INTRODUCTION
Intraductal papillary neoplasm of the bile duct (IPN-B) is a neoplastic lesion preceding invasive intrahepatic cholangiocarcinoma (ICC) and is a new definition of a tumor with papillary growth in the intra- or extra-hepatic bile duct[12]. Histologically, IPN-B is characterized by a prominent papillary growth of atypical biliary epithelium with distinct fibrovascular cores and, frequently, mucin-secretion. It has previously been described as biliary papillomatosis[3], bile duct cystadenocarcinoma[4], intrahepatic cholangiocarcinoma (ICC)[5] or a mucin hypersecreting bile duct tumor[6].
On the other hand, biliary intraepithelial neoplasia (BilIN) showing, microscopically, growth of atypical biliary epithelium, has been identified as another type of neoplastic lesion preceding ICC[7]. BilIN is known often to progress to tubular adenocarcinoma, while IPN-B is associated with mucinous carcinoma and tubular adenocarcinoma[128]. Biliary papillary tumors, including IPN-B, resemble, histologically, intraductal papillary mucinous neoplasms of the pancreas (IPMN-P)[910]. In both organs, these neoplasms arise within the duct system and show a predominantly intraductal growth pattern, commonly an overproduction of mucin and an association with invasive adenocarcinoma[11].
Based on gross morphology, ICC can divided into three types: mass-forming, periductal-infiltrating and intraductal growth types[12]. Of these, the intraductal growth type, which corresponds to IPN-B, is associated with the most favorable outcome[13]. One feature of IPN-B is its relatively good prognosis after complete hepatic resection[1415]. Therefore, it is important to make a precise diagnosis at an early stage and to perform early surgical resection.
We report the clinical and histological findings of a patient who was diagnosed as IPN-B without hepatolithiasis and underwent a hemihepatectomy.
CASE REPORT
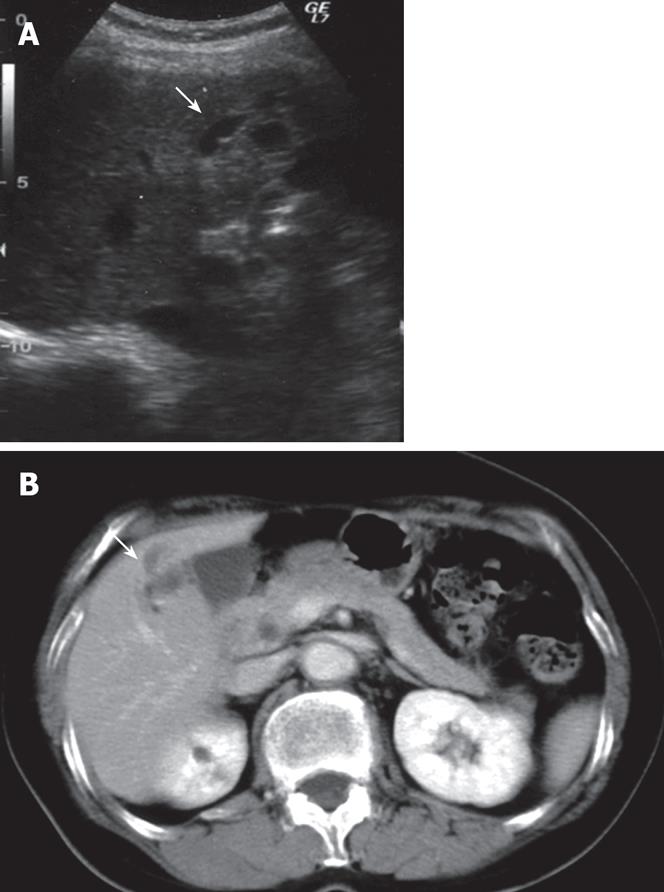
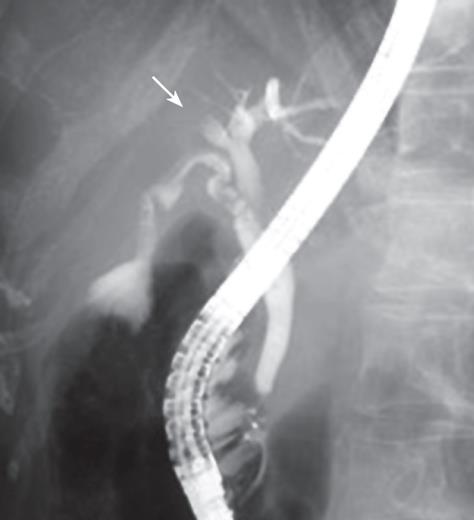
A 65-year-old woman was found to have dilatation of the intrahepatic bile duct in the right anterior segment during a general health examination in our hospital (Figure 1A). She had no history of liver disease, including hepatolithiasis. She was admitted to our hospital for detailed examination. Physical examination on admission revealed a height of 152.3 cm, a weight of 45 kg, a temperature of 37.1°C, a blood pressure of 117/61 mmHg, a pulse rate of 78/min, and a respiration rate of 22/min. Pertinent laboratory values included a white blood cell count (WBC) of 3180/&mgr;L, aspartate aminotransferase (AST) level of 30 IU/L, alanine aminotransferase (ALT) level of 30 IU/L, alkaline phosphatase (ALP) level of 400 IU/L, γ-glutamyl transferase (γ-GTP) level of 15 IU/L, CEA of 1.4 ng/mL, CA19-9 of 3 U/mL, and AFP of 5.6 ng/mL. Abdominal computed tomography (CT, Figure 1B) and magnetic resonance imaging (MRI) showed dilatation of the intrahepatic bile duct in the right anterior segment, but no solid mass around it. No metastases were found inside or outside the liver. Celiac angiography did not reveal any hypervascular tumors. Endoscopic retrograde cholangiopancreatography (ERCP) demonstrated an obstruction of the right bile duct at the root of the right hepatic duct but the common and left intrahepatic bile ducts were not dilated (Figure 2). These findings suggested that the dilatation of bile duct was due to the invasion of ICC and prompted us to plan a curative excision.
Figure 1 A: Abdominal ultrasonography (US) of the liver showing slight dilatation of bile ducts in the right liver (S5); B: Contrast-enhanced CT scan of the abdomen showing markedly dilatation of bile ducts in the liver (S5).
Solid masses were not observed.
Figure 2 Endoscopic retrograde cholangiopancreatography (ERCP) showing a biliary obstruction at the root of the hepatic duct, suggesting cholangiocarcinoma.
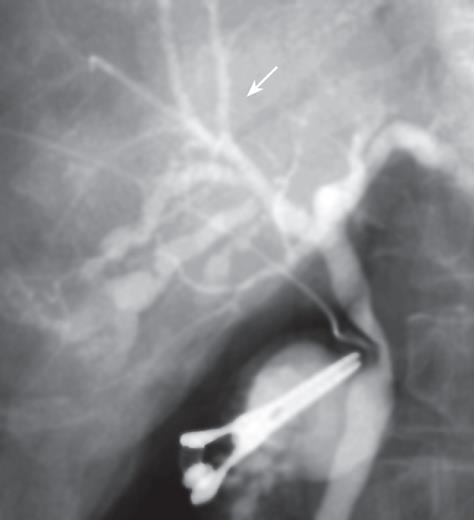
Neither ascites nor a palpable tumor of the liver was detected during laparotomy. Intraoperative US showed only dilatation of the bile duct (B5), as had been detected preoperatively. Subsequently, cholangiography demonstrated stenosis of the B5 bile duct and narrowness of the B8 bile duct (Figure 3). This feature was strikingly suspicious of cholangiocarcinoma, so we elected to perform a right-lobule hepatectomy. An intraoperative frozen section was negative for malignancy in the margin of the right bile duct.
Figure 3 Cholangiography showing stricture of the bile duct (B5) and narrowness of bile duct (B8) in the right liver.
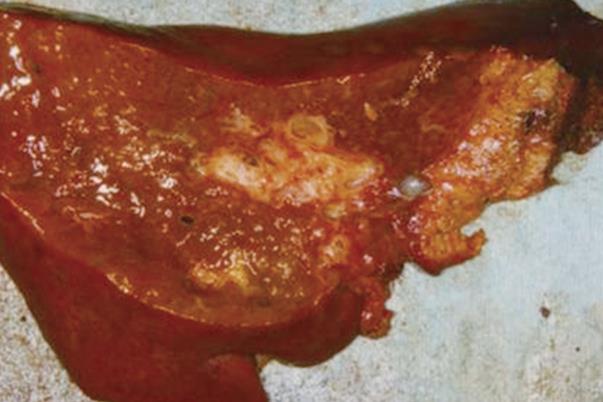
Macroscopic examination of the resected liver revealed bile duct dilatation in the right hepatic lobe (Figure 4). However, we could not identify any mass lesions and mucin was not observed macroscopically.
Figure 4 Macroscopic appearance of the resected right liver showing dilatation in the stump of the right intrahepatic duct, without a solid tumor component, which is surrounded by a fibrotic area.
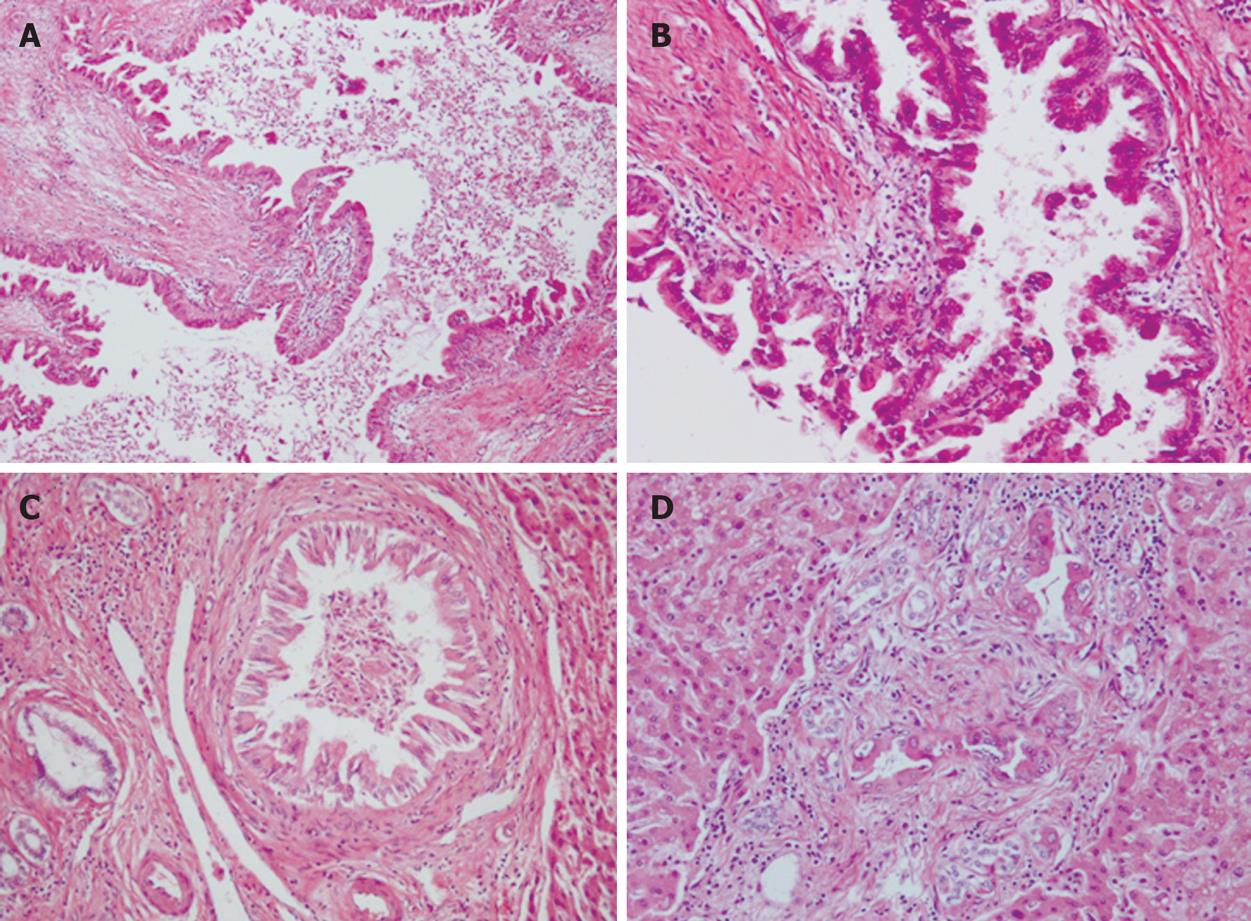
Microscopically, intrahepatic bile ducts were dilated with slight peri-ductal fibrosis. Within the dilated bile ducts, atypical biliary epithelium proliferated in a papillary fashion, associated with fine fibro-vascular cores (Figure 5A). Atypical cells showed nuclear enlargement, irregular nuclear membranes and distorted cellular polarity (Figure 5B). These atypical features corresponded to carcinoma in situ. Carcinoma cells proliferated continuously from the intrahepatic large bile ducts to small bile ducts (Figure 5C). Interestingly, carcinoma cells also were observed in proliferating bile ductules, which showed an irregular arrangement and slight dilatation (Figure 5D). Portal tracts showing irregular bile duct dilatation resembled Caroli’s disease; however, we could not identify any features of Caroli’s disease in the non-neoplastic area. No invasive growth could be detected.
Figure 5 Histopathological findings showing.
A: A view of the intrahepatic bile ducts dilated with peri-ductal fibrosis (× 100); B: Carcinoma cells continuously proliferating from the intrahepatic large bile ducts to small bile ducts (× 400); C: Carcinoma cells continuously proliferating from the intrahepatic large bile ducts to small bile ducts (× 200); D: Carcinoma cells in bile ductules around the small portal area (× 200).
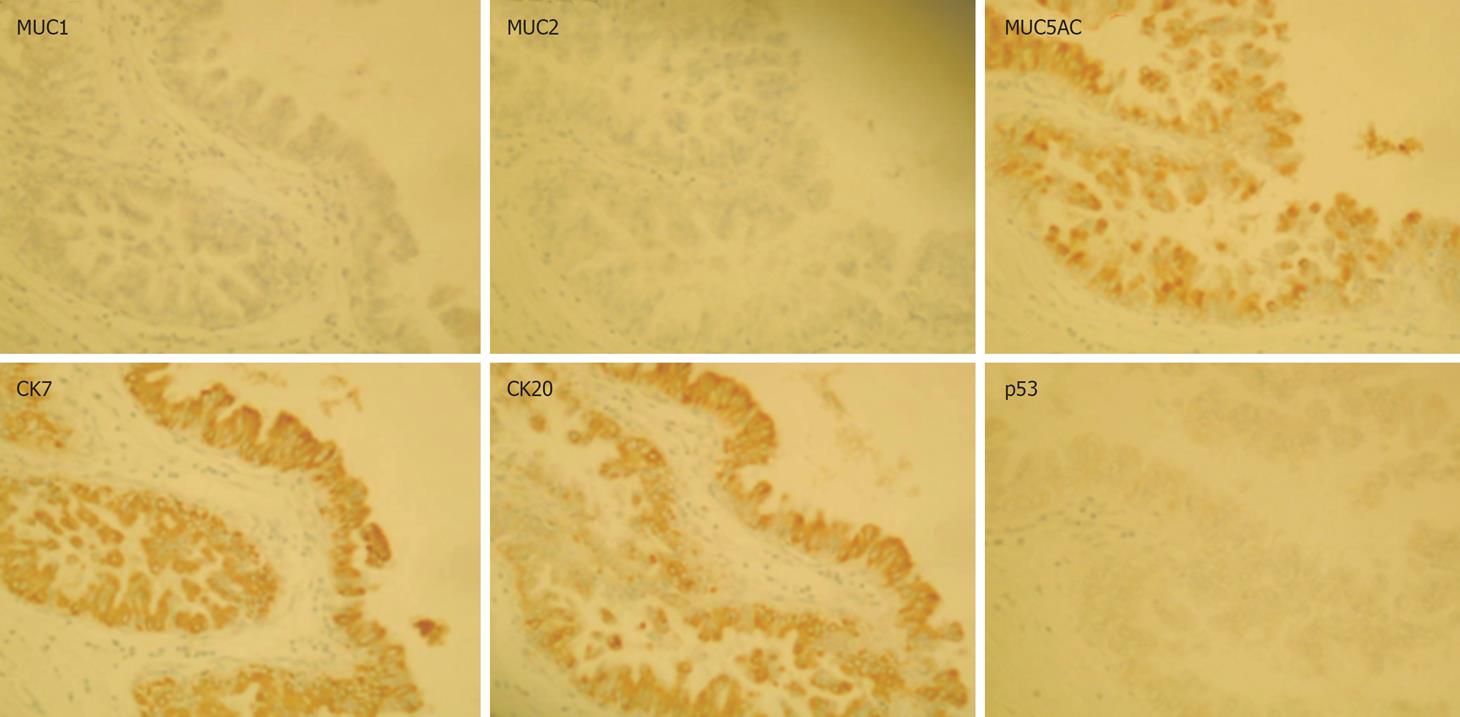
Immunohistochemical examination revealed that tumor cells were positive for CK7, CK20 and MUC5AC, and negative for MUC1, MUC2, and p53 (Figure 6). Finally, we diagnosed this lesion as IPN-B (carcinoma in situ), showing extensive intraductal spreading. The histopathological stage of the tumor according to the General Rules for Surgical and Pathological Studies on Cancer of Biliary Tract was the final stageI(pT1, pN0, P0, H1, M0) and final curability was evaluated as A. No event has been observed during the medical follow-up (an 18-mo period).
Figure 6 Immunohistological staining: MUC5AC, CK7 and CK20 are expressed in the tumor cells, whereas MUC1, MUC2 and p53 are not.
DISCUSSION
IPN-B is characterized by intrabiliary papillary growth, overproduction of mucin, multifocal occurrence, jaundice and cholangitis, and also has been reported to be accompanied by hepatolithiasis[12], which is recognized as being a risk factor for ICC. Recently, it has been reported that the number of cases of IPN-B without hepatolithiasis is much higher than those with it, and that it is hard to diagnose ICC with hepatolithiasis before laparotomy[1617]. In our case, when dilatation of the intrahepatic bile duct in the right anterior segment was detected by abdominal ultrasonography, neither a solid mass component nor hepatolithiasis was observed on imaging studies, and all laboratory data, including liver function and tumor markers (AFP, CEA, CA19-9), were within normal ranges. Therefore, we had difficulty in determining whether this case was malignant.
Several types of primary liver tumor with apparent cystic changes were considered in the differential diagnosis of our case[18]. For instance, biliary mucinous cystadenocarcinoma, intraductal neoplasm of liver (IPNL) and cholangiocarcinoma arising in a congenital cystic liver fibrosis are known examples.
We performed ERCP to evaluate the cause of intrahepatic cholangiectasis. This showed an obstruction of the right bile duct at the root of the right hepatic duct, which might have been due to a mucinous plug. ERCP is recommended to determine the presence and location of suspected intraductal tumors before laparotomy[19].
Furthermore, we believe that cholangiography is a reliable procedure for investigating the cause of bile duct dilatation and the image showed abnormalities of the bile ducts that were strikingly suspicious of the infiltration of carcinoma cells along the intrahepatic biliary epithelium. These results demonstrated that cholangiographic images are helpful in diagnosing IPN-B and determining the extent of dissection in case of surgical treatment. Generally, the prognosis of ICC (especially the non-papillary type) at an advanced stage is poor, whereas surgical excision of IPN-B has a good prognosis[1320]. Early detection of cancer in IPN-B improves survival, although it is hard to make an accurate assessment of the tumor invasion at an early stage, when a curative excision can be performed. As mentioned above, ERCP and cholangiography are useful for the diagnosis in situ carcinoma such as our case besides CT and MRI. Otherwise, bile cytology or biopsy of the bile duct as complementary diagnostic tools may be helpful to diagnose malignancy. It is necessary to cumulate the number of IPN-B cases and to observe their clinical manifestations and long term survival in advanced stage, compared to ICC.
We observed some remarkable findings in terms of histopathology. First, we needed to distinguish IPN-B from BilIN. In general, the former is characterized by flat or low papillary growth of atypical biliary epithelium, whereas the latter is characterized by papillary growth of that tissue. In our case, prominent papillary growth of atypical biliary epithelium was predominant, and this was appropriate for the diagnosis of IBN-B. As mentioned above, it is said that IPN-B could be the counterpart of pancreatic IPMN, and it is divided into various clinical stages: adenoma, carcinoma in situ (CIS) and periductal infiltrating type[910]. There are a lot of similarities in terms of the clinicopathological findings. This case corresponds to CIS, because dysplasia of biliary epithelium was mild in the small portal vein area and severe in the large bile duct. Secondly, regarding the dilatation of the bile duct, we had to distinguish the dilatation with tumor from focal Caroli’s disease or congenital hepatic fibrosis, etc. However, we considered that the dilatation was associated with a tumor because we were not able to find evidence of disease in other areas. Most IPN-Bs are characterized by mucin over-production and dilatation of bile duct with a remarkable papillary mass, but these are not detected in some cases comparable to ours. Finally, low papillary carcinoma cells extended superficially along the intrahepatic bile ducts, which was a very interesting feature.
Zen et al reported various expression patterns of MUCs and cytokeratins in neoplastic biliary epithelia of BilIN and IPN-B with progression to ICC in hepatolithiasis[21]. IPN-B with hepatolithiasis was characterized predominantly by the intestinal phenotype (MUC2+/CK20+). However, in the present case, the immunophenotype was of the MUC2-/CK20+/MUC1-/MUC5AC+/CK7+ pattern, which corresponds to that of the pancreaticobiliary type[22]. This type consists of carcinoma cells, including CIS but not adenoma, and is likely predominant in such a case without hepatolithiasis. According to recent analyses, biliary papillary tumors are characterized by the common expression of MUC2, CDX2 and CK20, and CK7 is expressed in neoplastic lesion of biliary papillary tumors[23–25]. Identification of immunophenotypes of MUCs and CKs may aid following up patients in terms of progression.
Although there are some reports of carcinogenesis of ICC arising from IPN-B or IPNL, the carcinogenic pathways in IPN-B are still unclear. However, it has been reported recently that the state of field cancerization may affect the carcinogenesis[26]. It is proposed to investigate the phenotypic and genetic changes in IPN-B for improving diagnosis and therapy.
Recently, it has been reported that highly invasive IPN-B may be involved in cases of ICC at an advanced stage, although IPN-B is generally characterized by lower malignancy and well differentiated histology. Further analyses of cases are necessary to establish its clinical features, therapy and prognosis.
Peer reviewer: Simon D Taylor-Robinson, MD, Department of Medicine A, Imperial College London, Hammersmith Hospital, Du Cane Road, London W12 0HS, United Kingdom