Published online Mar 14, 2008. doi: 10.3748/wjg.14.1603
Revised: January 7, 2008
Published online: March 14, 2008
AIM: To study the therapeutic value of combination of cryosurgery and 125iodine seed implantation for locally advanced pancreatic cancer.
METHODS: Forty-nine patients with locally advanced pancreatic cancer (males 36, females 13), with a median age of 59 years, were enrolled in the study. Twelve patients had liver metastases. In all cases the tumors were considered unresectable after a comprehensive evaluation. Patients were treated with cryosurgery, which was performed intraoperatively or percutaneously under guidance of ultrasound and/or computed tomography (CT), and 125iodine seed implantation, which was performed during cryosurgery or post-cryosurgery under guidance of ultrasound and/or CT. A few patients received regional celiac artery chemotherapy.
RESULTS: Thirteen patients received intraoperative cryosurgery and 36 received percutaneous cryosurgery. Some patients underwent repeat cryosurgery. 125Iodine seed implantation was performed during freezing procedure in 35 patients and 3-9 d after cryosurgery in 14 cases. Twenty patients, 10 of whom had hepatic metastases received regional chemotherapy. At 3 mo after therapy, CT was repeated to estimate tumor response to therapy. Most patients showed varying degrees of tumor necrosis. Complete response (CR) of tumor was seen in 20.4% patients, partial response (PR), in 38.8%, stable disease (SD), in 30.6%, and progressive disease (PD), in 10.2%. Adverse effects associated with cryosurgery included upper abdomen pain and increased serum amylase. Acute pancreatitis was seen in 6 patients one of whom developed severe pancreatitis. All adverse effects were controlled by medical management with no poor outcome. There was no therapy-related mortality. During a median follow-up of 18 mo (range of 5-40), the median survival was 16.2 mo, with 26 patients (53.1%) surviving for 12 mo or more. Overall, the 6-, 12-, 24- and 36-mo survival rates were 94.9%, 63.1%, 22.8% and 9.5%, respectively. Eight patients had survival of 24 mo or more. The patient with the longest survival (40 mo) is still living without evidence of tumor recurrence.
CONCLUSION: Cryosurgery, which is far less invasive than conventional pancreatic resection, and is associated with a low rate of adverse effects, should be the treatment of choice for patients with locally advanced pancreatic cancer. 125Iodine seed implantation can destroy the residual surviving cancer cells after cryosurgery. Hence, a combination of both modalities has a complementary effect.
- Citation: Xu KC, Niu LZ, Hu YZ, He WB, He YS, Li YF, Zuo JS. A pilot study on combination of cryosurgery and 125iodine seed implantation for treatment of locally advanced pancreatic cancer. World J Gastroenterol 2008; 14(10): 1603-1611
- URL: https://www.wjgnet.com/1007-9327/full/v14/i10/1603.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.1603
Pancreatic cancer is a rapidly growing tumor that is nearly always fatal. The majority of pancreatic cancers are detected at a late stage of illness, and only a minority of patients are candidates for curative surgical resection. Overall, the 1-and 5-year survival rates are only 20% and 5%, respectively[1–3]. Paclitaxel and gemcitabine are considered to be effective agents in pancreatic cancer, but their response rates are no more than 20%, and the effectiveness is less than 6 mo[45]. Therefore, it is necessary to seek novel treatment modalities[67]. This report examines the role of combined cryosurgery and 125iodine seed implantation in the treatment of locally advanced pancreatic cancer.
From March 2001 to November 2007, forty-nine patients with locally advanced pancreatic cancer underwent cryosurgery combined with 125iodine seed implantation. There were 36 males and 13 females, aged 28-89 years, with a median age of 59 years. Tumor size ranged from 2.2-7.1 cm in the largest diameter. Twelve patients had hepatic metastasis. In all patients, the diagnosis was based on ultrasound, computed tomography (CT) and MRI imaging, and 38 patients had a positive histology. Before hospitalization, 14 cases had received 4-6 cycles of chemotherapy (gencitabine, cisplatin, 5-FU). All patients received a comprehensive evaluation and were considered to be unresectable. The patients were provided information on cryosurgery guidelines, and the study received ethical approval.
Cryosurgery was performed with intraoperative or percutaneous approaches. Intraoperative cryosurgery: Patients were administrated general anesthesia and were positioned for an upper abdominal incision. The involved pancreas was exposed by trans-peritoneal mobilization of the bowel and stomach. Once the pancreatic mass was identified, an 18-gauge Tru-Cut biopsy needle was used to obtain one or two cores of tissue from the solid mass. If it was determined that the tumor was unresectable, after a thorough investigation, cryosurgery was performed under direct vision and under ultrasound guidance. A variable number (one to three) of 2 or 3 mm cryoprobes were placed directly into the pancreatic mass and positioned under ultrasound guidance. In general, lesions smaller than 3 cm could be frozen reliably with a single centrally placed 3-mm probe, whereas larger lesions required multiple probes. A double cycle of freeze/thaw procedure was used with an argon gas-based cryosurgical unit (EndoCare, Inc., CA, USA). Each cryoprobe was cooled to -160°C and the resulting iceball was monitored with ultrasound until the frozen region encompassed the entire mass of the tumor with at least a “0.5-cm safe border”. The tissue was then allowed to slowly thaw to 0°C. A second cycle of freezing/thawing was performed after repositioning of the cryoprobes. The cryoprobes were then removed and the still-frozen tract made by the cryoprobe was packed with thrombin-coated Gelfoam to control bleeding. Metastases of the liver were treated with cryosurgery at the same time[89].
Percutaneous cryosurgery: The procedure was performed under local anesthesia and under guidance of ultrasound or CT. Based on the location of the tumor, cryoprobe insertion was often carried out via the retroperitoneal approach. Generally, 2 or 3 mm cryoprobes were used. For tumors greater than 3cm in size, 2 to 3 probes were used. For liver metastases, simultaneous cryosurgery was performed using additional cryoprobes which were inserted through the right intercostal space. The cryosurgery procedure was similar to that performed intraoperatively[9].
Seed implantation: The procedure was performed either at the time of cryosurgery or after cryosurgery through the percutaneous approach under ultrasound or CT guidance. The 125iodine seeds were implanted at the tumor border. The number of seeds employed depended on the tumor size, with each seed implanted at a distance of 0.5 cm.
Postoperative management: The patients were instructed to stop eating for at least 3 d after the procedure. An analogue of somatostatin was given by intravenous infusion, usually for 3-4 d, or extended further until the abdominal pain subsided and the elevated serum amylase levels normalized. Aprotinin (Trasylol), an inhibitor of pancreatic enzymes, and a proton pump inhibitor were given by intravenous infusion to patients with abdominal pain and elevated serum amylase levels.
Adjuvant regional chemotherapy: Infusion of chemotherapeutic drugs was initiated one wk after cryosurgery, via a catheter in the celiac artery or hepatic artery. The treatment consisted of cycles of 5-FU 500 mg/m2, mitomycin C 8.5 mg/m2 and gemcitabine 500 mg/m2, every 2 wk.
Postoperative follow-up was performed at one mo after treatment and every 3 mo thereafter. On each visit, the patients were assessed by tumor marker assay, abdominal ultrasonography, and CT. Some patients were examined by positron emission tomography-CT PET-CT. The efficacy of cryosurgery was evaluated based on tumor size and survival of the patients. Changes in tumor mass were measured according to The Response Evaluation Criteria in Solid Tumors (RECIST) protocol[10], which is based on objective measurements of the tumor size before and after treatment. Complete response (CR) means that all targeted lesion had disappearance (scar) or reduced to less than 25% of the original size. Partial response (PR) means a greater than 30% decrease in the sum of the largest diameter of all targeted lesions. Stable disease (SD) means less than 30% decrease in the sum of the largest diameter of all targeted lesions. Progressive disease (PD) means an increase of greater than 20% in the sum of the largest diameter of all targeted lesions.
All radiologic studies were reviewed by the same radiologist with an expertise in pancreatic imaging. Ultrasound-guided biopsy was performed for lesions that were suspicious for recurrence. Cryosurgery was repeated if histology showed a positive result. The presence of a persistent nodule on imaging studies without tumor activity on PET-CT, with decreasing or normal tumor markers (CA19-9), or no changes in the absence of any other treatment for an interval of at least 6 mo after cryosurgery, was considered as remnant tumor. Tumor recurrence was determined by a positive histology, or by the combination of an increase in the cryotreated lesion on ultrasound, CT or PET-CT imaging, an increase in the tumor markers or by the discovery of metastases.
Survival was calculated using the Kaplan-Meier test[11]. Prognostic factors influencing survival were tested using the Log-rank, Tarone-Ware or Breslow test for univariate analysis and Cox regression[12]; Cox’s proportional hazard model with the forward-stepwise method (likelihood ratio) was used for multivariate analysis with various covariates. A significant difference was indicated by P < 0.05. Statistical analysis was performed using SPSS version 11.5 (SPSS, Chicago, USA).
Thirteen patients received intraoperative cryosurgery, and 36 underwent percutaneous cryosurgery. Among the patients who received percutaneous cryosurgery, 17 received a second course of cryosurgery and 3 received three courses of cryosurgery. 125Iodine seed implantation was performed during cryosurgery in 35 patients, and 3-9 d after cryosurgery in 14 cases. The median number of 125iodine seeds implanted was 34, with a range of 18-54 seeds. Twenty patients received adjuvant regional chemotherapy, 10 of whom had hepatic metastases. Five patients received 1 cycle of chemotherapy, ten received 2 cycles, three 3 cycles and two 4 cycles.
Response to treatment: Based on CT findings, at 3 mo after treatment, most patients showed varying degrees of tumor necrosis. The results of CR, PR, SD and PD were 20.4% (10/49), 38.8% (19/49), 30.6% (15/49) and 10.2% (5/49), respectively.
Adverse reactions: As shown in Table 1, 69.4% of patients had abdominal pain, which usually subsided in 2-3 d. About one-half of the patients (51.0%) had elevated serum amylase levels, which generally ranged 1-2 times of the normal reference values and lasted for 5-7 d. Acute pancreatitis with acute abdominal pain, and elevated serum amylase levels to four times or more was seen in 6 patients (12.2%), one of whom developed severe pancreatitis with intra-abdominal fluid effusion, and serum amylase levels 12 times of the normal reference values. All patients with pancreatitis recovered with conservative management. Three patients (6.1%) had intra-abdominal bleeding, however, abdominal fluid obtained by paracentesis did not have increased amylase levels. The intra-abdominal bleeding disappeared within four days. Nearly one-half of the patients (53.1%) had fever of 38-39.5°C, accompanied with chills. Fever persisted for 3-4 d, generally less than 7 d. Two patients had pulmonary infection, which recovered within 7-10 d with antibiotic therapy. Two patients aged 78 and 91 years, developed cerebral infarction and myocardial infarction respectively. There was no treatment-related mortality.
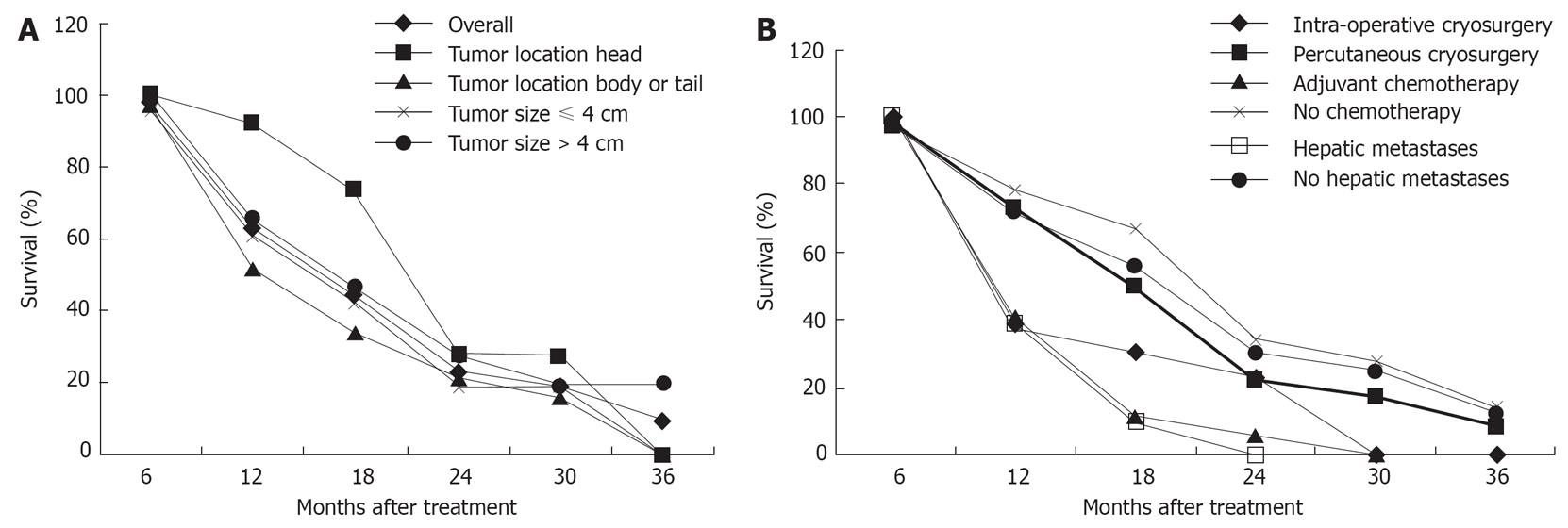
During a median follow-up of 18 mo (range of 5-40 mo), the median duration of survival was 16.2 mo. Twenty-six patients (53.1%) survived 12 mo or more, 8 of whom lived for 24 mo or more. The patient with the longest survival (40 mo) is still living without any evidence of tumor recurrence. A total of 36 patients died, in whom 17 died of cancer spread, 11 with hepatic metastases died of liver failure, 5 of cardio-cerebral vascular diseases and 3 of unknown causes. The 6-, 12-, 24- and 36-mo survival rates were 94.9%, 63.1%, 22.8% and 9.5% respectively (Figure 1A).
Univariate analysis was performed for factors influencing survival. Of the 5 variables tested, adjuvant chemotherapy and hepatic metastases were associated with a poor prognosis. The mode of cryosurgery (intra-operative vs percutaneous), tumor size (≤ 4 cm vs > 4 cm), and location (head vs body or tail) did not show independent significance for prognosis (Figure 1A and B).
The univariate analysis (Breslow test) of median survival in the different subgroups of patients with pancreatic cancer is shown in Table 2. The following factors were associated with longer median survival: cancer of pancreatic head, absence of hepatic metastases and absence of adjuvant chemotherapy.
| Patient subgroups | n | Median survival (mo) | P |
| Tumor location | |||
| Pancreatic head | 15 | 22 | 0.0204 |
| Pancreatic body or tail | 34 | 12 | |
| Tumor size | |||
| ≤ 4 cm | 24 | 13 | 0.7425 |
| > 4 cm | 25 | 14 | |
| Mode of cryosurgery | |||
| Inoperative | 13 | 11 | 0.1907 |
| Percutaneous | 36 | 14 | |
| Adjuvant chemotherapy | |||
| Yes | 20 | 11 | 0.0006 |
| No | 29 | 22 | |
| Hepatic metastases | |||
| Yes | 12 | 11 | 0.0088 |
| No | 37 | 19 |
A Cox model for multivariate regression analysis showed that apart from adjuvant chemotherapy, of the six factors tested, including patient’s age, gender, tumor size, location, mode of cryosurgery, number of 125iodine seeds implanted and hepatic metastases, only hepatic metastases was an independent prognostic factor (P = 0.007).
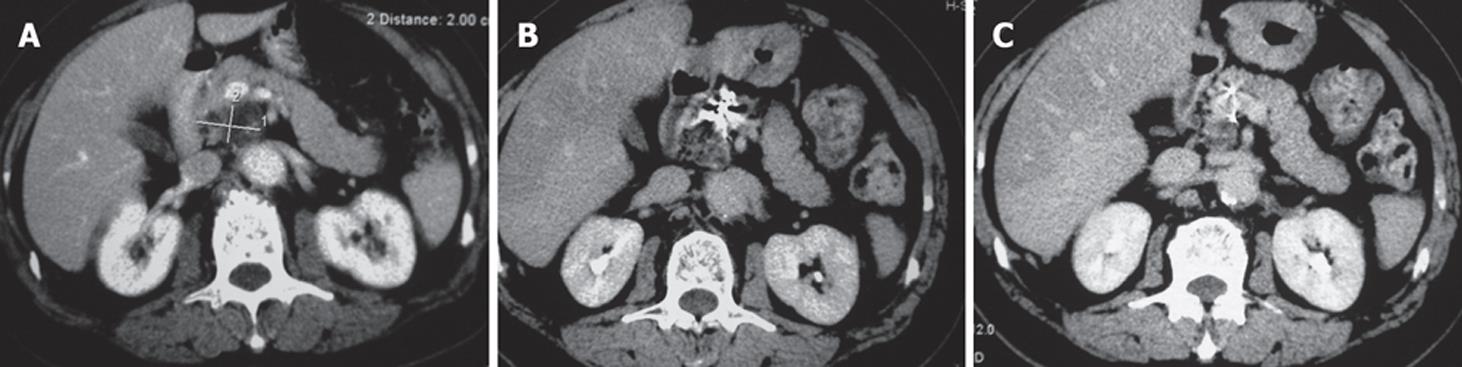
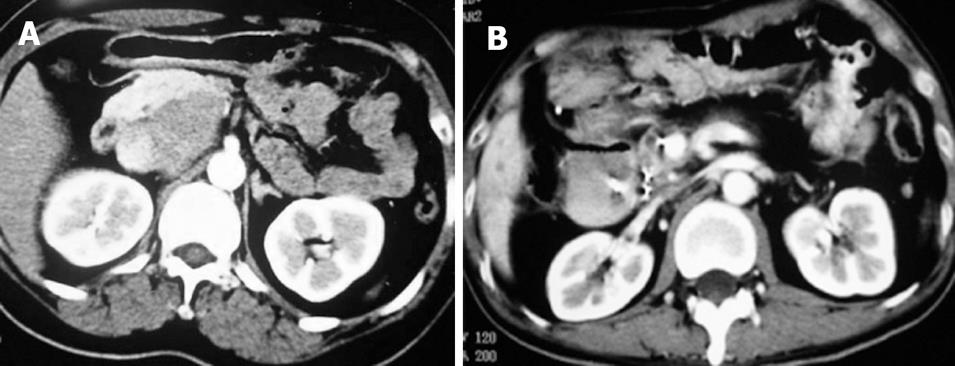
Case 1. Male, 80 years old. Ultrasound showed a 3 cm × 3 cm lesion in the pancreatic neck. Biopsy revealed cystadenocarcinoma. The patient underwent percutaneous cryosurgery with 125iodine seed implantation under CT guidance. Three mo after treatment, CT scan showed tumor necrosis, containing 125iodine particles. Current ultrasound and CT scan show that the original tumor has decreased to 1.5 cm × 1.1 cm in size (Figure 2). The patient has had recurrence-free survival of 40 mo.
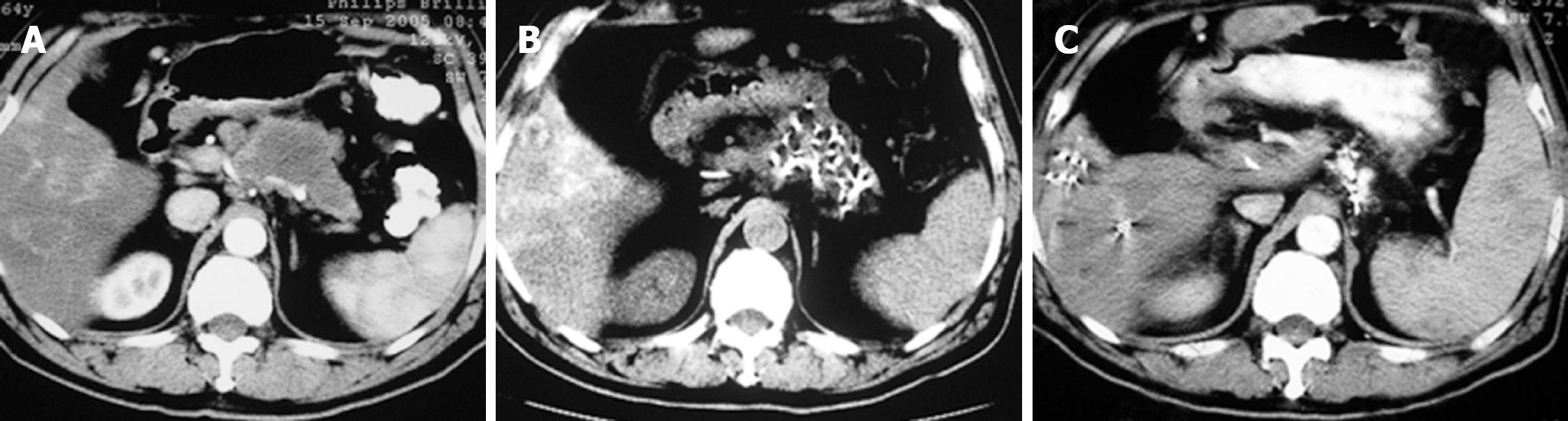
Case 2. Male, 61 years old. CT scan showed low-density areas, 4 cm × 5.5 cm in size in the body of pancreas and 3 intrahepatic lesions ranging from 2 cm to 5 cm in size. Biopsy showed adenocarcinoma. The serum CA19-9 was 512 IU. The patient underwent percutaneous cryosurgery and 125iodine seed implantation under CT/ ultrasound guidance for the pancreatic lesion and hepatic metastases. Repeat CT scan showed tumor shrinkage and stability of lesions in both the pancreas and liver (Figure 3). Ultrasound-guided biopsy showed no evidence of cancer. CA19-9 levels decreased to < 40 IU. The patient is now alive for 27 mo.
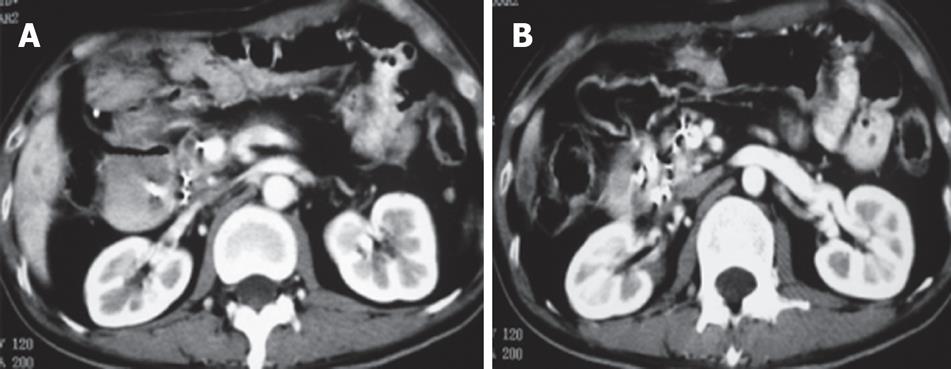
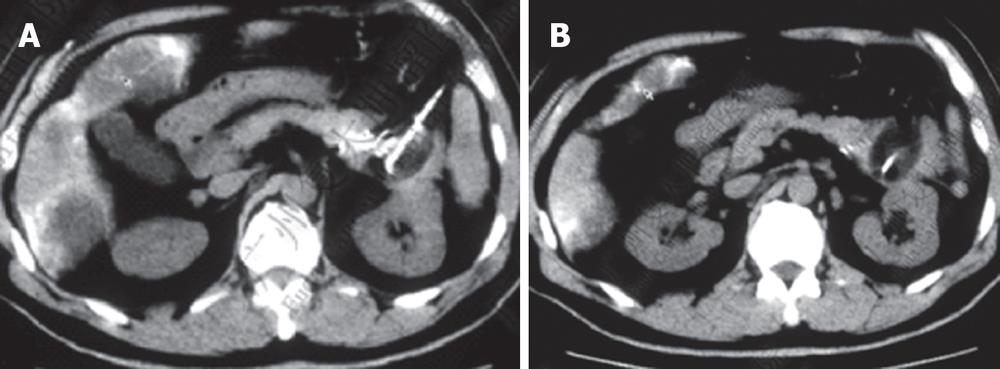
Case 3. Male, 36 years old. Ultrasound and CT revealed a mass in the pancreatic head with dilated common bile duct. Serum CA19-9 was 210 IU. The patient underwent laparotomy which revealed a mass, 5 cm × 5 cm in size in the pancreatic head. Biopsy showed moderately differentiated adenocarcinoma. A palliative cholecystojejunostomy was carried out to relieve the obstructive jaundice, and cryosurgery was performed under direct vision and ultrasound guidance. A repeat CT at three mo after treatment showed shrinkage and necrosis of the pancreatic mass with “honeycomb”-like change (Figure 4). CA19-9 had decreased to 48 IU. The patient survived for 19 mo.
Case 4. Male, 67 years old, with obstructive jaundice was found to have a mass, 5 cm × 3 cm in size in the pancreatic head with dilated common bile duct and gallbladder. Biopsy of the mass showed moderately differentiated mucinous adenocarcinoma. He was treated with percutaneous cryosurgery and 125iodine seed implantation. CT at 8 mo after treatment showed shrinkage and necrosis of the pancreatic mass (Figure 5).
Case 5. Female, 59 years old. CT scan showed a mass, 4 cm × 3 cm in size, in the pancreatic tail. Biopsy revealed adenocarcinoma. Percutaneous cryosurgery with 125iodine seed implantation was performed (Figure 6). Follow-up after 14 mo of treatment showed stable pancreatic tumor. The patient is currently alive 28 mo after diagnosis.
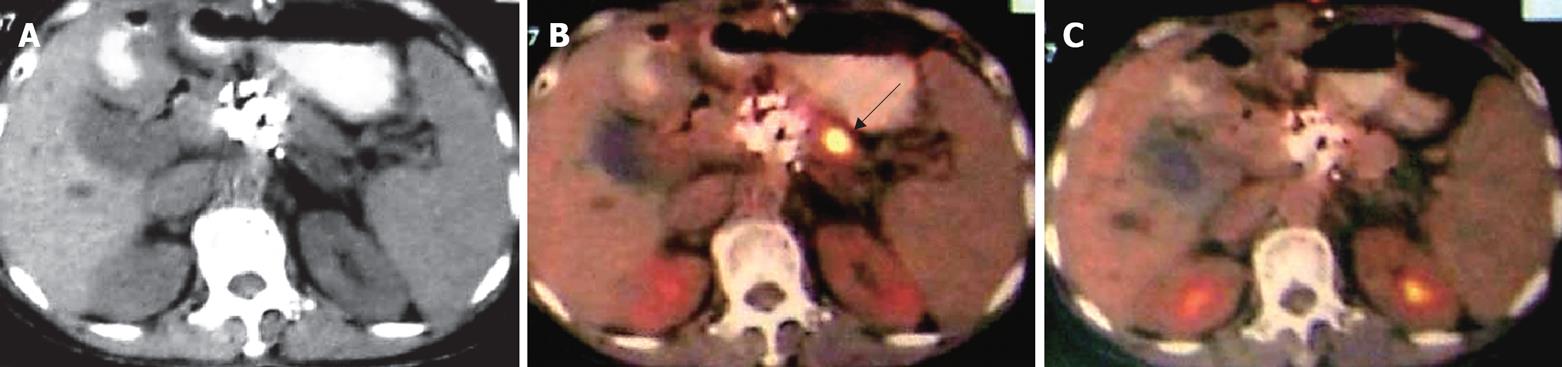
Case 6. Famale, 59 years old. Ultrasound and CT showed a mass of the pancreatic head, 4 cm × 4 cm in size. Biopsy showed poor-differentiated adenocarcinoma. She underwent percutaneous cryosurgery and 125iodine seed implantation. Twelve mo later, the tumor in the pancreatic head was stable, however a new lesion has appeared in the pancreatic body. The patient underwent a second course of percutaneous cryosurgery for the lesion in the pancreatic body. Follow-up PET-CT at 3 mo after the treatment, showed a significant decrease in the metabolic activity of the original lesion (Figure 7).
Cryosurgery has provided a novel therapeutic approach to the treatment of benign and malignant tumors, especially unresectable tumors[13]. A number of clinical trials have been published using this modality for the treatment of liver cancer, prostate cancer, kidney tumors, and breast cancer, with encouraging results[1415].
There are few reports on the use of cryosurgery for the treatment of pancreatic cancer. Kovach[16] reported 9 patients with unresectable pancreatic cancer who received a total of 10 sessions of intraoperative cryosurgery under ultrasound guidance. There was no cryosurgery-related mortality and no post-cryosurgery pancreatic fistulae or pancreatitis. Following treatment, patients experienced alleviation of pain and reduction in the use of analgesic agents. All patients were able to take normal diet at the time of discharge from the hospital. Patiutko[17] treated 30 patients with locally advanced pancreatic cancer with a combination of cryosurgery and radiation. All patients had effective control of pain, reduction in CA19-9, improvement of performance, and increase in the survival rate. Korpan[18] summarized the experience of cryosurgery for pancreatic cancer, and concluded that most patients obtained good results with this therapeutic modality.
The effectiveness of cryosurgery is dependent upon complete cryoablation to all the targeted tissue. Tumor persistence or recurrence at the site of cryoablation is often the result of incomplete destruction. Temperatures lower than -40°C are believed to be necessary for tumor ablation. Ice-balls targeted lesions are thus necessary for complete destruction of the tumor, because the outer several millimeters of the iceball circumference are at nonlethal temperatures. The 1-cm ice-ball extension beyond the tumor borders is required for adequate ablation[1920]. However, because the pancreatic volume is relatively small, cancer often involves most of the gland, and over-freezing increases the risk of complications, it is often difficult to ensure the “1 cm safe border”. Therefore, we decided to use the combination of cryosurgery with 125iodine seed implantation for the treatment of the pancreatic cancer. 125Iodine with a half-life of 59 d provides γ radiation for a short distance, resulting in the death of the targeted cells. Brachytherapy using 125iodine seed implantation has been successfully used for the treatment of prostate cancer and metastatic or recurrent cancer[21–24]. As a result, the use of 125iodine seed implantation is likely to be complementary to cryosurgery .
In the present study, 49 patients with locally advanced pancreatic cancer were treated with a combination of cryosurgery and 125iodine seed implantation. Thirteen patients underwent intraoperative cryosurgery and 36 patients percutaneous cryosurgery under ultrasound and CT guidance. The tumors showed different degrees of necrosis, and the CR, PR and SD were 20.4%, 38.8% and 30.6%, respectively, and only 10.2% demonstrated PD. During the median follow-up of 18 mo (5-40 mo), the median survival was 16.2 mo, of whom 26 patients (53.1%) survived 12 mo or more. The 6-, 12-, 24- and 36-mo survival rates were 94.9%, 63.1%, 22.8% and 9.5%, respectively.
Currently, the conventional therapies for locally advanced pancreatic cancer are chemotherapy and radiotherapy. Previous reports showed a median survival of 6-10 mo in patients with locally advanced disease treated with 5-FU-based chemoradiation. Patients with metastatic disease had a shorter survival (3-6 mo)[1]. A recently described combination regimen that is under investigation consists of gemcitabine, 5-FU, cisplatin, capecitabine and/or radiation[25–34]. These combination therapies produced a median progression-free survival ranging from 3-10 mo, and median survival of 7-16 mo, the objective response rate of the tumors was 22%-40%, and 1-year survival was 20%-78% (less than 60% in most reports) (Table 3). The results in our series were similar to those reported previously. However, it is important to note that in this series there were 8 cases who survived for 24 mo or more. The patient with the longest survival is living for 40 mo, with no evidence of recurrence. The findings indicate that combination of cryosurgery and 125iodine seed implantation offers the possibility of complete remission.
| Reporter | No. of patients (n) | Therapy | Median progression-free survival (mo) | Median survival (mo) | Objective response (%) | Survival at 12 mo after treatment (%) |
| El-Rayer[25] | 47 | Gemcitabine, cisplatin, and infusional fluorouracil | 34 | |||
| Tokuuye[26] | 53 | Small-field radiotherapy in combination with concomitant chemotherapy | 10.2 | 35.2 | ||
| Okusaka[27] | 34 | Gemcitabine + 5-FU | 3.2 | 7.1 | 25 | 14.3 |
| Yamazaki[28] | 22 | Concurrent chemoradiotherapygemcitabine | 16 | 32 | 78 | |
| Isacoff[29] | 50 | 5-FU, mitomycin dipyridamole | 26 | 54 | ||
| Park[30] | 45 | Gemcitabine + capecitabine | 5.4 | 10.4 | 40 | |
| Ko[31] | 25 | Gemcitabine + cisplatin, re-radiation + capecitabine | 10.5 | 13.5 | 62 | |
| Polyzos[32] | 32 | Gemcitabine + 5-FU, folic acid, somatostatin | 7 | 7 | 22 | 20 |
| Michael[33] | 30 | Gemcitabine + 13-cis | 7.8 | |||
| Furuse[34] | Intraoperative radiation, 5-FU infusion | 7.8 | 8.1 (2 yr) | |||
| Present series | 38 | Cryosurgery and 125iodine seed implantation | 12 | CR + PR 59.2 | 63.1 |
Using univariate and multivariate analysis, presence of hepatic metastasis was an independent prognostic factor and was associated with poor outcome. It was surprising to note that patients who were underwent to adjuvant regional chemotherapy had a lower survival. This finding could in part be related to patient selection; patients receiving chemotherapy had more severe illness, and one-half had hepatic metastases.
By univariate analysis, it was observed that patients with cancer of pancreatic head had longer median survival compared with patients with cancer of pancreatic body or tail. The reasons may be that cancer of pancreatic head is detected relatively earlier because of the development of obstructive jaundice.
It is believed that tumor size is of critical importance in cryotherapy[35]. However, tumor size could not be confirmed as an independent prognostic factor in our analysis. This finding may be related to the possibility that the combination of cryosurgery and 125iodine seed implantation may effectively destroy the entire tumor or a greater part of the targeted tissue, even in the presence of a large mass.
A great deal of attention has been paid to the safety of cryosurgery in the treatment of pancreatic cancer. Korpan[8] performed an experimental study on dogs who received pancreatic cryosurgery using the disc cryoprobe. None of the animals developed complications and there was no cryosurgery-related mortality. Moreover, there was no post-cryosurgery bleeding, pancreatic fistulae or secondary infection. In our series, no cryosurgery-related mortality was observed. The main adverse effects were abdominal pain, fever and increased serum amylase levels. Some patients developed acute pancreatitis, but none had a poor outcome. In addition, 125iodine seed implantation can be performed at the same time, and is not accompanied with the adverse effects observed with chemo-radiotherapy. As a whole, combination therapy of cryosurgery and 125iodine seed implantation is a less invasive procedure.
Korpan[818] pointed out that there were almost no known contraindications to the use of cryosurgery for pancreatic cancer. For most patients with pancreatic cancer, cryosurgery can substitute conventional surgery. These observations need to be confirmed by more studies. According to our experience, cryosurgery has several advantages in the treatment of unresectable pancreatic cancer: (1) The conventional management of unresectable pancreatic cancer involves a bypass operation without removal of the tumor. Cryosurgery can make up this shortcoming of conventional therapy, by converting the surgery from “palliative” to “radical”. (2) Cryosurgery is less invasive, and has lower rate of complications compared with conventional resection. (3) Unresectable tumors can be treated with percutaneous cryosurgery under ultrasound or CT guidance, with similar efficacy as intraoperative cryosurgery and is much less invasive to the patient.(4) During percutaneous cryosurgery, other modalities, such as 125iodine seed implantation, can be used simultaneously. (5) Metastatic tumors can be treated simultaneously, using the combination technique. (6) Immune enhancement or activation after cryosurgery may occur probably due to quantitative and qualitative changes in the surface antigen (component) of tumor cells[36]. That is called “cryoimmunity”[37]. (7) The cryoablated cancerous tissue has increased sensitivity to chemo/radiotherapy[3839].
In conclusion, although the present data is preliminary, it indicates that combination of cryosurgery and 125iodine seed implantation may play an important role in the treatment of locally advanced pancreatic cancer. These findings warrant further refinement of the technique as well as initiation of controlled clinical studies to better define the true value of combination treatment in pancreatic cancer.
Pancreatic cancer is the fifth leading cause of cancer-related death for both men and women. Patient survival depends on the extent of the disease and patient’s performance status at diagnosis. Patients who undergo surgical resection for localized non-metastatic pancreatic cancer have an approximately 20% longer survival rate, with a median survival of 12-20 mo. However, patients with locally advanced disease have a median survival of only 6-10 mo. The current approach of using chemoradiation, including gemcitabine, has failed to improve the outcome of this disease. Therefore, it is important to develop newer treatment modalities which are able to improve tumor control without the increasing toxicity in patients with locally advanced pancreatic cancer.
Recently, cryosurgery has provided encouraging results in the treatment of prostate cancer and liver cancer. However, there is limited clinical experience using cryosurgery for the treatment of pancreatic cancer. Moreover, the use of 125Iodine seed implantation has not been reported in the treatment of pancreatic cancer.
To our knowledge, this is the first report on the use of combined cryosurgery and 125iodine seed implantation in the treatment of locally advanced pancreatic cancer. Both cryosurgery and 125iodine seed implantation are local ablative techniques, with different mechanisms of action, and it is proposed that their combined use may have a complementary effect.
Cryosurgery and 125iodine seed implantation can be performed during surgery or percutaneously. Both techniques are mini-invasive modalities and can be adapted to treat unresectable tumor. In more than 80% of patients with pancreatic cancer, surgical resection is not feasible at the time of diagnosis. Of the patients who undergo an operation with curative intent, only 30%-50% have successful removal of the tumor. Therefore, cryosurgery and 125iodine seed implantation are of special significance in the management of unresectable pancreatic cancer.
Pancreatic cancer is derived mainly from ductal tissue with adenocarcinoma being the most common malignancy. There are very few pancreatic cancers which are classified as adenosquamous, giant cell cancers, and mucinous cystadenocarcinomas. Microscopically, these tumors may vary from well-differentiated to undifferentiated tumors. Seventy to 80 percent of respectable pancreatic cancers have already spread into lymph nodes at diagnosis. Ultrasonography and CT are the principal means of diagnosis of pancreatic cancer.
This is an interesting and well written paper of much practical value. The presentation is adequate and easy to understand. The results of this paper, despite the limited case number and the short follow-up, suggest that benefit exists in the treatment of locally advanced pancreatic cancer with combined cryosurgery and 125iodine seed implantation.
| 1. | Wolff RA, Abbruzzese JL, Evans DB. Neoplasms of the exocrine pancreas. Cancer medicine. 5th ed. Singapore: Harcourt Asia Pte Ltd 2000; 1436-1464. |
| 2. | Xu KC, Xu P. The treatment of pancreatic cancer. Modern Therapy of Digestive Disease. Shanghai: Shanghai Science-Technology-Education Pub 2001; 618-624. |
| 3. | Ducreux M, Boige V, Malka D. Treatment of advanced pancreatic cancer. Semin Oncol. 2007;34:S25-S30. |
| 4. | Wilkowski R, Thoma M, Bruns C, Wagner A, Heinemann V. Chemoradiotherapy with gemcitabine and continuous 5-FU in patients with primary inoperable pancreatic cancer. JOP. 2006;7:349-360. |
| 5. | Eickhoff A, Martin W, Hartmann D, Eickhoff JC, Mohler M, Galle PR, Riemann JF, Jakobs R. A phase I/II multicentric trial of gemcitabine and epirubicin in patients with advanced pancreatic carcinoma. Br J Cancer. 2006;94:1572-1574. |
| 6. | Wada K, Takada T, Amano H, Yoshida M, Miura F, Toyota N, Kato K, Isaka T, Nagashima I. Trend in the management of pancreatic adenocarcinoma--Japan vs. US and Europe. Nippon Geka Gakkai Zasshi. 2006;107:187-191. |
| 7. | Claude L, Mornex F. Chemoradiation in pancreatic carcinoma. Cancer Radiother. 2003;7:254-265. |
| 8. | Korpan NN. Pancreas cryosurgery. Basics of Cryosurgery. Wein NewYork: Springer-Verlag 2001; 151-154. |
| 9. | Xu KC, Niu LZ, Hu YZ, Zuo JS. Pancreatic cancer. Cryosurgery for Cancer. Shanghai: Shanghai Science-Technology-Education Pub 2007; 234-245. |
| 10. | Tsuchida Y, Therasse P. Response evaluation criteria in solid tumors (RECIST): new guidelines. Med Pediatr Oncol. 2001;37:1-3. |
| 11. | Lee CI, Yan X, Shi NZ. Nonparametric estimation of bounded survival functions with censored observations. Lifetime Data Anal. 1999;5:81-90. |
| 12. | Ziegler A, Lange S, Bender R. Survival analysis: Cox regression. Dtsch Med Wochenschr. 2007;132 Suppl 1:e42-e44. |
| 13. | Gage AA, Baust JG. Cryosurgery - a review of recent advances and current issues. Cryo Letters. 2002;23:69-78. |
| 14. | Xu KC, Niu LZ, He WB, Guo ZQ, Hu YZ, Zuo JS. Percutaneous cryoablation in combination with ethanol injection for unresectable hepatocellular carcinoma. World J Gastroenterol. 2003;9:2686-2689. |
| 15. | Mouraviev V, Polascik TJ. Update on cryotherapy for prostate cancer in 2006. Curr Opin Urol. 2006;16:152-156. |
| 16. | Kovach SJ, Hendrickson RJ, Cappadona CR, Schmidt CM, Groen K, Koniaris LG, Sitzmann JV. Cryoablation of unresectable pancreatic cancer. Surgery. 2002;131:463-464. |
| 17. | Patiutko IuI, Barkanov AI, Kholikov TK, Lagoshnyi AT, Li LI, Samoilenko VM, Afrikian MN, Savel'eva EV. The combined treatment of locally disseminated pancreatic cancer using cryosurgery. Vopr Onkol. 1991;37:695-700. |
| 18. | Korpan NN. Cryosurgery: ultrastructural changes in pancreas tissue after low temperature exposure. Technol Cancer Res Treat. 2007;6:59-67. |
| 19. | Mala T, Samset E, Aurdal L, Gladhaug I, Edwin B, Soreide O. Magnetic resonance imaging-estimated three-dimensional temperature distribution in liver cryolesions: a study of cryolesion characteristics assumed necessary for tumor ablation. Cryobiology. 2001;43:268-275. |
| 20. | Seifert JK, Gerharz CD, Mattes F, Nassir F, Fachinger K, Beil C, Junginger T. A pig model of hepatic cryotherapy. In vivo temperature distribution during freezing and histopathological changes. Cryobiology. 2003;47:214-226. |
| 21. | Martinez-Monge R, Nag S, Martin EW. 125Iodine brachytherapy for colorectal adenocarcinoma recurrent in the pelvis and paraortics. Int J Radiat Oncol Biol Phys. 1998;42:545-550. |
| 22. | Holm HH, Juul N, Pedersen JF, Hansen H, Stroyer I. Transperineal 125iodine seed implantation in prostatic cancer guided by transrectal ultrasonography. 1983. J Urol. 2002;167:985-988; discussion 988-989. |
| 23. | Kaye KW, Olson DJ, Payne JT. Detailed preliminary analysis of 125iodine implantation for localized prostate cancer using percutaneous approach. J Urol. 1995;153:1020-1025. |
| 24. | Kumar PP, Good RR, Jones EO, Hahn FJ, McCaul GF, Gallagher TF, Cox TA, Leibrock LG, Skultety MF. A new method for treatment of unresectable, recurrent brain tumors with single permanent high-activity 125iodine brachytherapy. Radiat Med. 1986;4:12-20. |
| 25. | El-Rayes BF, Zalupski MM, Shields AF, Vaishampayan U, Heilbrun LK, Jain V, Adsay V, Day J, Philip PA. Phase II study of gemcitabine, cisplatin, and infusional fluorouracil in advanced pancreatic cancer. J Clin Oncol. 2003;21:2920-2925. |
| 26. | Tokuuye K, Sumi M, Kagami Y, Murayama S, Ikeda H, Ikeda M, Okusaka T, Ueno H, Okada S. Small-field radiotherapy in combination with concomitant chemotherapy for locally advanced pancreatic carcinoma. Radiother Oncol. 2003;67:327-330. |
| 27. | Okusaka T, Ishii H, Funakoshi A, Ueno H, Furuse J, Sumii T. A phase I/II study of combination chemotherapy with gemcitabine and 5-fluorouracil for advanced pancreatic cancer. Jpn J Clin Oncol. 2006;36:557-563. |
| 28. | Yamazaki H, Nishiyama K, Koizumi M, Tanaka E, Ioka T, Uehara H, Iishi H, Nakaizumi A, Ohigashi H, Ishikawa O. Concurrent chemoradiotherapy for advanced pancreatic cancer: 1,000 mg/m2 gemcitabine can be administered using limited-field radiotherapy. Strahlenther Onkol. 2007;183:301-306. |
| 29. | Isacoff WH, Bendetti JK, Barstis JJ, Jazieh AR, Macdonald JS, Philip PA. Phase II trial of infusional fluorouracil, leucovorin, mitomycin, and dipyridamole in locally advanced unresectable pancreatic adenocarcinoma: SWOG S9700. J Clin Oncol. 2007;25:1665-1669. |
| 30. | Park BB, Park JO, Lee HR, Lee J, Choi DW, Choi SH, Heo JS, Lee JK, Lee KT, Lim do H. A phase II trial of gemcitabine plus capecitabine for patients with advanced pancreatic adenocarcinoma. Cancer Chemother Pharmacol. 2007;60:489-494. |
| 31. | Ko AH, Quivey JM, Venook AP, Bergsland EK, Dito E, Schillinger B, Tempero MA. A phase II study of fixed-dose rate gemcitabine plus low-dose cisplatin followed by consolidative chemoradiation for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2007;68:809-816. |
| 32. | Polyzos A, Tsavaris N, Vafiadis I, Polyzos K, Griniatsos J, Felekouras E, Nikiteas NI, Halikias S, Nikou G. Phase II study of gemcitabine plus 5-fluorouracil biologically modulated by folinic acid plus long-acting formulation of octreotide (LAR) in patients with advanced pancreatic cancer. J BUON. 2005;10:357-364. |
| 33. | Michael A, Hill M, Maraveyas A, Dalgleish A, Lofts F. 13-cis-Retinoic acid in combination with gemcitabine in the treatment of locally advanced and metastatic pancreatic cancer--report of a pilot phase II study. Clin Oncol (R Coll Radiol). 2007;19:150-153. |
| 34. | Furuse J, Ishii H, Okusaka T, Nagase M, Nakachi K, Ueno H, Ikeda M, Morizane C, Yoshino M. Phase I study of fixed dose rate infusion of gemcitabine in patients with unresectable pancreatic cancer. Jpn J Clin Oncol. 2005;35:733-738. |
| 35. | Seifert JK, Junginger T. Prognostic factors for cryotherapy of colorectal liver metastases. Eur J Surg Oncol. 2004;30:34-40. |
| 36. | Joosten JJ, Muijen GN, Wobbes T, Ruers TJ. In vivo destruction of tumor tissue by cryoablation can induce inhibition of secondary tumor growth: an experimental study. Cryobiology. 2001;42:49-58. |
| 37. | Mir LM, Rubinsky B. Treatment of cancer with cryochemo-therapy. Br J Cancer. 2002;86:1658-1660. |
| 38. | Homasson JP, Pecking A, Roden S, Angebault M, Bonniot JP. Tumor fixation of bleomycin labeled with 57 cobalt before and after cryotherapy of bronchial carcinoma. Cryobiology. 1992;29:543-548. |
| 39. | Mir LM, Rubinsky B. Treatment of cancer with cryochemo-therapy. Br J Cancer. 2002;86:1658-1660. |