Published online Mar 21, 2008. doi: 10.3748/wjg.14.1785
Revised: July 20, 2008
Published online: March 21, 2008
AIM: To explore whether cyclooxygenase 2 (COX-2) -765G>C polymorphism is associated with susceptibility of colorectal cancer (CRC) and to evaluate the risk of colorectal cancer in relation to environmental exposures and polymorphism.
METHODS: We conducted a case-control study of 137 patients with colorectal cancer and 199 cancer-free controls in northeast China. Multivariate logistic regression analysis was performed to calculate the adjusted odds ratio (OR) and 95% confidence interval (95% CI).
RESULTS: The -765G>C polymorphism was not independently associated with CRC risk. However, risk associated with the polymorphism differed by smoking and body mass index (BMI). Smoking and BMI associated risks were stronger among those with -765GG genotype, showing that smokers had a 2.682-fold greater risk of CRC than nonsmokers (51/43 vs 68/126, P = 0.006). Compared to those with a normal body mass index (BMI 18.5-22.9), those with overweight (BMI 23-24.9) had a 3.909-fold higher risk of CRC (OR = 3.909, 95% CI = 2.081-7.344; P < 0.001), while those with obesity (BMI > 25) had a 2.031- fold higher risk of CRC (OR = 1.107, 95% CI = 1.107-3.726; P = 0.022).
CONCLUSION: Although COX-2 -765G>C polymorphism is not associated with an increased risk of CRC, -765GG genotype appears to be related to an increased risk in the presence of smoking and higher BMI.
-
Citation: Xing LL, Wang ZN, Jiang L, Zhang Y, Xu YY, Li J, Luo Y, Zhang X.
Cyclooxygenase 2 polymorphism and colorectal cancer: -765G>C variant modifies risk associated with smoking and body mass index. World J Gastroenterol 2008; 14(11): 1785-1789 - URL: https://www.wjgnet.com/1007-9327/full/v14/i11/1785.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.1785
Cyclooxygenases (COXs) are rate-limiting enzymes for prostaglandin production [1]. Two isoforms were described, the constitutively expressed COX-1 and the inducible isoform COX-2[2]. COX-2 is expressed at low levels in most tissues but high in inflammatory states[3], and is induced by a variety of stimulators including cytokines[4], growth factors[5], as well as tumor promoters[6]. Prostaglandin synthesis by the COX-2 is regulatory compounds that play major roles in the inflammatory response[2]. Chronic inflammation is responsible for the development and progression of many common cancers. It is well established that patients with inflammatory bowel disease are at increased risk of developing colorectal cancer (CRC)[78].
COX-2 promoter region contains multiple regulatory elements, such as nuclear factor-Kb (NF-κB) binding site, nuclear factor interleukin-6(NF-IL6)/CCAAT/enhancer-binding protein(C/EBP) binding site, cyclic AMP-response element (CRE) and activation protein 1 (AP-1). The regulation of COX-2 gene expression could involve complex interaction among them[9]. Growing evidences indicate that genetic variants in the promoters of COX-2 gene may modulate risk for breast cancer[10], gastric adenocarcinoma[11], prostate cancer[12] and colorectal adenoma[13]. A common promoter variant, -765G>C (rs20417), a G to C transversion resulting in significantly lower promoter activity, and reduced levels of C-reactive protein (CRP), a systemic marker of inflammation[14].
In this study, we explored the association between the COX-2 promoter variant (-765G>C) and the risk for CRC. In view of that the environmental exposures are associated with an increased risk of CRC, we investigated interactions between the polymorphism and the environmental factors such as smoking status, intake of alcohol and BMI.
Unrelated subjects from Shenyang of China were enrolled for case-control studies of risk factors for CRC. The trial recruited 137 CRC patients and 199 healthy control subjects. Cases were patients with a histologically confirmed diagnosis of CRC in the First Affiliated Hospital of China Medical University and Shenyang Hospital of Anal Diseases, between 2005 and 2006. The CRC patients included 71 men and 66 women, and their median age was 61.29 years. The patients were grouped according to the TNM-classification (UICC) based on the postoperative histopathology evaluation.
Control subjects were randomly selected among the people admitted to the same hospital during the same period. There were 104 men and 95 women; the median age was 60.65 years. They had no histories of cancer.
All subjects were consent to participate in the study, and allow their blood samples to be analyzed. Detailed information on risk factors including tobacco and alcohol consumption and higher BMI were obtained with a baseline questionnaire. This study used the suggested WHO BMI cutoff points for Asians to assess several variables, respondents whose BMI was less than 23 kg/m2 were categorized as having normal weight, and respondents whose BMI was 23 kg/m2 or higher were categorized as being overweight and obese).
DNA extraction: Five mL of venous blood was collected from each subject. Genomic DNA was extracted using proteinase K digestion followed by a salting out procedure.
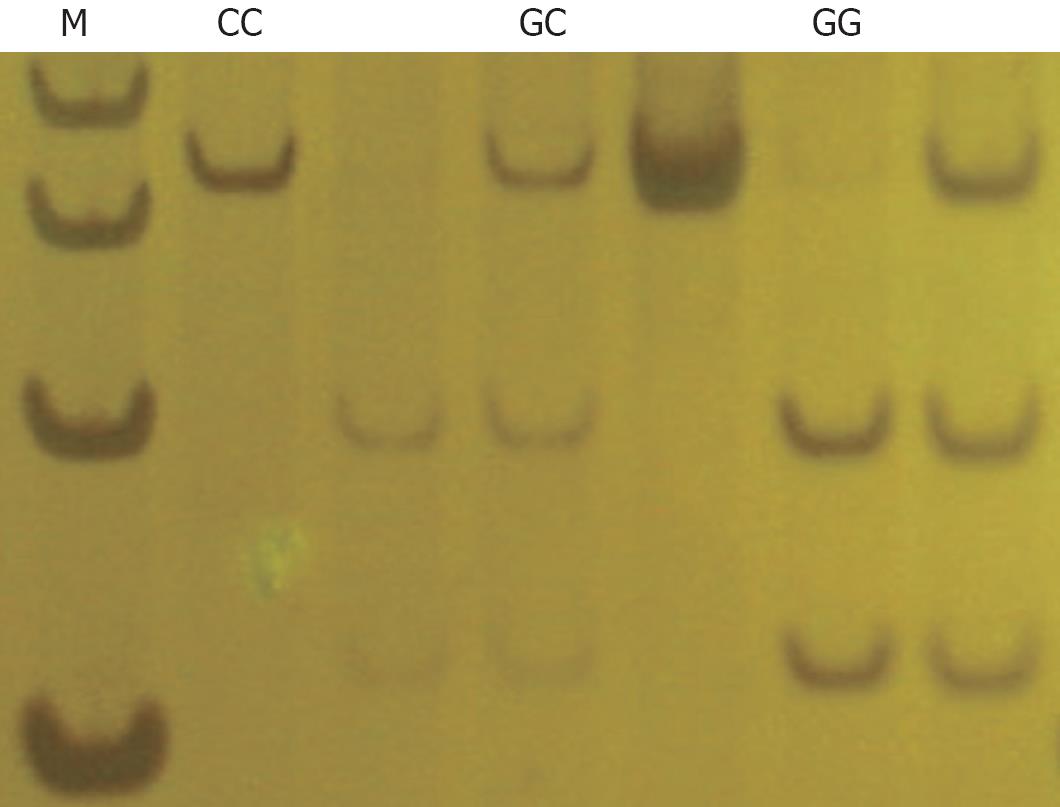
SNP genotyping: The COX-2 -765G>C genotypes were determined by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) assay. The primers were 5’-GGCTGTATATCTGCTCTATATGC -3’ (forward) and 5’-CCGCTTCCTTTGTCCATCAG-3’ (reverse). The target sequence was amplified in a 20 &mgr;L volume containing 20 ng DNA template, 2.0 &mgr;L 10 × PCR buffer, 0.5 U Taq-DNA-polymerase, 20 pmol of each primer and 1.6 &mgr;L 2.5 mmol/L dNTP. Amplification was performed for 1 min at 94°C and followed by 35 cycles of 30 s at 94°C, 30 s at 59°C, and 30 s at 72°C, and with a final step at 72°C for 1 min. The PCR products were then digested with Aci1 (New England BioLabs) and separated on 8% polyacrylamide gel electrophoresis. After electrophoresis, homozygous C allele was represented by a DNA band sized at 306 bp, whereas homozygous G allele was represented by a DNA band sized at 118 bp and 188 bp, and heterozygotes sized at 306 bp, 118 bp and 188 bp (Figure 1).
Odds ratios (ORs) and 95% confidence intervals (CIs) were estimated by logistic regression analyses based on the comparison of genotypes between CRC patients and healthy controls using SPSS version 13.0, adjusting for the potential confounders such as age, gender, tobacco use, alcohol use, and BMI. The asymptomatic Pearson’s χ2 test was used to assess Hardy-Weinberg equilibrium. Data were considered significant when P < 0.05.
Characteristics of the study population and the association with CRC are presented in Table 1. There were no significant differences in terms of distributions in age and gender between the cases and controls (P = 0.325 and 0.951, respectively), but the cases tended to have a higher body mass index (P < 0.001) and more likely to smoke cigarettes (P < 0.001).
| Controls/cases | OR 95% CI | |
| Sex | ||
| Male | 101/70 | 1 |
| Female | 98/67 | 0.986 (0.638-1.524) |
| Age (yr) | ||
| ≤ 60 | 98/60 | 1 |
| > 60 | 101/77 | 1.245 (0.804-1.928) |
| Smoking status | ||
| Non-smoker | 147/75 | 1 |
| Smoker | 52/62 | 2.337 (1.473-3.708)b |
| Alcohol duration (yr) | ||
| Never | 168/103 | 1 |
| 1-15 | 16/14 | 1.427 (0.669-3.046) |
| > 15 | 15/20 | 2.175 (1.066-4.437) |
| Body mass index (kg/m2) | ||
| 18.5-22.9 | 101/35 | 1 |
| 23-24.9 | 38/54 | 4.101 (2.329-7.220) |
| > 25 | 60/48 | 2.309 (1.345-3.962)d |
Distribution of the COX-2 -765G>C polymorphism genotypes in CRC patients and control subjects is shown in Table 2. Distribution of genotypes in controls was in good agreement with Hardy-Weinberg equilibrium (P = 0.838), so did in cases (P = 0.651). Overall, there was no association between CRC risk and COX-2 -765G>C genotype. Similarly, no significant association was observed between -765G>C polymorphism and clinicopathological parameters among CRC patients (Table 3).
| Controls/cases | OR1 95% CI | |
| COX-2 genotype | ||
| GG | 169/119 | 1 |
| GC | 29/17 | 0.867 (0.461-1.632) |
| CC | 1/1 |
| -765G>C GG/GC+CC | OR1 95% CI | |
| Age (yr) | ||
| < 60 | 49/11 | 1 |
| ≥ 60 | 70/7 | 0.445 (0.161-1.230) |
| Sex | ||
| Male | 60/10 | 1 |
| Female | 59/8 | 0.814 (0.300-2.204) |
| Lymph node metastasis | ||
| N(-) (n = 87) | 75/12 | 1 |
| N(+) | 41/5 | 0.762 (0.251-2.314) |
| TNM classification | ||
| StageI | 22/4 | 1 |
| > Stage II | 94/13 | 0.761 (0.226-2.558) |
| External membrane invasion | ||
| (+) | 91/11 | 1 |
| (-) | 25/6 | 1.985 (0.668-5.898) |
Risk associated with the COX-2 -765G>C variant differed by smoking and BMI (Table 4). There was a positive association between cigarette smoking and development of CRC in the study population. The -765GG genotype in smokers was associated with a relatively increased risk of colorectal cancer compared to non-smokers (OR = 2.682, 95% CI = 1.336-5.385; P = 0.006). The intake of alcohol was not positive for CRC development in this study. The effect of -765GG genotypes on CRC development was observed when individuals were stratified by BMI status. Compared with a normal BMI (18.5-22.9), those overweight (BMI 23-24.9) had a 3.909-fold higher risk of CRC (OR = 3.909, 95% CI = 2.081-7.344; P < 0.001), while the obese (BMI > 25) had a 2.031-fold higher risk of CRC (OR = 1.107, 95% CI = 1.107-3.726; P = 0.022).
| -765GG | -765GC+CC | |||
| Controls/cases | OR (95% CI) | Controls/cases | OR (95% CI) | |
| Smoking status | ||||
| Never | 126/68 | 1 | 21/7 | 1 |
| Smoker | 43/51 | 2.682 (1.336-5.385)b | 9/11 | 7.963 (0.615-103.051) |
| Alcohol duration (yr) | ||||
| Never | 143/89 | 1 | 25/14 | 1 |
| 1-15 | 13/12 | 0.750 (0.303-1.855) | 3/2 | 0.713 (0.044-11.599) |
| > 15 | 13/18 | 0.810 (0.259-2.531) | 2/2 | 0.199 (0.005-7.669) |
| Body mass index (BMI) | ||||
| 18.5-22.9 | 86/31 | 1 | 15/4 | 1 |
| 23-24.9 | 32/46 | 3.909 (2.081-7.344) | 6/8 | 5.128 (0.867-30.320) |
| > 25 | 51/42 | 2.031 (1.107-3.726)d | 9/6 | 6.281 (0.864-45.631) |
Numerous studies suggest that COX-2 plays an important role in the development of CRC[15]. However, the results of this study indicated that COX-2 -765G>C polymorphism was not associated with CRC in this study population, but smoking and BMI may modify the risk of CRC in COX-2 -765GG genotype.
Although the over-expression of COX-2 is closely related to the metastasis and invasion of CRC[16], and the COX-2 -765G>C polymorphism located in the putative Sp1 binding site may reduce promoter activity responsible for increasing susceptibility to CRC, our study failed to detect an association between this polymorphism and CRC.
Our null findings are consistent with the previous report of an absence of an association between -765G>C polymorphism and CRC in Spaniards[17]. Similarly, a study in Singapore Chinese suggests that the -765G>C genotype distribution in CRC patients and healthy controls was comparable, but a significant association between genotype and risk was observed among consumers of higher n-6 PUFAs[18].
In contrast, Tan et al[19] obtained contradictory positive results that the increased risk for CRC was associated with the COX-2 -765GC genotype in Chinese population. To explain this discrepancy, several points should be considered. Firstly, the different result in association between COX-2 -765G>C polymorphism and CRC may be due to the different study population. All our subjects were unrelated Han Chinese subjects, and drawn from a population pool in the northern part of China. Secondly, as the COX-2 -765C allele has lower promoter activity than the G allele, the over-expression of the COX-2 may lead to a higher risk of CRC and the G allele may be protective for CRC. In addition, the small sample size in the present study could not detect minor effect of the polymorphism on the development of CRC.
Substantial evidences indicate that significant exposure to cigarette smoke is associated with an elevated risk for CRC and could be a factor of an early onset of CRC[2021]. Cigarette smoke extract could promote tumor growth directly on colon cancer cells, the effect could likely be mediated by activation of COX-2 and up-regulation of the expression of VEGF, resulting in the induction of cellular proliferation and angiogenesis[22]. In our analysis, the COX-2-765GG genotype in smokers was associated with a significant increase in the risk of CRC compared to non-smokers.
Obesity is also a risk factor for CRC[2324], and it has been shown that body mass index was independent significant predictors of CRC[25]. In this study, a significant association was observed for interactions between the polymorphism and BMI and CRC risk, a BMI of > 23 kg/m2 was associated with an increase in the risk of CRC among -765GG genotype carriers. These findings support the hypothesis that smoking and BMI are significant risk factors for CRC. A stronger gene-environment interaction in CRC is expected.
The main limitation of our study is the lack of information on use of COX-2 inhibitors, which may cause bias in the effect of COX-2 on CRC. Another weakness of our study is the relative small study size, which may result in less precise estimation of gene-environment interaction.
In summary, our study found no association between COX-2 -765G>C polymorphism and CRC. However, our findings suggest that individuals with the -765GG genotype may be more sensitive to cigarette smoking and a higher BMI (> 23 kg/m2), perhaps attributable to the increased enzymatic activity of COX-2, and increased risk of CRC. This result is consistent with the observation that environmental factors were associated with development of CRC and that the association with smoking and BMI may differ by COX-2 -765G>C polymorphism.
Genetic and environmental factors are important in determining the susceptibility to colorectal cancer (CRC). COX-2, which is an enzyme responsible for the formation of prostaglandin H2 from arachidonic acid, regulates angiogenesis and plays a critical role in tumor progression and aggressiveness. To investigate the role of COX-2 in CRC development, the authors conducted a case-control study of CRC in northeast China.
Single nucleotide polymorphisms of COX-2 gene may affect the risk of cancer formation in humans, and cancer is a complex process involving genetic as well as environmental factors. The authors conducted this study to investigate the association between COX-2 polymorphism and CRC and to evaluate the potential interaction with exposures to smoking and BMI.
There are several reports on the association between COX-2 polymorphisms and CRC. In this study, although there was no association between CRC risk and COX-2 -765G>C genotype, risk associated with the COX-2 -765G>C variant differed by smoking and BMI. A stronger gene-environment interaction in CRC was observed.
In the present study, COX-2-765GG genotype appears to be associated with an increased risk in the presence of smoking and higher body mass index (BMI). The results can be used to the etiological studies and prevention of colorectal cancer.
Single nucleotide polymorphisms or SNPs (pronounced “snips”) are DNA sequence variations that occur when a single nucleotide (A, T, C or G) in the genome sequence is altered. Restriction fragment length polymorphism (RFLP) is a technique by which organisms may be differentiated by analysis of patterns derived from cleavage of their DNA.
The relationship between a polymorphism in cyclooxygenase-2 (COX-2) promoter region and the risk of colorectal cancer was studied in this manuscript. Although the studied polymorphism failed to show an association with colorectal cancer risk, the authors argued for positive association between this polymorphism and the risk of colorectal cancer in addition to tobacco use, alcohol intake, and obesity.
| 1. | Wendum D, Masliah J, Trugnan G, Flejou JF. Cyclooxygenase-2 and its role in colorectal cancer development. Virchows Arch. 2004;445:327-333. |
| 2. | Simmons DL, Botting RM, Hla T. Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol Rev. 2004;56:387-437. |
| 3. | Williams CS, Mann M, DuBois RN. The role of cyclooxy-genases in inflammation, cancer, and development. Oncogene. 1999;18:7908-7916. |
| 4. | Seibert K, Zhang Y, Leahy K, Hauser S, Masferrer J, Perkins W, Lee L, Isakson P. Pharmacological and biochemical demonstration of the role of cyclooxygenase 2 in inflammation and pain. Proc Natl Acad Sci USA. 1994;91:12013-12017. |
| 5. | Harrison JR, Lorenzo JA, Kawaguchi H, Raisz LG, Pilbeam C. Stimulation of prostaglandin E2 production by interleukin-1 alpha and transforming growth factor alpha in osteoblastic MC3T3-E1 cells. J Bone Miner Res. 1994;9:817-823. |
| 6. | Subbaramaiah K, Dannenberg AJ. Cyclooxygenase 2: a molecular target for cancer prevention and treatment. Trends Pharmacol Sci. 2003;24:96-102. |
| 7. | Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G7-G17. |
| 8. | Delaunoit T, Limburg PJ, Goldberg RM, Lymp JF, Loftus EV Jr. Colorectal cancer prognosis among patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2006;4:335-342. |
| 9. | Wang JM, Ko CY, Chen LC, Wang WL, Chang WC. Functional role of NF-IL6beta and its sumoylation and acetylation modifications in promoter activation of cyclooxygenase 2 gene. Nucleic Acids Res. 2006;34:217-231. |
| 10. | Gallicchio L, McSorley MA, Newschaffer CJ, Thuita LW, Huang HY, Hoffman SC, Helzlsouer KJ. Nonsteroidal antiinflammatory drugs, cyclooxygenase polymorphisms, and the risk of developing breast carcinoma among women with benign breast disease. Cancer. 2006;106:1443-1452. |
| 11. | Pereira C, Sousa H, Ferreira P, Fragoso M, Moreira-Dias L, Lopes C, Medeiros R, Dinis-Ribeiro M. -765G > C COX-2 polymorphism may be a susceptibility marker for gastric adenocarcinoma in patients with atrophy or intestinal metaplasia. World J Gastroenterol. 2006;12:5473-5478. |
| 12. | Shahedi K, Lindstrom S, Zheng SL, Wiklund F, Adolfsson J, Sun J, Augustsson-Balter K, Chang BL, Adami HO, Liu W. Genetic variation in the COX-2 gene and the association with prostate cancer risk. Int J Cancer. 2006;119:668-672. |
| 13. | Ali IU, Luke BT, Dean M, Greenwald P. Allellic variants in regulatory regions of cyclooxygenase-2: association with advanced colorectal adenoma. Br J Cancer. 2005;93:953-959. |
| 14. | Papafili A, Hill MR, Brull DJ, McAnulty RJ, Marshall RP, Humphries SE, Laurent GJ. Common promoter variant in cyclooxygenase-2 represses gene expression: evidence of role in acute-phase inflammatory response. Arterioscler Thromb Vasc Biol. 2002;22:1631-1636. |
| 15. | Sinicrope FA, Gill S. Role of cyclooxygenase-2 in colorectal cancer. Cancer Metastasis Rev. 2004;23:63-75. |
| 16. | Yao M, Lam EC, Kelly CR, Zhou W, Wolfe MM. Cyclooxy-genase-2 selective inhibition with NS-398 suppresses proliferation and invasiveness and delays liver metastasis in colorectal cancer. Br J Cancer. 2004;90:712-719. |
| 17. | Cox DG, Pontes C, Guino E, Navarro M, Osorio A, Canzian F, Moreno V. Polymorphisms in prostaglandin synthase 2/cyclooxygenase 2 (PTGS2/COX2) and risk of colorectal cancer. Br J Cancer. 2004;91:339-343. |
| 18. | Koh WP, Yuan JM, van den Berg D, Lee HP, Yu MC. Interaction between cyclooxygenase-2 gene polymorphism and dietary n-6 polyunsaturated fatty acids on colon cancer risk: the Singapore Chinese Health Study. Br J Cancer. 2004;90:1760-1764. |
| 19. | Tan W, Wu J, Zhang X, Guo Y, Liu J, Sun T, Zhang B, Zhao D, Yang M, Yu D. Associations of functional polymorphisms in cyclooxygenase-2 and platelet 12-lipoxygenase with risk of occurrence and advanced disease status of colorectal cancer. Carcinogenesis. 2007;28:1197-1201. |
| 20. | Tsong WH, Koh WP, Yuan JM, Wang R, Sun CL, Yu MC. Cigarettes and alcohol in relation to colorectal cancer: the Singapore Chinese Health Study. Br J Cancer. 2007;96:821-827. |
| 21. | Buc E, Kwiatkowski F, Alves A, Panis Y, Mantion G, Slim K. Tobacco smoking: a factor of early onset of colorectal cancer. Dis Colon Rectum. 2006;49:1893-1896. |
| 22. | Liu ES, Shin VY, Ye YN, Luo JC, Wu WK, Cho CH. Cyclooxygenase-2 in cancer cells and macrophages induces colon cancer cell growth by cigarette smoke extract. Eur J Pharmacol. 2005;518:47-55. |
| 23. | Doria-Rose VP, Newcomb PA, Morimoto LM, Hampton JM, Trentham-Dietz A. Body mass index and the risk of death following the diagnosis of colorectal cancer in postmenopausal women (United States). Cancer Causes Control. 2006;17:63-70. |
| 24. | Johnson IT, Lund EK. Review article: nutrition, obesity and colorectal cancer. Aliment Pharmacol Ther. 2007;26:161-181. |