequent observations performed during the follow-up from a minimum of 1 mo to a maximum of 42 mo. In 3 patients, there was no variation in the pre-existing 6 stenoses, all with pre-stenotic dilatation, but the obstructive symptoms disappeared.
Among the 5 patients without stenoses, none developed stenosis or obstructive symptoms and in one patient a regression of terminal ileum alteration was observed (Table 1). On average, CDAI improved during infliximab maintenance therapy, but no relationship was found between CDAI at time of induction or at last follow up observation and regression of stenoses.
The most relevant finding of the present study is the observation that during therapy with anti-TNF alpha antibodies there was no progression of pre-existing stenoses, no appearance of new ones, and complete regression of 1/22 (4.5%) small bowel stenoses after the induction phase and 8/15 (53.3%) small bowel stenoses after 6-22 maintenance infusions. In addition, despite the long duration of CD, no stenosis progressed to require surgery and in the presence of stenosis obstructive symptoms disappeared during infliximab treatment.
The limitations of this study are the small size of the population and the observational type of assessment. Confirmation in an ad hoc randomized controlled study performed in a larger sample size is required.
The strengths of this study are the prospective collection of data, the morphological assessment of CD stenosis with description of intestinal wall and lumen diameter and in comparison with previous reports, the relatively long duration of the follow-up in patients with small bowel stenosis treated with infliximab therapy.
A previously published review[3] of prospectively collected data in the TREAT registry and from the ACCENT I trial concluded long-term treatment with infliximab was not associated with increased risk of development of stenosis. These conclusions were based on clinical judgment, as radiological and endoscopic evaluations were performed at the discretion of the investigators, and not systematically in patients without obstructive symptoms. In addition, because patients with symptomatic stenosis were excluded from the study, the effect of infliximab on high-grade stenosis could not be assessed.
The present study refers to patients with CD of the small bowel treated with infliximab and belonging to a larger CD patient population assessed in a long-term prospective follow-up study, in which SICUS is used to evaluate the time course of small bowel lesions.
SICUS has enabled the normal values of wall thickness and luminal diameter of the small bowel and the reproducibility of measurements in healthy control subjects to be defined[9]. Furthermore, the accuracy of SICUS in the assessment of the number, site and extension of small bowel lesions has been validated with intraoperative findings[10].
In the present study, patients with symptomatic stenosis, some of whom have indications for surgery, and patients without stenosis, were prospectively followed up.
The observations obtained progressively in this case series indicate anti-TNF antibody therapy did not cause intestinal stenosis or obstructive symptoms. This finding is in contrast with previous reports of retrospective studies[12], in which obstructive adverse events occurred after infliximab administration. However, retrospective analysis of these observations did not reveal whether the obstructive complication was due to a previously existing symptomatic stenosis and refractory to other therapies leading up to the use of infliximab. In the present study, three patients received surgery during maintenance therapy. In one, who received stricturoplasty for stenosis, the lesion was already present and had surgical indications before infliximab treatment. Infliximab was administered in an attempt to avoid surgery in a patient with a previous history of intestinal resection and at risk of developing short bowel syndrome. After surgery, infliximab maintenance therapy was continued and after 2 years of follow-up no new stenosis has appeared and the patient shows no obstructive symptoms. In the second patient, who received surgery for enterocutaneous fistula, there was a temporaneous improvement after infliximab treatment and a symptomatic recurrence after the induction phase plus 3 cycles of maintenance therapy drug administration. In the third patient with entero-enteric fistulas, after an initial improvement of symptoms there was a severe recurrence after one year of maintenance therapy.
In three additional patients, despite the stenoses not changing after treatment, there was a disappearance of obstructive symptoms, and surgery was not required in the follow-up period. In the remaining six patients, there was complete regression of stenoses, irrespective of the CD type, whether stenosing or fistulizing, the site of the lesion, age of the patient, smoking status at follow-up, type and number of previous operations and pharmacological treatment, or the duration of CD.
It is reasonable to interpret the regression of the stenotic lesions as due to the anti-inflammatory effect of infliximab treatment.
A retrospective study evaluating the symptomatic response of obstructive symptoms for up to 18 mo reported a favorable response in 9 of 11 patients treated with infliximab and concomitant immunosuppressive drugs[13]. In the present study, only two patients of the 9 with stenoses receiving maintenance infliximab therapy received azathioprine concomitantly, indicating the favorable response was obtained after treatment with the biological agent, irrespective of the immunosuppressive drugs. In addition, regression of stenoses and obstructive symptoms was confirmed at all subsequent follow-up assessments. Such a favorable long-term effect could be a result of the maintenance treatment with infliximab that, when started immediately after endoscopic dilatation of small bowel stenosis, has been reported to prevent stenotic progression and obstructive complications in a 2-year follow up study[14].
Tissue inflammation and fibrosis in the gut wall are regulated by cytokines with opposite effects. TNF has both a pro-inflammatory and anti-fibrotic action in the intestinal mucosa[15]. Anti-TNF agents induce endoscopic[16] and histological[17] mucosal healing, but it is not known how they act in the deeper layers of the gut wall and whether, and how, they affect fibrosis at this level[18]. The regression of stenotic lesions after administration indicates anti-TNF agents may act as antifibrotic agents in the deeper layers of the gut wall. It is also of relevance that 3 stenotic lesions with prestenotic dilatations regressed during infliximab treatment. A prestenotic dilatation is usually considered to be the result of a long-term stabilized and non-compliant luminal stricture due to circumferential fibrotic thickening of the gut wall. The intimate texture of this increased gut wall thickness cannot be ascertained with SICUS. It seems, however, that Infliximab treatment can remodel it[18]. This event is possible because, in CD, fibrosis is mainly produced by mesenchymal cells, myocytes, interstitial cells of Cajal, and fibroblasts, all of which can proliferate and transform in fibrogenic cells or, under favorable conditions, redifferentiate into non-fibrogenic cells[19–27]. Several pro- and anti-fibrogenic factors and intestinal fibroblasts participate in the development of intestinal fibrosis[28] and should be antagonized to prevent fibrosis. In a murine model, chronic inflammation-induced intestinal fibrosis could be prevented by means of antisense NF-κB[29]. In the present study, it would appear that infliximab acts on the Crohn’s lesion of the gut wall by either stopping or reversing the evolution of the stenotic process. However, in the patients investigated, no relevant clinical factors with high inter- and intra-individual variability predicted the response of the stenotic lesions to infliximab, indicating the degree of reversible inflammation and fibrosis may differ from one subject to another and may differ from one stenosis to another in the same subject.
Complete or partial regression of the stenotic lesions did occur early after induction, or late after several, up to 22, cycles of infliximab administration.
The great time and cycle response variability in the non-progression and regression of the stenoses may imply the effect of infliximab on the lesion may depend on at which stage of the CD fibrogenetic process infliximab is administered. Supportive of this hypothesis is the experimental evidence infliximab downregulates basic fibroblast growth factor and vascular endothelial growth factors involved in the process of intestinal fibrogenesis in CD[30]. Theoretically, the earlier in the process of fibrogenesis infliximab is administered, the greater the probability to prevent stricture formation. Indeed, in two operated patients with early therapy after postsurgical recurrence there was a prompt regression of stenotic lesion, and in one of them, complete disappearance of pretreatment endoscopic and SICUS alterations were observed.
In conclusion, within the time-limit of the follow-up, it would appear infliximab is able to modify the expected time course of the disease in a relevant number of treated patients by stopping further development of, or causing regression of, stenotic lesions, thus being helpful to postpone or avoid surgical interventions.
Longer follow-up studies and properly structured clinical trials are needed to assess whether infliximab loses its response over time and whether the potential risks of infliximab therapy outweigh the possible benefits.
Strictures are common features in Crohn’s disease (CD). Several studies suggest that, in CD, fibrosis is mainly produced by mesenchymal cells, myocytes, interstitial cells of Cajal and fibroblasts, all of which can proliferate and transform into fibrogenic cells or, under favorable conditions, redifferentiate in non-fibrogenic cells. Anti-TNF agents induce mucosal healing. Concerns have been raised that rapid healing of narrowed segments induced by anti-TNF agents may further narrow the lumen. Several differing studies support the concept of an antifibrogenic role for infliximab, possibly down-regulating collagen production restoring migration and reducing collagen production of CD myofibroblasts.
Follow-up studies based on the use of available imaging techniques to investigate, from different points of view, in vivo, the CD-involved intestinal wall and the response to different treatments.
The strengths of this study are: (1) The prospective collection of data; (2) the morphological assessment of CD stenosis; (3) the relatively long duration of the follow-up of patients with small bowel stenosis who had been treated with infliximab therapy; and (4) the study was not supported by any pharmaceutical company and the authors have no conflicts of interests.
These data suggest the presence of stenosis in patients with severe CD does not contraindicate anti-TNF treatment. If these data can be confirmed in an ad hoc randomized controlled study performed in a larger sample size, treatment with infliximab: (1) Could be indicated in patients with stenotic lesions requiring surgery; (2) may delay or even remove the need for surgery, and thus, favorably change the natural history of CD in patients with stenotic lesions of the small bowel.
Small intestine contrast ultrasonography (SICUS) was not different from other structures (i.e bladder, gallbladder, stomach). Filling the small intestine with fluid enables visualization of the intestinal wall and lumen. Macrogol links to water that remains in the intestinal lumen. After the ingestion of 375 mL-500 mL of macrogol solution, it is possible to visualize the entire small intestine from the Treitz to the ileo-cecal valve.
This is an interesting small series showing infliximab does not appear to have the major adverse effect on Crohn’s strictures that was at one time feared.
| 1. | Toy LS, Scherl EJ, Kornbluth A, Marion JF, Greenstein AJ, Agus S, Gerson C, Fox N, Present DH. Complete bowel obstruction following initial response to infliximab therapy for Crohn’s disease: A series of a newly described complications. Gastroenterology. 2000;118:2974. |
| 2. | Vasilopoulos S, Kugathasan S, Saeian K, Emmons JE, Hogan WJ, Otterson MF, Telford GL, Binion DG. Intestinal strictures complicating initially successful infliximab treatment for luminal Crohn’s disease. Am J Gastroenterol. 2000;95:2503. |
| 3. | Lichtenstein GR, Olson A, Travers S, Diamond RH, Chen DM, Pritchard ML, Feagan BG, Cohen RD, Salzberg BA, Hanauer SB. Factors associated with the development of intestinal strictures or obstructions in patients with Crohn’s disease. Am J Gastroenterol. 2006;101:1030-1038. |
| 4. | Louis E, Collard A, Oger AF, Degroote E, Aboul Nasr El Yafi FA, Belaiche J. Behaviour of Crohn's disease according to the Vienna classification: changing pattern over the course of the disease. Gut. 2001;49:777-782. |
| 5. | Freeman HJ. Natural history and clinical behavior of Crohn's disease extending beyond two decades. J Clin Gastroenterol. 2003;37:216-219. |
| 6. | Cosnes J, Nion-Larmurier I, Beaugerie L, Afchain P, Tiret E, Gendre JP. Impact of the increasing use of immunosuppressants in Crohn's disease on the need for intestinal surgery. Gut. 2005;54:237-241. |
| 7. | Cosnes J, Cattan S, Blain A, Beaugerie L, Carbonnel F, Parc R, Gendre JP. Long-term evolution of disease behavior of Crohn's disease. Inflamm Bowel Dis. 2002;8:244-250. |
| 8. | Pallotta N, Baccini F, Corazziari E. Contrast ultrasonography of the normal small bowel. Ultrasound Med Biol. 1999;25:1335-1340. |
| 9. | Pallotta N, Baccini F, Corazziari E. Small intestine contrast ultrasonography. J Ultrasound Med. 2000;19:21-26. |
| 10. | Pallotta N, Tomei E, Viscido A, Calabrese E, Marcheggiano A, Caprilli R, Corazziari E. Small intestine contrast ultrasonography: an alternative to radiology in the assessment of small bowel disease. Inflamm Bowel Dis. 2005;11:146-153. |
| 11. | Calabrese E, La Seta F, Buccellato A, Virdone R, Pallotta N, Corazziari E, Cottone M. Crohn's disease: a comparative prospective study of transabdominal ultrasonography, small intestine contrast ultrasonography, and small bowel enema. Inflamm Bowel Dis. 2005;11:139-145. |
| 12. | Shivananda S, Lennard-Jones J, Logan R, Fear N, Price A, Carpenter L, van Blankenstein M. Incidence of inflammatory bowel disease across Europe: is there a difference between north and south? Results of the European Collaborative Study on Inflammatory Bowel Disease (EC-IBD). Gut. 1996;39:690-697. |
| 13. | Holtmann MH, Neurath MF. Anti-TNF strategies in stenosing and fistulizing Crohn's disease. Int J Colorectal Dis. 2005;20:1-8. |
| 14. | Barberani F, Boschetto S, Gigliozzi A, Giovannone M, Tosoni M. Safety and effectiveness of endoscopic pneumatic dilatation (EPD) + Infliximab combined therapy in stenosing Crohn’s disese. Gastroenterology. 2006;130:A658. |
| 15. | Ulloa L, Doody J, Massague J. Inhibition of transforming growth factor-beta/SMAD signalling by the interferon-gamma/STAT pathway. Nature. 1999;397:710-713. |
| 16. | D'haens G, Van Deventer S, Van Hogezand R, Chalmers D, Kothe C, Baert F, Braakman T, Schaible T, Geboes K, Rutgeerts P. Endoscopic and histological healing with infliximab anti-tumor necrosis factor antibodies in Crohn's disease: A European multicenter trial. Gastroenterology. 1999;116:1029-1034. |
| 17. | Baert FJ, D'Haens GR, Peeters M, Hiele MI, Schaible TF, Shealy D, Geboes K, Rutgeerts PJ. Tumor necrosis factor alpha antibody (infliximab) therapy profoundly down-regulates the inflammation in Crohn's ileocolitis. Gastroenterology. 1999;116:22-28. |
| 18. | Di Sabatino A, Pender SL, Jackson CL, Prothero JD, Gordon JN, Picariello L, Rovedatti L, Docena G, Monteleone G, Rampton DS. Functional modulation of Crohn's disease myofibroblasts by anti-tumor necrosis factor antibodies. Gastroenterology. 2007;133:137-149. |
| 19. | Van Assche G, Geboes K, Rutgeerts P. Medical therapy for Crohn's disease strictures. Inflamm Bowel Dis. 2004;10:55-60. |
| 20. | Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts. II. Intestinal subepithelial myofibroblasts. Am J Physiol. 1999;277:C183-C201. |
| 21. | Baugh MD, Perry MJ, Hollander AP, Davies DR, Cross SS, Lobo AJ, Taylor CJ, Evans GS. Matrix metalloproteinase levels are elevated in inflammatory bowel disease. Gastroenterology. 1999;117:814-822. |
| 22. | Pender SL, MacDonald TT. Matrix metalloproteinases and the gut - new roles for old enzymes. Curr Opin Pharmacol. 2004;4:546-550. |
| 23. | von Lampe B, Barthel B, Coupland SE, Riecken EO, Rosewicz S. Differential expression of matrix metalloproteinases and their tissue inhibitors in colon mucosa of patients with inflammatory bowel disease. Gut. 2000;47:63-73. |
| 24. | Louis E, Ribbens C, Godon A, Franchimont D, De Groote D, Hardy N, Boniver J, Belaiche J, Malaise M. Increased production of matrix metalloproteinase-3 and tissue inhibitor of metalloproteinase-1 by inflamed mucosa in inflammatory bowel disease. Clin Exp Immunol. 2000;120:241-246. |
| 25. | Graham MF. Pathogenesis of intestinal strictures in Crohn’s disease. Inflamm Bowel Dis. 1995;1:220-227. |
| 26. | McKaig BC, Hughes K, Tighe PJ, Mahida YR. Differential expression of TGF-beta isoforms by normal and inflammatory bowel disease intestinal myofibroblasts. Am J Physiol Cell Physiol. 2002;282:C172-C182. |
| 27. | Rieder F, Brenmoehl J, Leeb S, Scholmerich J, Rogler G. Wound healing and fibrosis in intestinal disease. Gut. 2007;56:130-139. |
| 28. | Sans M, Masamunt MC. Fibrogenesis and inflammatory bowel disease. Gastroenterol Hepatol. 2007;30:36-41. |
| 29. | Lawrance IC, Wu F, Leite AZ, Willis J, West GA, Fiocchi C, Chakravarti S. A murine model of chronic inflammation-induced intestinal fibrosis down-regulated by antisense NF-kappa B. Gastroenterology. 2003;125:1750-1761. |
| 30. | Di Sabatino A, Ciccocioppo R, Benazzato L, Sturniolo GC, Corazza GR. Infliximab downregulates basic fibroblast growth factor and vascular endothelial growth factor in Crohn's disease patients. Aliment Pharmacol Ther. 2004;19:1019-1024. |