Published online Mar 21, 2008. doi: 10.3748/wjg.14.1690
Revised: February 15, 2008
Published online: March 21, 2008
Constitutive activation of the insulin-like growth factor (IGF)-signaling axis is frequently observed in human hepatocellular carcinoma (HCC). Especially the overexpression of the fetal growth factor IGF-II, IGF-Ireceptor (IGF-IR), and cytoplasmic downstream effectors such as insulin-receptor substrates (IRS) contribute to proliferation, anti-apoptosis, and invasive behavior. This review focuses on the relevant alterations in this signaling pathway and independent in vivo models that support the central role IGF-II signaling during HCC development and progression. Since this pathway has become the center of interest as a target for potential anti-cancer therapy in many types of malignancies, various experimental strategies have been developed, including neutralizing antibodies and selective receptor kinase inhibitors, with respect to the specific and efficient reduction of oncogenic IGF-II/IGF-IR-signaling.
- Citation: Breuhahn K, Schirmacher P. Reactivation of the insulin-like growth factor-II signaling pathway in human hepatocellular carcinoma. World J Gastroenterol 2008; 14(11): 1690-1698
- URL: https://www.wjgnet.com/1007-9327/full/v14/i11/1690.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.1690
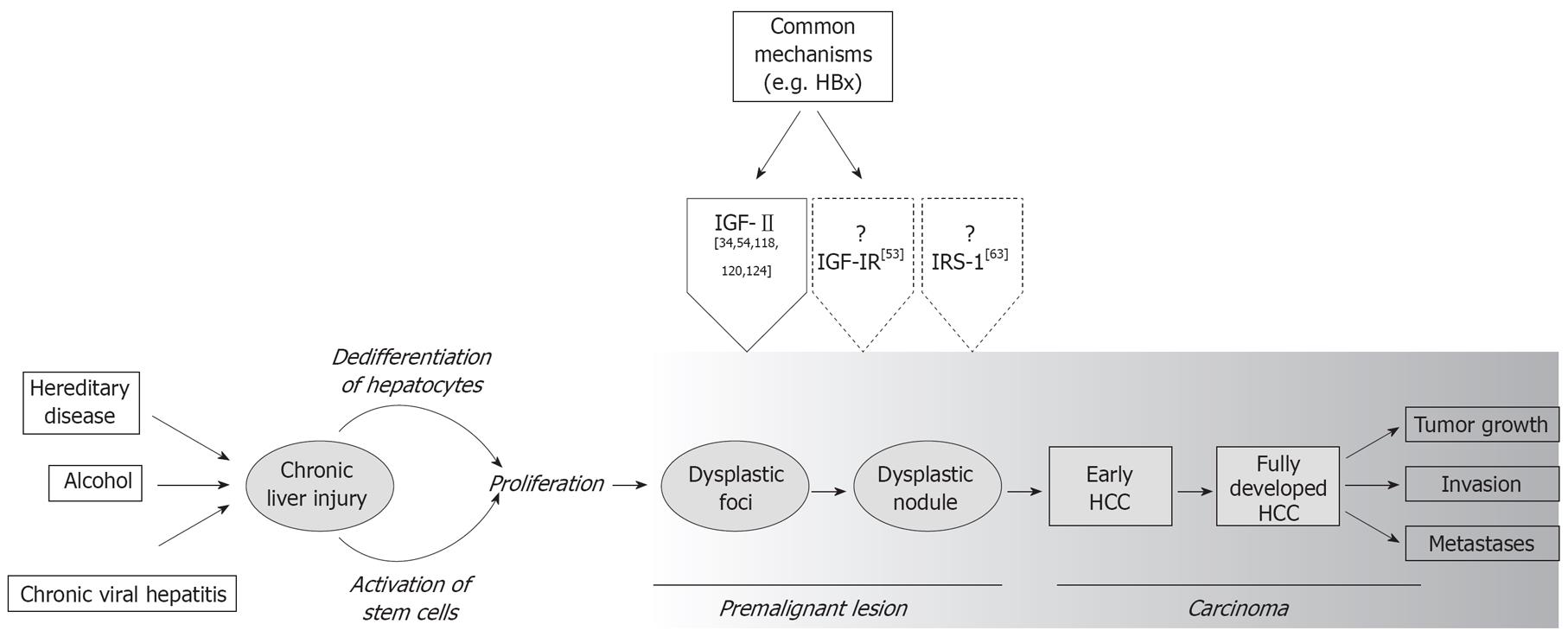
Human hepatocellular carcinoma (HCC) is considered the fifth most frequent malignancy worldwide and the third most common cause for cancer mortality with an increasing incidence in Asia and Africa, but also in industrial countries[1]. In more than 80% of cases, a well-defined etiology such as viral infection with hepatitis B- and C-viruses (HBV and HCV), aflatoxin B1 intoxication, chronic alcohol abuse, or hereditary diseases is associated with its development (Figure 1); however, clinical diagnosis of HCC is difficult due to the lack of reliable serum markers. Moreover, the therapeutic options for HCC patients are sobering due to the high angioinvasive capacity of the tumor.
Although the underlying molecular mechanisms responsible for the development and progression of HCC have not been completely delineated, it has become clear that aberrant activation of growth factor signaling pathways is a pivotal event in hepatocarcinogenesis. Besides the hepatocyte growth factor (HGF)/MET, Wingless (Wnt/frizzled/β-catenin), transforming growth factor α (TGFα)/EGF-R, and transforming growth factor β (TGFβ)/TβR-signaling, dysregulation of the evolutionary highly conserved insulin-like growth factor (IGF) pathway is critically involved in proliferation and anti-apoptosis of HCC cells associated with uncontrolled tumor growth and chemoresitance[2]. In fact, based on its central regulatory position in tumor cell homeostasis, this signaling axis is considered a promising therapeutic anti-cancer target in many human malignancies. This review focuses on the molecular changes of IGF-signaling detected in human HCC, animal model systems that underline the central role of IGF-II-signaling in hepatocarcinogenesis, as well as resulting therapeutic strategies for the treatment of human liver cancer.
The key molecules in this pathway are the ligands IGF-I and IGF-II, IGF-binding proteins (IGFBP1-6), membrane-associated receptors [IGF-Ireceptor (IGF-IR), mannose-6-phosphate receptor/IGF-II receptor (IGF-IIR)], and insulin receptor substrates (IRS-1-6).
IGF-Iand IGF-II are small, secreted molecules that are predominantly produced by the liver and which stimulate different cell types in both an autocrine and paracrine manner. These factors display differing expression kinetics as the expression of IGF-II declines while the bioavailability of IGF-Iincreases shortly after birth. Besides the transcriptional regulation (e.g. genomic imprinting of the igf-II gene promotor), ligand bioavailability is further influenced by the presence of IGFBPs in tissues and serum[3]. Secreted IGFBPs bind extracellular IGFs with affinities comparable to IGF-IR and therefore modulate ligand bioactivity. For instance, 70% of IGF-II is bound to IGFBP-3, which is the most abundant BP in serum[4]; however, depending on the cellular context, both inhibitory as well as stimulatory effects of IGFBPs on IGF-signaling have been described. All IGFBPs are substrates for proteases and their bioavailability/bioactivity is regulated by limited proteolytic cleavage with an impact on IGF-dependent physiological processes[5]. However, IGF-independent biological effects under pathophysiological conditions have also been described for several IGFPBs[6].
The signaling of IGF-Iand IGF-II is mediated by IGF-IR, a heterotetrameric protein (two α- and β-chains), which consists of an extracellular ligand binding site and an intracellular tyrosine kinase domain. IGF-IR binds IGF-Iwith 15- to 20-fold higher affinity than IGF-II[7]. Ligand binding and receptor tyrosine kinase (RTK)-dependent phosphorylation of intracellular substrates such as IRS and Src homology collagen (Shc) then lead to the activation of the phosphatidylinositol 3-kinase (PI3-kinase)/protein kinase B (PKB/AKT)-axis and the Ras/mitogen activated protein kinase (MAPK)-pathway[8]. IRS proteins are a family of six (IRS-1 to IRS-6) related adaptors that integrate and coordinate signaling of the insulin receptor (IR) and also the IGF-IR. They are responsible for most of the biological activities of IGF-IR[9].
In addition, IGF-II (but not IGF-I) efficiently binds and activates a distinct isoform of the insulin receptor lacking exon 11 (IR-A)[10–12]. IR and IGF-IR are highly homologous RTKs (up to 84% in the tyrosine kinase domain and 100% at the ATP-binding site)[13], but there are substantial functional differences between both molecules: while both receptors exert metabolic effects, IGF-IR is anti-apoptotic, mitogenic, and it facilitates a malignant phenotype[14]. However, IR-A plays a central role not only in metabolic processes, but also in IGF-II-induced migration in cells lacking IGF-IR[11]. These different biological effects are possibly based on ligand/receptor abundance, protein turnover or currently undiscovered peculiarities of the distinct signaling axes. In addition, recent findings show that structural features in the domain governing ligand specificity do distinguish IGF-IR from IR[15].
In contrast, IGF-IIR which is structurally unrelated to IGF-IR, does not exhibit cytoplasmic kinase activity[16]. Although this receptor does not directly contribute to IGF-signaling, it regulates IGF-II turnover and bioavailability through receptor-mediated endocytosis and subsequent degradation[17]. IGF-Iand insulin cross-react very weakly with IGF-IIR and therefore are not regulated by its (inhibitory) activity[18].
Alterations in the IGF-signaling pathway have been described in several adult and pediatric human tumors such as Wilms tumors[19], as well as colon[2021], lung[22], breast[2324], and prostate cancer[25]. The reactivation of IGF-signaling in HCC predominantly occurs at the level of IGF-II expression, which is secreted by the tumor cells themselves, which is suggestive of autocrine mechanisms of stimulation[2627]. This growth factor is highly expressed in the fetal liver and early after birth, but its expression is strongly reduced in adulthood in humans, mice and rats[28–30]. Several studies have shown elevated expression levels for IGF-II in preneoplastic lesions (Dysplastic Nodules) and very high levels in HCC (Table 1, Figure 1), which is mainly based on aberrant activation of the epigenetically regulated igf-II promoters P1-P4[31]. Indeed, HCCs showing high level of expression of IGF-II exhibit reconstitution of the fetal type transcription pattern due to a loss of promotor-specific imprinting and hypomethylation[2632–35]. Furthermore, viral proteins have been reported to facilitate IGF-II overexpression in HBV- and HCV-associated HCCs. For example, the HBV-derived HBx protein and the HCV-derived core gene product induce IGF-II expression through interaction with transcription factors activity such as Sp1 and Egr1[3637]. In addition, the inactivation of tumor suppressor genes such as p53 by aflatoxin-induced mutations in codon 249 increases IGF-II expression through the formation of transcriptional complexes[38].
Besides the direct transcriptional induction of IGF-II expression, additional mechanisms may contribute to elevated IGF-II bioavailability in HCC cells. Firstly, reduced levels of IGFBP-1, -2, -3, and -4 in HCCs were found to be associated with IGF overexpression[3940]. These IGFBP-based effects on IGF-concentration may be even more complex, since a reduced degradation of IGFBPs by matrix-metalloproteinases (MMPs) was regulated by tissue inhibitors of MMPs (TIMPs). The regulation of TIMP-1, which is repressed in many HCCs, is associated with changes in IGF-II abundance[4142]. Secondly, the downregulation or inactivation of IGF-IIR theoretically leads to increased concentrations of IGF-II based on insufficient internalization and degradation. Here, the reduced expression of IGF-IIR, the loss of heterozygosity (LOH) at the igf-IIr gene locus, homozygous deletions, and missense mutations with an impact on ligand binding have been described with respect to HCCs[43–49]. However, other studies did not detect any genetic alterations at the igf-IIr locus, which may be due to methodological and population-based differences[50–52]. Moreover, few studies described elevated IGF-IIR levels in HCCs[5354]. Independent of the underlying molecular mechanism, IGF-II overexpression denominates a group of HCCs with fewer tumor infiltrating lymphocytes, a lower apoptosis rate[55] and extrahepatic metastasis[56]. Thus, serum IGF-II availability was proposed as a tumor marker discriminating HCC from cirrhosis[57].
IGF-I- and IGF-II-mediated signaling may occur through IGF-IR and IR holoreceptor dimers as well as through IGF-IR/IR hemireceptor complexes[5859]. Particularly IGF-II has been shown to efficiently activate both IGF-IR and IR-A. However, our own results suggested that the presence of IR was not essential for IGF-II-mediated oncogenic properties in liver tumor cells, since efficient siRNA-dependent inhibition of IR (all isoforms) did not lead to changes in proliferation, apoptosis, or migration in HCC cells (unpublished data). Therefore, in HCC cells IGF-IR is the relevant receptor for protumorigenic IGF-II signaling. This finding is supported by the fact that IGF-IR is highly expressed in many human malignancies and that only IGF-IR-signaling is crucial for oncogenic transformation and tumor cell survival[60]. Indeed, while IGF-IR levels were constitutively low in normal hepatocytes, IGF-IR was overexpressed in HCC and HCC cell lines (Table 1). Just as it was observed for elevated IGF-II expression, viral-based molecular mechanisms and mutational inactivation of tumor suppressor genes caused IGF-IR overexpression: HBV-derived HBx protein as well as p53 mutations in codon 249 induce IGF-IR[6162], suggesting that these protumorigenic events modulate several IGF-pathway constituents such as IGF-II and IGF-IR to reach maximal (oncogenic) signaling efficiency.
Lastly, IRS-1, -2, and -4 are overexpressed in most HCCs (Table 1). So far, most analyses are reported for IRS-1, showing that elevated IRS-1 levels mediate anti-apoptosis[63], tumor cell growth[64], and mitosis[65]. Further, it has been found that the HCV-derived core protein reduced IRS-1 expression in HCC cell lines[66]. To our knowledge, no molecular mechanisms responsible for the elevated IRS-1 expression (e.g. other viral proteins) have been described so far. Whether other IRS family members serve identical functions in HCC cells has not yet been analyzed.
In summary, several lines of evidence suggest a ‘multi-hit’ model for the oncogenic activation of IGF-II signaling in HCC. Firstly, the sum of protumorigenic events detected in HCCs (e.g. increased IGF-II, IGF-IR, and IRS bioavailability) indicates the potential for multiple hits in one single tumor. Secondly, viral proteins and the inactivation of tumor suppressor genes induce several IGF-II pathway constituents. Although increased bioavailability of IGF-II appears to be the dominant mechanism in human hepatocarcinogenesis, many hits in this pathway may be necessary to obtain full malignant competence.
The pivotal oncogenic function of IGF-II-signaling in hepatocarcinogenesis is supported by several animal models. Transgenic mice expressing IGF-II (20-30-fold increased levels in serum) develop hypoglycemia and many types of malignancies, which are most frequently HCC[67]. In contrast, overexpression of IRS-1 is associated with increased DNA-synthesis, but liver tumor development was not detected[68]. In knockout model systems the disruption of the igf-IIr gene leads to elevated IGF-II levels; but since these animals exhibit lethal organ abnormalities (e.g. organomegaly), no further studies concerning liver tumor development have been carried out[69–71].
In addition to these IGF-pathway-specific transgenic and knockout animals, additional models, initially not intended for the examination of the IGF-axis, supported the functional relevance of especially dysregulated IGF-II in hepatocarcinogenesis. Both mice with liver-directed expression of SV40T-Ag or HBV presurface gene products (preS1 and preS2) developed HCCs, which is associated with a high level of IGF-II expression[72]. Moreover, transgenic mice overexpressing the woodchuck hepatitis virus/c-MYC[73], c-MYC[74], and TGFα[75] developed HCCs accompanied by elevated IGF-II expression in the tumors. Equally, liver tumors in p53-null animals exhibited increased amounts of IGF-II as compared to normal littermates after delivery of polyoma virus middle T antigen (PyMT)[76].
Cross-breeding experiments underlined the importance of IGF-II-signaling in hepatocarcinogenesis. Interbreeding of IGF-II knock-out mice with SV40T-Ag animals resulted in a reduced frequency (up to 15-fold) and size of liver tumors as compared to animals only expressing the oncogene[77], suggesting an important role of IGF-II-signaling in tumor progression. This anti-tumorigenic effect for IGF-II-deficiency in tumor models was supported by similar results in animals expressing SV40T-Ag in Langerhans cells showing widely identical results[30]. In a more indirect approach, TIMP1 overexpression reduced IGF-II-driven HCC development in SV40T-Ag transgenic animals based on reduced tumor cell proliferation and vascularization[417879]. However, it is also noteworthy that mice expressing the c-MYC oncogene and which are deficient for IGF-IR only showed a marginally reduced HCC incidence compared to animals expressing the oncogene alone[74].
The functional connection between the viral infection of hepatocytes and IGF-II abundance was supported by studies utilizing the woodchuck model system. After woodchuck hepatitis virus (WHV) infection, a high level of IGF-II expression was detected in precancerous woodchuck liver and in up to 45% of HCCs, which correlates with repressed viral DNA replication and n-MYC expression in early precancerous lesions[3480]. Further studies revealed that IGF-II availability protected from n-MYC-induced apoptosis especially under serum-free conditions[81]. Therefore, the selection for cells with high IGF-II levels may rescue a more unfavorable tumor phenotype and therefore promote tumor progression. Lastly, a reactivation of IGF-II expression in experimentally induced liver tumors using different chemical substances (3’-Me-DAB, 2-AAF, DENA) has been described in rats[82–84].
These data clearly show that IGF-II overexpression and intactness of the IGF-II/IGF-IR pathway is also a common event in murine liver tumor development, independent of the underlying molecular mechanisms (e.g. oncogene activity, regeneration processes, chemically induced carcinogenesis)[72].
IGF-II is highly expressed during prenatal development and early after birth but levels rapidly decline in adulthood[2829]. Since IGF-II signaling is frequently reactivated in human hepatocarcinogenesis, inhibition of this pathway unlikely affects normal liver function under physiological conditions and therefore represents a favorable therapeutic strategy. Several techniques have been developed to modulate the activity of IGF-(II) signaling in different tumor cell types[85]. Many approaches, such as neoexpression of dominant-negative receptor mutants (dnIGF-IR) or transfection of IGF-IR-specific antisense oligodeoxynucleotides, attained convincing inhibitory effects on IGF/IGF-IR signaling in vitro and in vivo[85]. However, neutralizing antibodies binding IGF-IR and IGF-IR-specific small inhibitory molecules are currently the most promising therapeutic and clinically relevant approaches[60].
Recently, numerous blocking antibodies recognizing different membrane-bound RTKs such as EGF-R/HER1 (Cetuximab/Erbitux) and HER2 (Trastuzumab/Herceptin) have been developed[86]. Besides IGF-II-binding antibodies that physically inhibit ligand/receptor interaction[8788], many neutralizing antibodies specific for IGF-IR have been described such as alpha-IR3[89], mAb391[90], scFv-FC[91], CP-751,871[92], IMC-A12[93], 7H2HM[94] EM164[95], h7C10[96], 4G11[97], 19D12[98], R1507[60], AMG479[60], and 19D12[60]. Reduced IGF/IGF-IR signaling is presumably based upon lysosome-dependent degradation of IGF-IR[9091]. Since proteasome inhibitors (e.g., Brefeldin) as well as protein synthesis inhibitors (e.g., cyclohexamide) did not affect antibody-dependent downregulation of the receptor[9091], it has been speculated that anti-IGF-IR antibodies hampered steady-state protein turnover based on endosomal accumulation of antibody/receptor complexes[99]. Although the anti-tumor effects of these antibodies were tested for several different cell types in preclinical studies, no comprehensive analyses regarding the anti-tumorigenic impact on HCC cells have been published to date. However, it is noteworthy that for other tumor entities, clinical trials for antibodies targeting IGF-IR have been launched such as CP-751, 871 (Pfizer), IMC-A12 (ImClone Systems), R1507 (Roche), and AMG479 (Amgen)[60].
In addition to neutralizing antibodies, small molecule inhibitors targeting RTKs such as EGF-R/HER1 (Gefitinib/Iressa), BCR/ABL fusion product (Glivec/Imatinib), or cellular kinases (the multi-kinase inhibitor Sorafenib/Nexavar recognizing VEGF-R, PDGF-R, c-kit, Raf, and RET) have been developed. Since IGF-IR and the IR are structurally related, highly specific IGF-IR inhibitors are necessary to prevent diabetogenic effects in patients. Published IGF-IR-selective RTK-inhibitors are tyrphostins (AG538[100101], AG1024[102], AG1034[102]), cyclolignans (picropodophyllin[103104]), 6-5 ring-fused compounds[105], pyrrole derivatives (NVP-AEW541[106107], NVP-ADW742[108109]), PQIP[110], BMS536924[111], and BMS-554417[112]. Anti-tumorigenic effects of some inhibitors on HCC cells have been demonstrated. The application of NVP-AEW541[113] and picropodophyllin (Nussbaum et al, unpublished data) was shown to reduce tumor cell proliferation and increase apoptosis. Equally, IGF-II induced tumor cell motility was reduced by picropodophyllin (Nussbaum et al, unpublished data). In addition, the inhibition of IGF-IR-signaling by a combination of AG1024 and EGF-R-signaling by RTK-inhibitors or blocking antibodies synergistically reduced tumor growth[114115]. However, NVP-ADW742 affects the viability of hepatocytes in a concentration-dependent manner. This RTK-inhibitor potentiated bile acid-induced cell death in normal hepatocytes, suggesting liver toxicity in patients with aberrant bile flow[116]. Because IGF-IR signaling is almost absent in normal hepatocytes, it is questionable whether these effects were IGF-dependent or independent. Thus, the effects of IGF-IR-specific inhibition on normal and diseased liver have to be analyzed carefully.
Although the anti-tumorigenic effects of IGF-IR-specific small molecules have been analyzed in numerous tumor cell types in preclinical setups[60], to our knowledge, no clinical trials have been initiated to date.
Several components of the IGF-signaling axis, such as IGF-II, IGF-IR and IRS, are frequently dysregulated in human hepatocarcinogenesis. The oncogenic reactivation of IGF-II-signaling has been verified in several in vivo models and supports the therapeutic relevance of this pathway. However, aberrant growth factor bioactivity involved in tumor development cannot be understood in a mono-dimensional manner since an intense cross-talk between IGF-IR signaling and other oncogenic pathways have been described[2]. Indeed, first functional studies revealed the necessity for multi-modal approaches for optimal anti-tumorigenic results and dose reduction. Therefore, it is questionable whether the highest specificity is the ‘gold standard’ for efficient treatment of malignancies, especially with respect to the development of (IGF-IR specific) RTK inhibitors. Thus, inhibitors targeting IGF-IR and other RTKs or combinations of different specific substances targeting distinct pathways might be attractive therapeutic approaches in the future.
| 1. | El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557-2576. |
| 2. | Breuhahn K, Longerich T, Schirmacher P. Dysregulation of growth factor signaling in human hepatocellular carcinoma. Oncogene. 2006;25:3787-3800. |
| 3. | Murphy LJ. Insulin-like growth factor-binding proteins: functional diversity or redundancy? J Mol Endocrinol. 1998;21:97-107. |
| 4. | Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16:3-34. |
| 5. | Maile LA, Holly JM. Insulin-like growth factor binding protein (IGFBP) proteolysis: occurrence, identification, role and regulation. Growth Horm IGF Res. 1999;9:85-95. |
| 6. | Firth SM, Baxter RC. Cellular actions of the insulin-like growth factor binding proteins. Endocr Rev. 2002;23:824-854. |
| 7. | Germain-Lee EL, Janicot M, Lammers R, Ullrich A, Casella SJ. Expression of a type I insulin-like growth factor receptor with low affinity for insulin-like growth factor II. Biochem J. 1992;281:413-417. |
| 8. | Kurmasheva RT, Houghton PJ. IGF-I mediated survival pathways in normal and malignant cells. Biochim Biophys Acta. 2006;1766:1-22. |
| 9. | Myers MG Jr, Sun XJ, White MF. The IRS-1 signaling system. Trends Biochem Sci. 1994;19:289-293. |
| 10. | Denley A, Bonython ER, Booker GW, Cosgrove LJ, Forbes BE, Ward CW, Wallace JC. Structural determinants for high-affinity binding of insulin-like growth factor II to insulin receptor (IR)-A, the exon 11 minus isoform of the IR. Mol Endocrinol. 2004;18:2502-2512. |
| 11. | Denley A, Brierley GV, Carroll JM, Lindenberg A, Booker GW, Cosgrove LJ, Wallace JC, Forbes BE, Roberts CT Jr. Differential activation of insulin receptor isoforms by insulin-like growth factors is determined by the C domain. Endocrinology. 2006;147:1029-1036. |
| 12. | Frasca F, Pandini G, Scalia P, Sciacca L, Mineo R, Costantino A, Goldfine ID, Belfiore A, Vigneri R. Insulin receptor isoform A, a newly recognized, high-affinity insulin-like growth factor II receptor in fetal and cancer cells. Mol Cell Biol. 1999;19:3278-3288. |
| 13. | Ullrich A, Gray A, Tam AW, Yang-Feng T, Tsubokawa M, Collins C, Henzel W, Le Bon T, Kathuria S, Chen E. Insulin-like growth factor I receptor primary structure: comparison with insulin receptor suggests structural determinants that define functional specificity. EMBO J. 1986;5:2503-2512. |
| 14. | Baserga R, Hongo A, Rubini M, Prisco M, Valentinis B. The IGF-I receptor in cell growth, transformation and apoptosis. Biochim Biophys Acta. 1997;1332:F105-F126. |
| 15. | Lou M, Garrett TP, McKern NM, Hoyne PA, Epa VC, Bentley JD, Lovrecz GO, Cosgrove LJ, Frenkel MJ, Ward CW. The first three domains of the insulin receptor differ structurally from the insulin-like growth factor 1 receptor in the regions governing ligand specificity. Proc Natl Acad Sci USA. 2006;103:12429-12434. |
| 16. | Kornfeld S. Structure and function of the mannose 6-phosphate/insulinlike growth factor II receptors. Annu Rev Biochem. 1992;61:307-330. |
| 17. | Oka Y, Rozek LM, Czech MP. Direct demonstration of rapid insulin-like growth factor II Receptor internalization and recycling in rat adipocytes. Insulin stimulates 125I-insulin-like growth factor II degradation by modulating the IGF-II receptor recycling process. J Biol Chem. 1985;260:9435-9442. |
| 18. | Roth RA. Structure of the receptor for insulin-like growth factor II: the puzzle amplified. Science. 1988;239:1269-1271. |
| 19. | Reeve AE, Eccles MR, Wilkins RJ, Bell GI, Millow LJ. Expression of insulin-like growth factor-II transcripts in Wilms’ tumour. Nature. 1985;317:258-260. |
| 20. | Baghdiguian S, Verrier B, Gerard C, Fantini J. Insulin like growth factor I is an autocrine regulator of human colon cancer cell differentiation and growth. Cancer Lett. 1992;62:23-33. |
| 21. | Lambert S, Collette J, Gillis J, Franchimont P, Desaive C, Gol-Winkler R. Tumor IGF-II content in a patient with a colon adenocarcinoma correlates with abnormal expression of the gene. Int J Cancer. 1991;48:826-830. |
| 22. | Favoni RE, de Cupis A, Ravera F, Cantoni C, Pirani P, Ardizzoni A, Noonan D, Biassoni R. Expression and function of the insulin-like growth factor I system in human non-small-cell lung cancer and normal lung cell lines. Int J Cancer. 1994;56:858-866. |
| 23. | Toropainen E, Lipponen P, Syrjanen K. Expression of insulin-like growth factor I (IGF-I) in female breast cancer as related to established prognostic factors and long-term prognosis. Eur J Cancer. 1995;31A:1443-1448. |
| 24. | Toropainen EM, Lipponen PK, Syrjanen KJ. Expression of insulin-like growth factor II in female breast cancer as related to established prognostic factors and long-term prognosis. Anticancer Res. 1995;15:2669-2674. |
| 25. | Chan JM, Stampfer MJ, Giovannucci E, Gann PH, Ma J, Wilkinson P, Hennekens CH, Pollak M. Plasma insulin-like growth factor-I and prostate cancer risk: a prospective study. Science. 1998;279:563-566. |
| 26. | Cariani E, Lasserre C, Seurin D, Hamelin B, Kemeny F, Franco D, Czech MP, Ullrich A, Brechot C. Differential expression of insulin-like growth factor II mRNA in human primary liver cancers, benign liver tumors, and liver cirrhosis. Cancer Res. 1988;48:6844-6849. |
| 27. | Lund P, Schubert D, Niketeghad F, Schirmacher P. Autocrine inhibition of chemotherapy response in human liver tumor cells by insulin-like growth factor-II. Cancer Lett. 2004;206:85-96. |
| 28. | Kiess W, Yang Y, Kessler U, Hoeflich A. Insulin-like growth factor II (IGF-II) and the IGF-II/mannose-6-phosphate receptor: the myth continues. Horm Res. 1994;41 Suppl 2:66-73. |
| 29. | Li X, Cui H, Sandstedt B, Nordlinder H, Larsson E, Ekstrom TJ. Expression levels of the insulin-like growth factor-II gene (IGF2) in the human liver: developmental relationships of the four promoters. J Endocrinol. 1996;149:117-124. |
| 30. | Christofori G, Naik P, Hanahan D. A second signal supplied by insulin-like growth factor II in oncogene-induced tumorigenesis. Nature. 1994;369:414-418. |
| 31. | Vu TH, Hoffman AR. Promoter-specific imprinting of the human insulin-like growth factor-II gene. Nature. 1994;371:714-717. |
| 32. | Tang SH, Yang DH, Huang W, Zhou HK, Lu XH, Ye G. Hypomethylated P4 promoter induces expression of the insulin-like growth factor-II gene in hepatocellular carcinoma in a Chinese population. Clin Cancer Res. 2006;12:4171-4177. |
| 33. | Li X, Nong Z, Ekstrom C, Larsson E, Nordlinder H, Hofmann WJ, Trautwein C, Odenthal M, Dienes HP, Ekstrom TJ. Disrupted IGF2 promoter control by silencing of promoter P1 in human hepatocellular carcinoma. Cancer Res. 1997;57:2048-2054. |
| 34. | Fu XX, Su CY, Lee Y, Hintz R, Biempica L, Snyder R, Rogler CE. Insulinlike growth factor II expression and oval cell proliferation associated with hepatocarcinogenesis in woodchuck hepatitis virus carriers. J Virol. 1988;62:3422-3430. |
| 35. | Gray A, Tam AW, Dull TJ, Hayflick J, Pintar J, Cavenee WK, Koufos A, Ullrich A. Tissue-specific and developmentally regulated transcription of the insulin-like growth factor 2 gene. DNA. 1987;6:283-295. |
| 36. | Lee S, Park U, Lee YI. Hepatitis C virus core protein transactivates insulin-like growth factor II gene transcription through acting concurrently on Egr1 and Sp1 sites. Virology. 2001;283:167-177. |
| 37. | Lee YI, Lee S, Lee Y, Bong YS, Hyun SW, Yoo YD, Kim SJ, Kim YW, Poo HR. The human hepatitis B virus transactivator X gene product regulates Sp1 mediated transcription of an insulin-like growth factor II promoter 4. Oncogene. 1998;16:2367-2380. |
| 38. | Lee YI, Lee S, Das GC, Park US, Park SM, Lee YI. Activation of the insulin-like growth factor II transcription by aflatoxin B1 induced p53 mutant 249 is caused by activation of transcription complexes; implications for a gain-of-function during the formation of hepatocellular carcinoma. Oncogene. 2000;19:3717-3726. |
| 39. | Gong Y, Cui L, Minuk GY. The expression of insulin-like growth factor binding proteins in human hepatocellular carcinoma. Mol Cell Biochem. 2000;207:101-104. |
| 40. | Huynh H, Chow PK, Ooi LL, Soo KC. A possible role for insulin-like growth factor-binding protein-3 autocrine/paracrine loops in controlling hepatocellular carcinoma cell proliferation. Cell Growth Differ. 2002;13:115-122. |
| 41. | Martin DC, Fowlkes JL, Babic B, Khokha R. Insulin-like growth factor II signaling in neoplastic proliferation is blocked by transgenic expression of the metalloproteinase inhibitor TIMP-1. J Cell Biol. 1999;146:881-892. |
| 42. | Terada T, Okada Y, Nakanuma Y. Expression of immunoreactive matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases in human normal livers and primary liver tumors. Hepatology. 1996;23:1341-1344. |
| 43. | Piao Z, Choi Y, Park C, Lee WJ, Park JH, Kim H. Deletion of the M6P/IGF2r gene in primary hepatocellular carcinoma. Cancer Lett. 1997;120:39-43. |
| 44. | Byrd JC, Devi GR, de Souza AT, Jirtle RL, MacDonald RG. Disruption of ligand binding to the insulin-like growth factor II/mannose 6-phosphate receptor by cancer-associated missense mutations. J Biol Chem. 1999;274:24408-24416. |
| 45. | De Souza AT, Hankins GR, Washington MK, Fine RL, Orton TC, Jirtle RL. Frequent loss of heterozygosity on 6q at the mannose 6-phosphate/insulin-like growth factor II receptor locus in human hepatocellular tumors. Oncogene. 1995;10:1725-1729. |
| 46. | De Souza AT, Hankins GR, Washington MK, Orton TC, Jirtle RL. M6P/IGF2R gene is mutated in human hepatocellular carcinomas with loss of heterozygosity. Nat Genet. 1995;11:447-449. |
| 47. | Devi GR, De Souza AT, Byrd JC, Jirtle RL, MacDonald RG. Altered ligand binding by insulin-like growth factor II/mannose 6-phosphate receptors bearing missense mutations in human cancers. Cancer Res. 1999;59:4314-4319. |
| 48. | Sue SR, Chari RS, Kong FM, Mills JJ, Fine RL, Jirtle RL, Meyers WC. Transforming growth factor-beta receptors and mannose 6-phosphate/insulin-like growth factor-II receptor expression in human hepatocellular carcinoma. Ann Surg. 1995;222:171-178. |
| 49. | Yamada T, De Souza AT, Finkelstein S, Jirtle RL. Loss of the gene encoding mannose 6-phosphate/insulin-like growth factor II receptor is an early event in liver carcinogenesis. Proc Natl Acad Sci USA. 1997;94:10351-10355. |
| 50. | Enomoto A, Esumi M, Yamashita K, Takagi K, Takano S, Iwai S. Abnormal nucleotide repeat sequence in the TGF-betaRII gene in hepatocellular carcinoma and in uninvolved liver tissue. J Pathol. 2001;195:349-354. |
| 51. | Saeki A, Tamura S, Ito N, Kiso S, Matsuda Y, Yabuuchi I, Kawata S, Matsuzawa Y. Lack of frameshift mutations at coding mononucleotide repeats in hepatocellular carcinoma in Japanese patients. Cancer. 2000;88:1025-1029. |
| 52. | Wada I, Kanada H, Nomura K, Kato Y, Machinami R, Kitagawa T. Failure to detect genetic alteration of the mannose-6-phosphate/insulin-like growth factor 2 receptor (M6P/IGF2R) gene in hepatocellular carcinomas in Japan. Hepatology. 1999;29:1718-1721. |
| 53. | Cantarini MC, de la Monte SM, Pang M, Tong M, D’Errico A, Trevisani F, Wands JR. Aspartyl-asparagyl beta hydroxylase over-expression in human hepatoma is linked to activation of insulin-like growth factor and notch signaling mechanisms. Hepatology. 2006;44:446-457. |
| 54. | Fan ZR, Yang DH, Cui J, Qin HR, Huang CC. Expression of insulin like growth factor II and its receptor in hepatocellular carcinogenesis. World J Gastroenterol. 2001;7:285-288. |
| 55. | Breuhahn K, Vreden S, Haddad R, Beckebaum S, Stippel D, Flemming P, Nussbaum T, Caselmann WH, Haab BB, Schirmacher P. Molecular profiling of human hepatocellular carcinoma defines mutually exclusive interferon regulation and insulin-like growth factor II overexpression. Cancer Res. 2004;64:6058-6064. |
| 56. | Dong ZZ, Yao DF, Yao DB, Wu XH, Wu W, Qiu LW, Jiang DR, Zhu JH, Meng XY. Expression and alteration of insulin-like growth factor II-messenger RNA in hepatoma tissues and peripheral blood of patients with hepatocellular carcinoma. World J Gastroenterol. 2005;11:4655-4660. |
| 57. | Tsai JF, Jeng JE, Chuang LY, You HL, Ho MS, Lai CS, Wang LY, Hsieh MY, Chen SC, Chuang WL. Serum insulin-like growth factor-II and alpha-fetoprotein as tumor markers of hepatocellular carcinoma. Tumour Biol. 2003;24:291-298. |
| 58. | Sakai K, Clemmons DR. Glucosamine induces resistance to insulin-like growth factor I (IGF-I) and insulin in Hep G2 cell cultures: biological significance of IGF-I/insulin hybrid receptors. Endocrinology. 2003;144:2388-2395. |
| 59. | Denley A, Carroll JM, Brierley GV, Cosgrove L, Wallace J, Forbes B, Roberts CT Jr. Differential activation of insulin receptor substrates 1 and 2 by insulin-like growth factor-activated insulin receptors. Mol Cell Biol. 2007;27:3569-3577. |
| 60. | Tao Y, Pinzi V, Bourhis J, Deutsch E. Mechanisms of disease: signaling of the insulin-like growth factor 1 receptor pathway-therapeutic perspectives in cancer. Nat Clin Pract Oncol. 2007;4:591-602. |
| 61. | Kim SO, Park JG, Lee YI. Increased expression of the insulin-like growth factor I (IGF-I) receptor gene in hepatocellular carcinoma cell lines: implications of IGF-I receptor gene activation by hepatitis B virus X gene product. Cancer Res. 1996;56:3831-3836. |
| 62. | Lee YI, Han YJ, Lee SY, Lee YI, Park SK, Park YJ, Moon HB, Shin JH, Lee JH. Activation of insulin-like growth factor II signaling by mutant type p53: physiological implications for potentiation of IGF-II signaling by p53 mutant 249. Mol Cell Endocrinol. 2003;203:51-63. |
| 63. | Tanaka S, Wands JR. Insulin receptor substrate 1 overexpression in human hepatocellular carcinoma cells prevents transforming growth factor beta1-induced apoptosis. Cancer Res. 1996;56:3391-3394. |
| 64. | Tanaka S, Wands JR. A carboxy-terminal truncated insulin receptor substrate-1 dominant negative protein reverses the human hepatocellular carcinoma malignant phenotype. J Clin Invest. 1996;98:2100-2108. |
| 65. | Mohr L, Tanaka S, Wands JR. Ethanol inhibits hepatocyte proliferation in insulin receptor substrate 1 transgenic mice. Gastroenterology. 1998;115:1558-1565. |
| 66. | Pazienza V, Clement S, Pugnale P, Conzelman S, Foti M, Mangia A, Negro F. The hepatitis C virus core protein of genotypes 3a and 1b downregulates insulin receptor substrate 1 through genotype-specific mechanisms. Hepatology. 2007;45:1164-1171. |
| 67. | Rogler CE, Yang D, Rossetti L, Donohoe J, Alt E, Chang CJ, Rosenfeld R, Neely K, Hintz R. Altered body composition and increased frequency of diverse malignancies in insulin-like growth factor-II transgenic mice. J Biol Chem. 1994;269:13779-13784. |
| 68. | Tanaka S, Mohr L, Schmidt EV, Sugimachi K, Wands JR. Biological effects of human insulin receptor substrate-1 overexpression in hepatocytes. Hepatology. 1997;26:598-604. |
| 69. | Lau MM, Stewart CE, Liu Z, Bhatt H, Rotwein P, Stewart CL. Loss of the imprinted IGF2/cation-independent mannose 6-phosphate receptor results in fetal overgrowth and perinatal lethality. Genes Dev. 1994;8:2953-2963. |
| 70. | Ludwig T, Eggenschwiler J, Fisher P, D’Ercole AJ, Davenport ML, Efstratiadis A. Mouse mutants lacking the type 2 IGF receptor (IGF2R) are rescued from perinatal lethality in Igf2 and Igf1r null backgrounds. Dev Biol. 1996;177:517-535. |
| 71. | Wang ZQ, Fung MR, Barlow DP, Wagner EF. Regulation of embryonic growth and lysosomal targeting by the imprinted Igf2/Mpr gene. Nature. 1994;372:464-467. |
| 72. | Schirmacher P, Held WA, Yang D, Chisari FV, Rustum Y, Rogler CE. Reactivation of insulin-like growth factor II during hepatocarcinogenesis in transgenic mice suggests a role in malignant growth. Cancer Res. 1992;52:2549-2556. |
| 73. | Liu P, Terradillos O, Renard CA, Feldmann G, Buendia MA, Bernuau D. Hepatocarcinogenesis in woodchuck hepatitis virus/c-myc mice: sustained cell proliferation and biphasic activation of insulin-like growth factor II. Hepatology. 1997;25:874-883. |
| 74. | Cadoret A, Desbois-Mouthon C, Wendum D, Leneuve P, Perret C, Tronche F, Housset C, Holzenberger M. c-myc-induced hepatocarcinogenesis in the absence of IGF-I receptor. Int J Cancer. 2005;114:668-672. |
| 75. | Harris TM, Rogler LE, Rogler CE. Reactivation of the maternally imprinted IGF2 allele in TGFalpha induced hepatocellular carcinomas in mice. Oncogene. 1998;16:203-209. |
| 76. | Lewis BC, Klimstra DS, Socci ND, Xu S, Koutcher JA, Varmus HE. The absence of p53 promotes metastasis in a novel somatic mouse model for hepatocellular carcinoma. Mol Cell Biol. 2005;25:1228-1237. |
| 77. | Haddad R, Held WA. Genomic imprinting and Igf2 influence liver tumorigenesis and loss of heterozygosity in SV40 T antigen transgenic mice. Cancer Res. 1997;57:4615-4623. |
| 78. | Martin DC, Ruther U, Sanchez-Sweatman OH, Orr FW, Khokha R. Inhibition of SV40 T antigen-induced hepatocellular carcinoma in TIMP-1 transgenic mice. Oncogene. 1996;13:569-576. |
| 79. | Martin DC, Sanchez-Sweatman OH, Ho AT, Inderdeo DS, Tsao MS, Khokha R. Transgenic TIMP-1 inhibits simian virus 40 T antigen-induced hepatocarcinogenesis by impairment of hepatocellular proliferation and tumor angiogenesis. Lab Invest. 1999;79:225-234. |
| 80. | Yang D, Alt E, Rogler CE. Coordinate expression of N-myc 2 and insulin-like growth factor II in precancerous altered hepatic foci in woodchuck hepatitis virus carriers. Cancer Res. 1993;53:2020-2027. |
| 81. | Ueda K, Ganem D. Apoptosis is induced by N-myc expression in hepatocytes, a frequent event in hepadnavirus oncogenesis, and is blocked by insulin-like growth factor II. J Virol. 1996;70:1375-1383. |
| 82. | Norstedt G, Levinovitz A, Moller C, Eriksson LC, Andersson G. Expression of insulin-like growth factor I (IGF-I) and IGF-II mRNA during hepatic development, proliferation and carcinogenesis in the rat. Carcinogenesis. 1988;9:209-213. |
| 83. | Ueno T, Takahashi K, Matsuguchi T, Ikejiri K, Endo H, Yamamoto M. Reactivation of rat insulin-like growth factor II gene during hepatocarcinogenesis. Carcinogenesis. 1988;9:1779-1783. |
| 84. | Wang Z, Ruan YB, Guan Y, Liu SH. Expression of IGF-II in early experimental hepatocellular carcinomas and its significance in early diagnosis. World J Gastroenterol. 2003;9:267-270. |
| 85. | Breuhahn K, Nussbaum T, Singer S, Schirmacher P. The insulin-like growth factor (IGF) signaling pathway: strategies for successfull therapeutic tasks in cancer treatment. Current Cancer Therapy Reviews. 2006;2:157-167. |
| 86. | Gschwind A, Fischer OM, Ullrich A. The discovery of receptor tyrosine kinases: targets for cancer therapy. Nat Rev Cancer. 2004;4:361-370. |
| 87. | Araki K, Sangai T, Miyamoto S, Maeda H, Zhang SC, Nakamura M, Ishii G, Hasebe T, Kusaka H, Akiyama T. Inhibition of bone-derived insulin-like growth factors by a ligand-specific antibody suppresses the growth of human multiple myeloma in the human adult bone explanted in NOD/SCID mouse. Int J Cancer. 2006;118:2602-2608. |
| 88. | Feng Y, Zhu Z, Xiao X, Choudhry V, Barrett JC, Dimitrov DS. Novel human monoclonal antibodies to insulin-like growth factor (IGF)-II that potently inhibit the IGF receptor type I signal transduction function. Mol Cancer Ther. 2006;5:114-120. |
| 89. | Jacobs S, Cook S, Svoboda ME, Van Wyk JJ. Interaction of the monoclonal antibodies alpha IR-1 and alpha IR-3 with insulin and somatomedin-C receptors. Endocrinology. 1986;118:223-226. |
| 90. | Hailey J, Maxwell E, Koukouras K, Bishop WR, Pachter JA, Wang Y. Neutralizing anti-insulin-like growth factor receptor 1 antibodies inhibit receptor function and induce receptor degradation in tumor cells. Mol Cancer Ther. 2002;1:1349-1353. |
| 91. | Sachdev D, Li SL, Hartell JS, Fujita-Yamaguchi Y, Miller JS, Yee D. A chimeric humanized single-chain antibody against the type I insulin-like growth factor (IGF) receptor renders breast cancer cells refractory to the mitogenic effects of IGF-I. Cancer Res. 2003;63:627-635. |
| 92. | Cohen BD, Baker DA, Soderstrom C, Tkalcevic G, Rossi AM, Miller PE, Tengowski MW, Wang F, Gualberto A, Beebe JS. Combination therapy enhances the inhibition of tumor growth with the fully human anti-type 1 insulin-like growth factor receptor monoclonal antibody CP-751,871. Clin Cancer Res. 2005;11:2063-2073. |
| 93. | Lu D, Zhang H, Koo H, Tonra J, Balderes P, Prewett M, Corcoran E, Mangalampalli V, Bassi R, Anselma D. A fully human recombinant IgG-like bispecific antibody to both the epidermal growth factor receptor and the insulin-like growth factor receptor for enhanced antitumor activity. J Biol Chem. 2005;280:19665-19672. |
| 94. | Beck A, Bussat MC, Zorn N, Robillard V, Klinguer-Hamour C, Chenu S, Goetsch L, Corvaïa N, Van Dorsselaer A, Haeuw JF. Characterization by liquid chromatography combined with mass spectrometry of monoclonal anti-IGF-1 receptor antibodies produced in CHO and NS0 cells. J Chromatogr B Analyt Technol Biomed Life Sci. 819(2):203-218. |
| 95. | Maloney EK, McLaughlin JL, Dagdigian NE, Garrett LM, Connors KM, Zhou XM, Blattler WA, Chittenden T, Singh R. An anti-insulin-like growth factor I receptor antibody that is a potent inhibitor of cancer cell proliferation. Cancer Res. 2003;63:5073-5083. |
| 96. | Goetsch L, Gonzalez A, Leger O, Beck A, Pauwels PJ, Haeuw JF, Corvaia N. A recombinant humanized anti-insulin-like growth factor receptor type I antibody (h7C10) enhances the antitumor activity of vinorelbine and anti-epidermal growth factor receptor therapy against human cancer xenografts. Int J Cancer. 2005;113:316-328. |
| 97. | Jackson-Booth PG, Terry C, Lackey B, Lopaczynska M, Nissley P. Inhibition of the biologic response to insulin-like growth factor I in MCF-7 breast cancer cells by a new monoclonal antibody to the insulin-like growth factor-I receptor. The importance of receptor down-regulation. Horm Metab Res. 2003;35:850-856. |
| 98. | Wang Y, Hailey J, Williams D, Wang Y, Lipari P, Malkowski M, Wang X, Xie L, Li G, Saha D. Inhibition of insulin-like growth factor-I receptor (IGF-IR) signaling and tumor cell growth by a fully human neutralizing anti-IGF-IR antibody. Mol Cancer Ther. 2005;4:1214-1221. |
| 99. | Di Guglielmo GM, Drake PG, Baass PC, Authier F, Posner BI, Bergeron JJ. Insulin receptor internalization and signalling. Mol Cell Biochem. 1998;182:59-63. |
| 100. | Blum G, Gazit A, Levitzki A. Substrate competitive inhibitors of IGF-1 receptor kinase. Biochemistry. 2000;39:15705-15712. |
| 101. | Blum G, Gazit A, Levitzki A. Development of new insulin-like growth factor-1 receptor kinase inhibitors using catechol mimics. J Biol Chem. 2003;278:40442-40454. |
| 102. | Parrizas M, Gazit A, Levitzki A, Wertheimer E, LeRoith D. Specific inhibition of insulin-like growth factor-1 and insulin receptor tyrosine kinase activity and biological function by tyrphostins. Endocrinology. 1997;138:1427-1433. |
| 103. | Girnita A, Girnita L, del Prete F, Bartolazzi A, Larsson O, Axelson M. Cyclolignans as inhibitors of the insulin-like growth factor-1 receptor and malignant cell growth. Cancer Res. 2004;64:236-242. |
| 104. | Vasilcanu D, Girnita A, Girnita L, Vasilcanu R, Axelson M, Larsson O. The cyclolignan PPP induces activation loop-specific inhibition of tyrosine phosphorylation of the insulin-like growth factor-1 receptor. Link to the phosphatidyl inositol-3 kinase/Akt apoptotic pathway. Oncogene. 2004;23:7854-7862. |
| 105. | Li W, Favelyukis S, Yang J, Zeng Y, Yu J, Gangjee A, Miller WT. Inhibition of insulin-like growth factor I receptor autophosphorylation by novel 6-5 ring-fused compounds. Biochem Pharmacol. 2004;68:145-154. |
| 106. | Garcia-Echeverria C, Pearson MA, Marti A, Meyer T, Mestan J, Zimmermann J, Gao J, Brueggen J, Capraro HG, Cozens R. In vivo antitumor activity of NVP-AEW541-A novel, potent, and selective inhibitor of the IGF-IR kinase. Cancer Cell. 2004;5:231-239. |
| 107. | Scotlandi K, Manara MC, Nicoletti G, Lollini PL, Lukas S, Benini S, Croci S, Perdichizzi S, Zambelli D, Serra M. Antitumor activity of the insulin-like growth factor-I receptor kinase inhibitor NVP-AEW541 in musculoskeletal tumors. Cancer Res. 2005;65:3868-3876. |
| 108. | Mitsiades CS, Mitsiades NS, McMullan CJ, Poulaki V, Shringarpure R, Akiyama M, Hideshima T, Chauhan D, Joseph M, Libermann TA. Inhibition of the insulin-like growth factor receptor-1 tyrosine kinase activity as a therapeutic strategy for multiple myeloma, other hematologic malignancies, and solid tumors. Cancer Cell. 2004;5:221-230. |
| 109. | Warshamana-Greene GS, Litz J, Buchdunger E, Garcia-Echeverria C, Hofmann F, Krystal GW. The insulin-like growth factor-I receptor kinase inhibitor, NVP-ADW742, sensitizes small cell lung cancer cell lines to the effects of chemotherapy. Clin Cancer Res. 2005;11:1563-1571. |
| 110. | Ji QS, Mulvihill MJ, Rosenfeld-Franklin M, Cooke A, Feng L, Mak G, O’Connor M, Yao Y, Pirritt C, Buck E. A novel, potent, and selective insulin-like growth factor-I receptor kinase inhibitor blocks insulin-like growth factor-I receptor signaling in vitro and inhibits insulin-like growth factor-I receptor dependent tumor growth in vivo. Mol Cancer Ther. 2007;6:2158-2167. |
| 111. | Wittman M, Carboni J, Attar R, Balasubramanian B, Balimane P, Brassil P, Beaulieu F, Chang C, Clarke W, Dell J. Discovery of a (1H-benzoimidazol-2-yl)-1H-pyridin-2-one (BMS-536924) inhibitor of insulin-like growth factor I receptor kinase with in vivo antitumor activity. J Med Chem. 2005;48:5639-5643. |
| 112. | Haluska P, Carboni JM, Loegering DA, Lee FY, Wittman M, Saulnier MG, Frennesson DB, Kalli KR, Conover CA, Attar RM. In vitro and in vivo antitumor effects of the dual insulin-like growth factor-I/insulin receptor inhibitor, BMS-554417. Cancer Res. 2006;66:362-371. |
| 113. | Hopfner M, Huether A, Sutter AP, Baradari V, Schuppan D, Scherubl H. Blockade of IGF-1 receptor tyrosine kinase has antineoplastic effects in hepatocellular carcinoma cells. Biochem Pharmacol. 2006;71:1435-1448. |
| 114. | Desbois-Mouthon C, Cacheux W, Blivet-Van Eggelpoel MJ, Barbu V, Fartoux L, Poupon R, Housset C, Rosmorduc O. Impact of IGF-1R/EGFR cross-talks on hepatoma cell sensitivity to gefitinib. Int J Cancer. 2006;119:2557-2566. |
| 115. | Huether A, Hopfner M, Baradari V, Schuppan D, Scherubl H. EGFR blockade by cetuximab alone or as combination therapy for growth control of hepatocellular cancer. Biochem Pharmacol. 2005;70:1568-1578. |
| 116. | Dent P, Han SI, Mitchell C, Studer E, Yacoub A, Grandis J, Grant S, Krystal GW, Hylemon PB. Inhibition of insulin/IGF-1 receptor signaling enhances bile acid toxicity in primary hepatocytes. Biochem Pharmacol. 2005;70:1685-1696. |
| 117. | Boyault S, Rickman DS, de Reynies A, Balabaud C, Rebouissou S, Jeannot E, Herault A, Saric J, Belghiti J, Franco D. Transcriptome classification of HCC is related to gene alterations and to new therapeutic targets. Hepatology. 2007;45:42-52. |
| 118. | D’Errico A, Grigioni WF, Fiorentino M, Baccarini P, Lamas E, De Mitri S, Gozzetti G, Mancini AM, Brechot C. Expression of insulin-like growth factor II (IGF-II) in human hepatocellular carcinomas: an immunohistochemical study. Pathol Int. 1994;44:131-137. |
| 119. | Ng IO, Lee JM, Srivastava G, Ng M. Expression of insulin-like growth factor II mRNA in hepatocellular carcinoma. J Gastroenterol Hepatol. 1998;13:152-157. |
| 120. | Sedlaczek N, Hasilik A, Neuhaus P, Schuppan D, Herbst H. Focal overexpression of insulin-like growth factor 2 by hepatocytes and cholangiocytes in viral liver cirrhosis. Br J Cancer. 2003;88:733-739. |
| 121. | Sohda T, Oka Y, Iwata K, Gunn J, Kamimura S, Shijo H, Okumura M, Yun K. Co-localisation of insulin-like growth factor II and the proliferation marker MIB1 in hepatocellular carcinoma cells. J Clin Pathol. 1997;50:135-137. |
| 122. | Nishiyama M, Wands JR. Cloning and increased expression of an insulin receptor substrate-1-like gene in human hepatocellular carcinoma. Biochem Biophys Res Commun. 1992;183:280-285. |
| 123. | Boissan M, Beurel E, Wendum D, Rey C, Lecluse Y, Housset C, Lacombe ML, Desbois-Mouthon C. Overexpression of insulin receptor substrate-2 in human and murine hepatocellular carcinoma. Am J Pathol. 2005;167:869-877. |